Abstract
The calpains are a family of Ca2+-dependent cysteine proteases implicated in various biological processes. In this family, calpain 6 (Capn6) is unique in that it lacks the active-site cysteine residues requisite for protease activity. During the search for genes downstream of the endothelin 1 (ET-1) signaling in pharyngeal-arch development, we identified Capn6. After confirming that the expression of Capn6 in pharyngeal arches is downregulated in ET-1-null embryos by in situ hybridization, we investigated its function. In Capn6-transfected cells, cytokinesis was retarded and was often aborted to yield multinucleated cells. Capn6 overexpression also caused the formation of microtubule bundles rich in acetylated α-tubulin and resistant to the depolymerizing activity of nocodazole. Green fluorescent protein-Capn6 overexpression, immunostaining for endogenous Capn6, and biochemical analysis demonstrated interaction between Capn6 and microtubules, which appeared to be mainly mediated by domain III. Furthermore, RNA interference-mediated Capn6 inactivation caused microtubule instability with a loss of acetylated α-tubulin and induced actin reorganization, resulting in lamellipodium formation with membrane ruffling. Taken together, these results indicate that Capn6 is a microtubule-stabilizing protein expressed in embryonic tissues that may be involved in the regulation of microtubule dynamics and cytoskeletal organization.
The calpains are a family of intracellular cysteine proteases whose activities are highly dependent on Ca2+ ions (9, 11, 16, 35, 36, 38). Fourteen members of the calpain family are known in mammals, whereas a large number of molecules with structural similarity have been reported to constitute a superfamily beyond species. Calpain 1 (Capn1) (μ-calpain) and Capn2 (m-calpain) are the representative members most extensively studied. They heterodimerize with a small regulatory subunit, Capn4. These “classical” calpains are ubiquitously expressed and are implicated in various cellular functions, such as migration, apoptosis, cell growth, and cell cycle progression. p94/Capn3 is a skeletal-muscle-specific calpain whose loss-of-function mutation causes limb girdle muscular dystrophy type 2A (11, 16, 35). Capn10 has been identified as a molecule associated with an increased risk for type 2 diabetes (11, 16). To date, many proteins have been proposed as candidates for their enzymatic substrates, although the physiologically relevant substrates remain largely unknown (11).
Many large-molecule calpains share a common four-domain structure consisting of domains I to IV (9, 11, 16, 35, 36, 38). Domain II is divided into subdomains IIa and IIb, which generate a substrate-binding cleft between them. The crystal structure of Capn2 has revealed that the cysteine residue in subdomain IIa and the histidine and asparagine residues in subdomain IIb form a catalytic triad (13, 30, 37). Domain III is related to the C2 domain, a Ca2+- and phospholipid-binding module (33). Domain IV is characterized by the presence of multiple EF-hand motifs in Capn1, -2, -3, -8, -9, -11, and -12, whereas other calpains have distinct structures in their C-terminal regions. In Capn5 and Capn6, the C-terminal structure is defined as the T domain, based on homology to Caenorhabditis elegans TRA-3, a nematode sex determination factor (35), but the function of this domain is unclear.
In the calpain family, Capn6 is unique in that it lacks the active-site catalytic cysteine residue and may not be a proteolytic enzyme (4). Capn6 is located on the X chromosome and is expressed during embryogenesis (4, 5, 26). In particular, the mandibular arch, somite and developing skeletal muscle, heart, epithelial border of the fourth ventricle, lobar bronchi, capsule of the lung and kidney, and chorionic plate of the placenta highly express Capn6 from the mid- to late-embryonic stages (5). In contrast, Capn6 expression appears to be downregulated after birth (5). These findings led us to postulate some important roles for Capn6 in embryonic development; however, no functional characterization has been reported for Capn6.
Various developmental processes are controlled by genetic hierarchies involved in interactions among different cell populations. In mandibular-arch development, endothelin 1 (ET-1), a 21-amino-acid peptide originally identified as a vascular-endothelium-derived vasoconstrictor (25, 42), has emerged as a regulator of the dorsoventral axis patterning through the induction of Dlx5 and Dlx6, homeobox genes belonging to the vertebrate Distal-less homologues (2, 7, 19, 21, 22, 29). However, key molecules downstream of this genetic hierarchy controlling pharyngeal-arch development remain largely unknown. While exploring such downstream genes by microarray analysis, we encountered Capn6 as a candidate molecule. Its expression pattern in the pharyngeal arch and unique molecular form among the calpain family members inclined us to pursue its role in pharyngeal-arch development. To start with, we decided to unveil the molecular function of this molecule. Here, we demonstrate that Capn6 is a microtubule-stabilizing protein. Capn6 overexpression causes a failure of cytokinesis, leading to the formation of multinucleated cells. Immunocytochemical and biochemical analyses revealed the association of Capn6 with microtubules, which appeared to be mediated by domain III. In addition, RNA interference (RNAi)-mediated Capn6 inactivation resulted in a loss of stable microtubules and induced actin reorganization, resulting in lamellipodium formation with membrane ruffling. These results suggest that Capn6 may be involved in the regulation of microtubule stability and actin organization. During pharyngeal-arch development, Capn6 may act as a downstream molecule of ET-1 signaling through its effect on cytoskeletal organization.
MATERIALS AND METHODS
Microarray analysis.
Total RNA was extracted from excised embryonic day 10.5 (E10.5) mandibular arches of ET-1−/− embryos with ISOGEN (Nippon Gene). Each cDNA sample was synthesized from the total RNA with a Superscript kit (Clontech) and was hybridized to a GeneChip array containing about 12,500 mouse genes. The hybridized arrays were scanned and analyzed with the Affymetrix GeneChip System. The relative abundance of each gene was estimated from the average difference of intensities. Change was calculated by taking the difference in average differences between the ET-1+/± (wild-type and homozygous) and ET-1−/− samples. The score of increase, decrease, or no change of expression for individual genes was defined on the basis of ranking the difference calls from three intergroup comparisons (3 × 3) as follows: no change = 0, marginal increase/decrease = 1/−1, and increase/decrease = 2/−2. The final rank was assigned by summing up the nine values corresponding to the difference calls, and the values varied from −18 to 18. The cutoff value for the final determination of increase/decrease was set as 9/−9.
Plasmids.
The 3′ untranslated region of Capn6 was amplified by reverse transcription (RT)-PCR of cDNA samples from E10.5 mandibular arches and subcloned into the pCRII TOPO vector (Invitrogen) for the generation of antisense riboprobes. For the expression of green fluorescent protein (GFP) fusion protein, the Capn6 open reading frame and its PCR-amplified deletion mutants were subcloned in frame into the pEGFP-C2 expression vector (Clontech). Wild-type Capn6 expression vectors were constructed by cutting out the GFP gene from pEGFP-Capn6 expression plasmids. For the glutathione S-transferase (GST) fusion protein, Capn6 and its deletion mutants were subcloned in frame into the PGEX-4T-1 vector (Amersham). All of the constructs were verified by sequencing.
Whole-mount in situ hybridization.
The ET-1−/− mouse was described previously (20). Embryos were harvested at E10.5 and fixed in 4% paraformaldehyde-phosphate-buffered saline (PBS). Whole-mount in situ hybridization was performed as described by Wilkinson using a digoxigenin-labeled Capn6 riboprobe (41).
Generation of Capn6 antibody.
Polyclonal antibody against mouse Capn6 was generated at Transgenic Inc. by injecting a rabbit with a keyhole limpet hemocyanin-conjugated synthetic oligopeptides corresponding to the C-terminal domain of Capn6 (amino acids 605 to 617). The bleeds were affinity purified using Affi-Gel 10 gel (Bio-Rad) coupled with the oligopeptides.
Cell culture and transfection.
NIH 3T3, HeLa, and HEK 293T cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and antibiotics at 37°C in 5% CO2. For transfection, cells were grown to 50 to 90% confluence and were treated with a mixture of plasmid DNA and LipofectAMINE PLUS or LipofectAMINE 2000 reagent (Invitrogen). After 2 to 3 h of incubation, the cells were refed with medium containing fetal calf serum and were allowed to recover for 24 to 48 h. Cells transfected with plasmids encoding GFP derivatives were observed by fluorescence microscopy. For stable transformants of GFP-tubulin, linearized pEGFP-Tub vector (BD Biosciences) was transfected into NIH 3T3 cells and selected with G418. Several independent clones were picked up, and expression was confirmed by fluorescence microscopy and Western blotting.
Immunofluorescence microscopy.
Cells were washed with preincubated general tubulin buffer (GTB) {80 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 7, 1 mM MgCl2, 1 mM EGTA} at 37°C, fixed with 4% paraformaldehyde in GTB, permeabilized with 0.2% Triton X-100 in GTB, and washed with GTB at room temperature. After being blocked with 5% skim milk in GTB, the cells were incubated with primary antibodies as follows; the rabbit polyclonal anti-Capn6 antibody, rabbit polyclonal anti-GFP antibody (MBL), and mouse monoclonal anti-α-tubulin and anti-acetylated α-tubulin antibodies (Sigma). Then, the cells were washed and stained with the secondary antibodies (fluorescein isothiocyanate- or rhodamine-conjugated donkey anti-mouse or rabbit immunoglobulin G; Jackson Immunoresearch). For actin staining, 2.5 units/ml rhodamine-phalloidin (Invitrogen) was added to the reaction buffer containing fluorescein isothiocyanate-conjugated secondary antibody. The cells were viewed using a fluorescence microscope (Nikon TE300) or a confocal microscope (Nikon D-ECLIPSE C1). Three-dimensional views (see Fig. 3F and G) were produced from 28 images in a two-dimensional Z stack spanning about 0.15 μm of the center of a single cell using EZ-C1 2.30 (Nikon) software.
FIG. 3.
Subcellular localization of GFP-Capn6 in HeLa cells at various stages of the cell cycle and its stabilizing effect on microtubules. (A to G) Cells were transfected with expression plasmids containing GFP (A and F) or GFP-Capn6 (B to E and G). After 24 h, the cells were stained with the anti-GFP antibody and anti-α-tubulin (A to E) or anti-acetylated α-tubulin (F and G) antibody. Notably, perinuclear microtubule bundling was observed in GFP-Capn6-transfected cells at interphase (B). During mitosis, GFP-Capn6 was distributed in association with the mitotic spindle (C). At telophase, GFP-Capn6 colocalized to the central spindle (D). At the late stage of cytokinesis, GFP-Capn6 colocalized to the midbody, whereas a large portion of the GFP signals were distributed throughout the cytoplasm (E). Three-dimensional Z-stack images show that the acetylated α-tubulin contents (Ac-tubulin), especially in the perinuclear region, were increased in GFP-Capn6-transfected cells (F and G). The scale bars indicate 20 μm. (H) HeLa cells were transfected with GFP (lane 1) or GFP-Capn6 (lane 2), lysed 16 h after transfection, and blotted with anti-acetylated α-tubulin, anti-α-tubulin, and anti-β-actin antibodies. HeLa cells were treated with DMSO (lane 3), 500 nM paclitaxel (lane 4), or 5 μM nocodazole (lane 5) for 1 h and blotted similarly to serve as controls for changes in acetylated α-tubulin levels. Acetylated-α-tubulin levels were increased in GFP-Capn6-transfected cells compared with GFP-transfected cells, whereas total α-tubulin levels were not increased. Blotting for β-actin served as an internal control. Similar results were obtained in three independent experiments. Representative data are shown.
Time-lapse video microscopy.
Cells were grown on 35-mm culture dishes set in a control chamber maintained at 5% CO2-95% air at 37°C. Cell images were obtained from 12 to 16 h after transfection at 5-min (for GFP-Capn6 overexpression experiments) or 1-min (for RNAi experiments) intervals on a TE300 microscope (Nikon) using a 20× Nikon objective lens (numerical aperture, 0.45) and an ORCA 100 cooled charge-coupled-device camera (Hamamatsu) and analyzed by AquaCosmos imaging software (Hamamatsu).
Western blotting.
For whole-cell lysate preparation, cells were solubilized in PBS containing 1% Triton X-100, 0.1% sodium deoxycholate, 0.02% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, 0.5 mM vanadate, and protease inhibitor cocktail (Sigma). For crude fractionation, cells were suspended in 80 mM PIPES, pH 7.0, 0.1% NP-40, 1 mM MgCl2, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM vanadate, and protease inhibitor cocktail (Sigma). After incubation for 15 min on ice, the suspension was passed through a 27-gauge syringe needle and centrifuged at 800 × g for 5 min at 4°C. The supernatant was saved as the soluble fraction. The pellet was resuspended in PBS containing 1% Triton X-100 and protease inhibitors, sonicated for 10 s, and subjected to protein analysis. The protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce), and equal volumes of proteins were separated by 7.5% SDS-polyacrylamide gel electrophoresis (PAGE) and then electrotransferred to a polyvinylidene difluoride (PVDF) membrane. After being blocked with 5% skim milk in 0.1% Tween 20 in Tris-buffered saline, pH 7.6, the blots were probed with primary antibodies as follows: rabbit polyclonal anti-Capn6 antibody; rabbit polyclonal anti-GFP antibody (MBL); rabbit polyclonal anti-μ-calpain (Capn1) antibody (Sigma); mouse monoclonal anti-α-tubulin, anti-acetylated α-tubulin, and anti-β-actin antibodies (Sigma); and goat polyclonal anti-MAP4 antibody (Santa Cruz). The membranes were then washed with 0.1% Tween 20 in Tris-buffered saline, pH 7.6, and incubated with peroxidase-conjugated anti-rabbit, anti-mouse, or anti-goat immunoglobulin G (DAKO). The signals were detected using the ECL chemiluminescence detection system (Amersham Bioscience).
Microtubule cosedimentation assay.
Cells were lysed in 80 mM PIPES, pH 7.0, 1% Triton X-100, 1 mM MgCl2, 1 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM vanadate, and protease inhibitor cocktail (Sigma) by passing them through a 30-gauge syringe needle. After incubation on ice for 30 min to depolymerize the microtubules, the lysate was centrifuged at 20,000 × g for 80 min at 4°C to remove cellular debris. The supernatant was divided into two tubes, and 20 μM paclitaxel (Taxol) or vehicle (dimethyl sulfoxide [DMSO]) was added to each sample. After incubation for 30 min at 37°C, the reaction mixture was centrifuged at 20,000 × g for 40 min at room temperature. The resultant pellets were resuspended in lysis buffer, separated by 7.5% SDS-PAGE, and subjected to Western blotting analysis with whole lysate and supernatants.
Microtubule binding assay on filter paper.
GST-fused full-length Capn6 protein was tested for microtubule binding on filter paper according to the method previously described by Nakaseko et al. (28). Briefly, GST-Capn6 fusion proteins were purified from bacterial lysates by using glutathione beads and subjected to SDS-PAGE. Then, the proteins were transferred to a PVDF filter and incubated in 100 mM PIPES, pH 6.9, 2 mM EGTA, 1 mM MgSO4, 0.1% Tween 20, and 5% skim milk, followed by an additional incubation with paclitaxel-stabilized bovine brain microtubules (50 μg/ml; Cytoskeleton Inc.) in the same buffer. The filter was then washed and immunoblotted with anti-tubulin antibody. Purified MAP2 (Cytoskeleton Inc.) served as a positive control.
GST pull-down assay.
For cell lysate preparation, NIH 3T3 cells were solubilized in pull-down buffer (20 mM Tris-HCl, pH 7.6, 1% Triton X-100, 0.25% sodium deoxycholate, 0.25 M NaCl) containing 1 mM phenylmethylsulfonyl fluoride, 0.5 mM vanadate, and protease inhibitor cocktail (Sigma). The solubilized cell lysates were frozen and thawed twice and sonicated 25 times for 10 s each time. Unbroken cells and cellular debris were removed by centrifugation at 20,000 × g at 4°C for 10 min. After incubation on ice for 15 min, the lysates were incubated with 20 μM paclitaxel and 1 mM GTP at 37°C for 30 min to stabilize the microtubules. Then, GST fusion proteins bound to the beads were mixed with lysates and incubated at 4°C for 6 h. The beads were washed three times with pull-down buffer containing protease inhibitors, and the bound proteins were eluted by adding 2.5× sample buffer and then boiled for 5 min. These samples were subjected to 10% (vol/vol) SDS-PAGE, and proteins were detected by Western blotting with anti-α-tubulin or anti-acetylated α-tubulin antibodies. The amounts of GFP fusion proteins were grossly estimated by Ponceau staining.
siRNA experiments.
Two different stealth small interfering RNA (siRNA) duplexes designated no. 1298 and 1715, which targeted nucleotides 1298 to 1322 and 1715 to 1739 of the mouse Capn6 mRNA sequence, respectively, and control siRNA containing the same nucleotides as no. 1298 but with scrambled sequence were synthesized by Invitrogen. The sense sequences of 1298, 1715, and control siRNAs were 5′-GGUUCCGUCUUCACCAUCUGUAUAU-3′, 5′-GGACCACUGACAUUCCUAUUAUCAU-3′, and 5′-GGUUGCUUCCACUACGUCAUUCUAU-3′, respectively. The siRNAs were transfected into NIH 3T3 cells using OligofectAMINE (Invitrogen) according to the manufacturer's protocol. In some experiments, GFP-tubulin-expressing NIH 3T3 cells were used. The efficiency of gene knockdown was evaluated by RT-PCR with primers specific for the mouse Capn6 mRNA (sense, 5′-GAATGTGGACATCCTACATG-3′; antisense, 5′-GCTGTCCTATTACTGAATAG-3′) and Western blotting. Expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin served as internal controls for RT-PCR and Western blotting, respectively.
RESULTS
Endothelin-1-dependent Capn6 expression in pharyngeal-arch development.
To explore target genes downstream of the ET-1 signaling involved in pharyngeal-arch development, we performed oligonucleotide microarray analysis on E10.5 ET-1−/− and ET-1+/± mandibular arches to identify genes downstream of the ET-1 signaling in mandibular-arch development. Among 132 genes with decreases in the E10.5 ET-1−/− mandibular arch in comparison to the ET-1+/± control (data not shown), Capn6 was found to be decreased by ∼2.8-fold.
To confirm the downregulation of Capn6 in the ET-1−/− mandibular arch, we performed whole-mount in situ hybridization. Capn6 expression was detected in the mandibular arch, heart, limb buds, and somites during embryogenesis, suggesting its tissue-specific role during embryonic development (Fig. 1A and C). In the ET-1−/− mutant, Capn6 expression was downregulated in the mandibular-arch mesenchyme, whereas its expression in the most dorsal mandibular epithelium was preserved (Fig. 1B and D). Capn6 expression in other regions was not affected in the ET-1−/− mutant (Fig. 1B).
FIG. 1.
Expression of Capn6 in mouse embryos. Whole-mount in situ hybridization was performed on E10.5 wild-type (A and C) and ET-1−/− (B and D) embryos using the Capn6 probe. Whole bodies (A and B) and excised mandibular arches (C and D) are shown. Capn6 is normally expressed in the mandibular arches, heart, and limb buds. In the ET-1−/− mutant, the expression of Capn6 in the mandibular arches is specifically downregulated, whereas Capn6 expression in other regions is not affected. ep, epithelium; fl, forelimb bud; hl, hindlimb bud; ht, heart; md, mandibular arch; me, mesenchyme; nt, neural tube; sm, somites.
Capn6 induces multinucleation by interfering with cytokinesis.
To characterize the function of Capn6, we fused Capn6 to GFP and overexpressed it in HeLa cells. Among cells transfected with GFP-Capn6, ∼20% became bi- or trinucleated 48 h after transfection, whereas only ∼3% of control GFP-transfected cells became multinucleated (Fig. 2A to C). Overexpression of wild-type Capn6 also induced multinucleation with comparable frequency (data not shown), suggesting that this effect was not due to GFP fusion. When HEK 293T cells were transfected, almost all Capn6-transfected cells became multinucleated within 7 to 10 days, sometimes with ∼10 nuclei (data not shown).
FIG. 2.
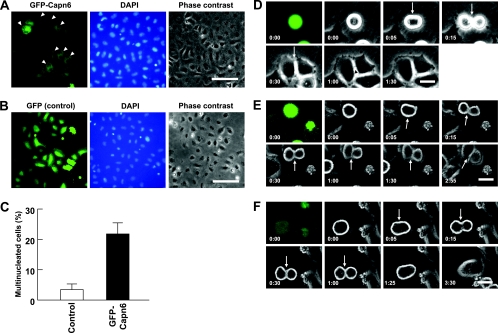
GFP-Capn6 overexpression impairs cytokinesis and causes the formation of multinucleated cells. (A and B) Multinucleation of HeLa cells by GFP-Capn6 overexpression. Many GFP-Capn6-transfected cells become binucleated (arrowheads) 48 h after transfection (A), whereas most control GFP-transfected cells are mononucleated (B). The scale bars indicate 100 μm. (C) Comparison of multinucleated-cell numbers between control and GFP-Capn6-transfected cells 48 h after transfection. The data represent the mean and standard deviation of four independent experiments. (D to F) Representative images from time-lapse recordings of HeLa cells transiently expressing GFP (D) or GFP-Capn6 (E and F). (D) In control GFP-expressing cells, the cleavage furrow (arrows) started to ingress within 5 min and daughter cells flattened out within 30 min after the onset of anaphase. The cytoplasmic bridge (arrowhead) disappeared within 90 min. (E) In many GFP-Capn6-expressing cells, furrow formation started normally, but its progression was retarded. (F) In some cases, GFP-Capn6 overexpression caused regression of the cleavage furrow to yield binucleated cells. Time is in h/min after anaphase onset (0:00 time point). The scale bars indicate 20 μm.
To explore the cause of the multinucleation induced by Capn6, we followed the fate of Capn6-transfected HeLa cells under time-lapse video microscopy. In all the control GFP-expressing cells examined (n = 13), the cleavage furrow started to ingress within 5 min and daughter cells flattened out within 30 min after the onset of anaphase (Fig. 2D; see Video S1 in the supplemental material). Thereafter, the cytoplasmic bridge disappeared within 90 min (Fig. 2D). Among GFP-Capn6-expressing mitotic cells examined (n = 15), 12 cells were able to complete cell division eventually, but the progression of furrow ingression was obviously retarded in 10 of the 12 cells (Fig. 2E; see Video S2 in the supplemental material). Eight of 12 daughter pairs retained a peanut-shaped morphology at 90 min after anaphase onset, whereas 2 daughter pairs were still tethered to each other with the cytoplasmic bridge beyond 90 min. The other two cells, in which GFP signals were relatively low, showed no apparent retardation of cytokinesis (data not shown). In 3 of 15 cells examined, GFP-Capn6 overexpression caused regression of the cleavage furrow at various time points to yield binucleated cells (Fig. 2F; see Video S3 in the supplemental material). All the Capn6-overexpressing cells commenced mitosis and cytokinesis apparently normally, with the cleavage furrow being appropriately positioned (Fig. 2E and F; see Video S4 in the supplemental material) indicating that Capn6 does not affect the onset of anaphase and cleavage furrow formation. Instead, Capn6 overexpression can disrupt the progression and completion of cytokinesis, often leading to the formation of multinucleated cells.
GFP-Capn6 colocalizes to and stabilizes the microtubule network.
The failure of cytokinesis in Capn6-overexpressing cells led us to speculate that Capn6 may associate with cytoskeletal proteins involved in cytokinesis. To investigate this possibility, we examined the intracellular distribution of GFP-Capn6. In contrast to the diffuse distribution of control GFP protein throughout the cytoplasm and nucleus (Fig. 3A and F and 4A), GFP-Capn6 was mainly distributed in the perinuclear region in HeLa cells (Fig. 3B and G) or in a fascicular pattern in NIH 3T3 cells (Fig. 4C). Costaining with anti-tubulin antibody revealed that these distributions largely overlapped with thick microtubule bundles, whose formation appeared to be facilitated by Capn6 overexpression (Fig. 3B and 4C).
FIG. 4.
GFP-Capn6 colocalizes to the microtubule network and induces microtubule bundling. NIH 3T3 cells were transfected with expression plasmids containing GFP (A and B) or GFP-Capn6 (C and D). After 16 h, the cells were treated with DMSO (vehicle) (A and C) or 500 nM nocodazole (B and D) for 15 min and stained with anti-GFP and anti-α-tubulin antibodies. GFP-Capn6 induced thick microtubule bundling (C), which was resistant to the destabilizing effect of nocodazole (D). The scale bars indicate 20 μm.
During mitosis, GFP-Capn6 was distributed in association with the mitotic spindle (Fig. 3C). At telophase, GFP-Capn6 was most intensely colocalized to microtubules in the central spindle (Fig. 3D). This colocalization was sustained in the midbody toward the end of cytokinesis, although a large portion of the GFP signals appeared to be distributed throughout the cytoplasm (Fig. 3E).
To determine the nature of microtubule bundles induced by Capn6 overexpression, we examined the level of tubulin acetylation, a posttranslational modification characteristic of stable microtubules (32). Immunostaining with specific antibody revealed that microtubules in GFP-Capn6-overexpressing cells contained high levels of acetylated α-tubulin (Fig. 3G), whereas cells expressing GFP alone did not (Fig. 3F). In particular, the distribution of acetylated α-tubulin corresponded to Capn6-induced microtubule bundles in the perinuclear region in many transfected HeLa cells (Fig. 3G).
To confirm the increase in acetylated α-tubulin by Capn6, immunoblotting was performed on GFP- and GFP-Capn6-transfected HeLa cells. Acetylated α-tubulin levels were increased in GFP-Capn6-transfected cells compared to GFP-transfected cells, whereas total α-tubulin levels were not increased (Fig. 3H). Paclitaxel, a microtubule-stabilizing agent, and nocodazole, a microtubule-disrupting agent, induced increase and decrease in acetylated α-tubulin levels, respectively (Fig. 3H), indicating that the increase in acetylated α-tubulin in GFP-Capn6-transfected cells may reflect increased stability of microtubules.
In addition, the stability of microtubule bundles was tested by treatment with low-dose nocodazole. In cells expressing GFP alone, the microtubule network was largely disrupted by 500 nM nocodazole (Fig. 4A and B). In contrast, microtubule bundles induced by GFP-Capn6 were resistant to 500 nM nocodazole (Fig. 4C and D). These results indicate that Capn6-induced microtubule bundling is likely due to increased stability of microtubules.
Capn6 is a calpain family member that lacks active-site cysteine and histidine residues critical for proteolysis. It is therefore unlikely that the microtubule-stabilizing effect of Capn6 is mediated by protease activity common to other calpains. Indeed, the calpain inhibitors E-64 and Z-LLal did not inhibit the Capn6-induced formation of microtubule bundles (data not shown).
Endogenous Capn6 associates with microtubules.
To further characterize the nature of Capn6, we raised a polyclonal antibody against a synthetic oligopeptide corresponding to the C-terminal region of Capn6. This antibody recognized a band of ∼74 kDa, corresponding to the expected molecular mass of Capn6, in untransfected cell lysates and more strongly in Capn6-transfected cell lysates (Fig. 5A), although it also detected several additional bands of unknown origin. The specificity of this ∼74-kDa band was also confirmed by RNAi-mediated knockdown (see Fig. 8B).
FIG. 5.
Detection of Capn6 by rabbit polyclonal antibody. (A) Whole-cell lysates of untrasfected (lane 1) and Capn6-transfected (lane 2) NIH 3T3 cells were immunoblotted. The Capn6 antibody recognizes a band of ∼74 kDa in untransfected cell lysates and more strongly in Capn6-transfected cell lysates. (B) NIH 3T3 cell lysates were separated into 0.1% NP-40-insoluble (lane I) and -soluble (lane S) fractions and immunoblotted with the indicated antibodies. The ∼74-kDa band was found in the insoluble fraction with acetylated α-tubulin (Ac-tubulin) and MAP4. (C to F) Endogenous Capn6 colocalizes to the microtubule network. NIH 3T3 cells were treated with DMSO (vehicle) (C and F), 500 nM paclitaxel (D), or 500 nM nocodazole (E) for 30 min, and double stained with the anti-Capn6 (C to E) or anti-Capn1 (F) and anti-α-tubulin antibodies. The scale bars indicate 20 μm.
FIG. 8.
RNAi-mediated inactivation of Capn6 destabilizes microtubules. NIH 3T3 cells were transfected with Capn6-targeted or control siRNAs. Forty-eight hours after transfection, the effects of siRNAs were evaluated. (A and B) RT-PCR for Capn6 mRNA (A) and Western blotting for Capn6 protein (B). Stealth siRNAs targeting two different regions of the Capn6 transcript (1298 and 1715 for nucleotides 1298 to 1322 and 1715 to 1739, respectively) downregulated Capn6 mRNA and protein levels. (C) Immunostaining with anti-Capn6 antibody. Signals superimposed on microtubules were evident in paclitaxel-treated control siRNA-transfected cells (a), but not in paclitaxel-treated cells transfected with 1298 (b) or 1715 (c) siRNA, although background cytosolic staining was comparable in the three groups. (D) NIH 3T3 cells were transfected with control (a), 1298 (b), or 1715 (c) siRNA and immunostained for acetylated α-tubulin (Ac-tubulin). The levels of acetylated α-tubulin decreased in cells transfected with Capn6-targeted siRNA. (E) NIH 3T3 cells stably expressing GFP-tubulin were transfected with control (a), 1298 (b), or 1715 (c) siRNA and immunostained for GFP. a′, b′, and c′ are magnified images of the boxed areas in a, b, and c, respectively. Microtubule network structures were largely disrupted in cells transfected with Capn6-targeted siRNA. The scale bars indicate 20 μm (C, D, and a to c in panel E) and 5 μm (a′ to c′ in panel E). (F) Western blotting of control and Capn6 siRNA-transfected NIH 3T3 cell extracts with anti-acetylated α-tubulin, anti-α-tubulin, and anti-β-actin antibodies. Acetylated α-tubulin levels decreased in Capn6 siRNA (1298 and 1715)-transfected cells compared with control siRNA-transfected cells, whereas total α-tubulin levels did not decrease. Blotting for β-actin served as an internal control. Similar results were obtained in three independent experiments. Representative data are shown.
To characterize the nature of Capn6 in terms of microtubule association, cell lysates were subjected to crude fractionation and Western blotting. Although microtubules are prone to depolymerization in the presence of 0.1% NP-40 at low temperature, MAP4, a protein associated with cold-stable microtubules (40), and a portion of acetylated α-tubulin were detected in the insoluble fraction (Fig. 5B). Capn1, a classical calpain localized in the cytoplasm, was found only in the soluble fraction (Fig. 5B). Under these conditions, Capn6 was predominantly detected in the insoluble fraction (Fig. 5B), which is consistent with the idea that Capn6 may associate with microtubules.
Correspondingly, immunostaining of NIH 3T3 cells with anti-Capn6 antibody detected signals mainly in the cytoplasm in a filamentous pattern, at least partially (Fig. 5C). This fine structure did not correlate with actin microfilaments visualized by rhodamine-labeled phalloidin (data not shown) but was largely superimposed on the intracellular microtubule network detected by anti-α-tubulin antibody (Fig. 5C). The correlation between Capn6 and microtubule signals was intensified in the presence of the microtubule-stabilizing agent paclitaxel (Fig. 5D). Conversely, treatment with nocodazole caused loss of the filamentous pattern of immunostaining and disrupted the colocalization between Capn6 and microtubules (Fig. 5E). In contrast to the predominant distribution of Capn6 to microtubules, Capn1 was localized in the cytoplasm with a granular pattern, although the signals could be partially overlapped with microtubules (Fig. 5F).
Capn6 interacts biochemically with microtubules.
To test whether Capn6 can biochemically interact with microtubules, we performed a microtubule cosedimentation assay on NIH 3T3 cell lysate. After incubation of the cell lysate on ice for microtubule depolymerization, centrifugation yielded soluble supernatant containing monomerized tubulin. As shown in Fig. 6A, endogenous Capn6 was recovered in the microtubule-containing pellet in the presence of paclitaxel, although a large quantity of Capn6 remained in the supernatants. MAP4 also coprecipitated with microtubules in the pellet, whereas Capn1 remained in supernatants regardless of paclitaxel; these served as positive and negative controls, respectively (Fig. 6A). When GFP-Capn6 was overexpressed in NIH 3T3 cells, it was cosedimented with microtubules after the addition of paclitaxel, whereas it remained in the soluble fraction without paclitaxel treatment (Fig. 6B). In contrast, control GFP was not correlated with the amount of α-tubulin sedimented in the pellet, although a trace signal was detected in each lane (Fig. 6B).
FIG. 6.
Capn6 biochemically interacts with microtubules. (A and B) Lysates of untransfected (A) or GFP-Capn6-transfected (B) NIH 3T3 cells were subjected to microtubule cosedimentation assay. (A) Endogenous Capn6 was detected in the microtubule-containing pellet in the presence of paclitaxel, although a large quantity of Capn6 remained in the supernatants (Sup). MAP4 also coprecipitated with microtubules in the pellet, whereas Capn1, detected as autolytic products, remained in the supernatants regardless of the presence of paclitaxel. (B) Overexpressed GFP-Capn6 was recovered in the pellet in the presence of paclitaxel. In contrast, control GFP did not correlate with the amount of precipitated α-tubulin in the pellet. Arrow, GFP-Capn6; arrowhead, GFP. (C) Unfused GST (lane 1), GST fused to full-length Capn6 (lane 2), and purified MAP2 (lane 3) were subjected to a microtubule binding assay on filter paper. GST-Capn6 and MAP2 (positive control), but not unfused GST, bound to microtubules as detected by anti-α-tubulin antibody. The filter was sequentially reblotted with anti-GST, anti-Capn6, and anti-MAP2 antibodies to confirm the presence of intact proteins.
The interaction between Capn6 and microtubules was further confirmed by a microtubule binding assay on filter paper and a GST pull-down assay. GST-Capn6 and unfused GST were run in SDS-PAGE, transferred to a PVDF filter, and then incubated with paclitaxel-stabilized microtubules. Immunoblotting with anti-tubulin antibody demonstrated that GST-Capn6, but not unfused GST, interacted with microtubules on the filter (Fig. 6C, lanes 1 and 2). MAP2, used as a positive control, also interacted with microtubules (Fig. 6C, lane 3). Furthermore, GST-Capn6, but not GST alone, pulled down paclitaxel-stabilized microtubules from NIH 3T3 cell lysates (Fig. 7C).
FIG. 7.
Mapping of Capn6 domains interacting with microtubules. (A) Structures of GFP-tagged full-length Capn6 and its deletion mutants. (B) Localization of GFP-Capn6 mutants. NIH 3T3 cells were transfected with expression plasmids encoding GFP-Capn6 mutants, treated with 500 nM paclitaxel for 1 h, and stained with anti-GFP and anti-α-tubulin antibodies. Note that only GFP-Capn6 mutants containing domain III (amino acids 327 to 503) colocalized to microtubule bundles. The scale bars indicate 20 μm. (C) GST pull-down assay for microtubule binding. Microtubules were detected by anti-α-tubulin and anti-acetylated tubulin antibodies. α-Tubulin was pulled down from NIH 3T3 cell lysates by GST-full-length Capn6, GST-Capn6(327-503) (domain III), and GST-Capn6(504-641) (domain T), but not by GST-Capn6(1-56) (domain I), GST-Capn6(57-326) (domain II), or GST alone (right). Blotting with anti-acetylated tubulin showed that stable microtubules were more likely to bind to domain III than to domain T. The amounts of GFP fusion proteins were grossly estimated by Ponceau staining (left). The arrows indicate GST fusion proteins.
Domain III is responsible for Capn6-microtubule interaction.
The structure of Capn6 protein consists of four domains (domains I, II, III, and T). To determine the domain(s) responsible for the interaction with microtubules, we constructed expression plasmids encoding GFP-tagged Capn6 deletion mutants (Fig. 7A) and transfected them into NIH 3T3 cells. In the presence of paclitaxel, GFP-tagged full-length Capn6 and Capn6(1-503), lacking domain T, colocalized to thickened microtubule bundles (Fig. 7B, a and b). In contrast, GFP-Capn6(1-326), lacking both domain III and domain T, did not colocalize to microtubules (Fig. 7B c). When individual domains were fused to GFP, only the construct containing domain III, GFP-Capn6(327-503), exhibited colocalization to microtubule bundles (Fig. 7B, d to g). Although domain III is sufficient for colocalization to microtubules, both 1-503 and 327-503 mutants did not induce multinucleation (data not shown), indicating that domain T is also necessary for full functional activity.
The association of each domain with microtubules was also examined by GST pull-down assay. GST-Capn6, but not unfused GST, pulled down paclitaxel-stabilized microtubules from NIH 3T3 cell lysates (Fig. 7C). Microtubules were also pulled down by GST-Capn6(327-503) (domain III) and GST-Capn6(504-641) (domain T), but not by GST-Capn6(1-56) (domain I) and GST-Capn6(57-326) (domain II) (Fig. 7C). Blotting with anti-acetylated α-tubulin revealed preferential binding of stabilized microtubules to domain III compared with domain T (Fig. 7C). These results suggest that the interaction of Capn6 with microtubules is mainly mediated by domain III, although domain T may also contribute to this association.
Suppression of Capn6 by siRNA destabilizes microtubules and affects actin organization.
To investigate the role of endogenous Capn6 in the organization of the microtubule network, we used RNAi to selectively knock down Capn6 expression in NIH 3T3 cells. Stealth siRNAs targeting two different regions of the Capn6 transcript (nucleotides 1298 to 1322 and 1715 to 1739) downregulated Capn6 mRNA (Fig. 8A) and protein (Fig. 8B) to different extents. Immunostaining with anti-Capn6 antibody revealed the disappearance of microtubule-superimposed signals in Capn6-downregulated cells (Fig. 8C). In proportion to the extent of Capn6 downregulation, Capn6 siRNAs decreased the level of acetylated α-tubulin within the microtubule network of transfected cells (Fig. 8D). To confirm the effect of Capn6 downregulation on the microtubule organization, we introduced siRNAs into NIH 3T3 cells stably expressing GFP-tubulin. In Capn6 siRNA-transfected cells, the filamentous organization of the microtubule network was largely disrupted, resulting in relatively homogeneous distribution of GFP-tubulin with a preference for the perinuclear region (Fig. 8E). Decreases in acetylated-α-tubulin levels in Capn6-downregulated cells were also confirmed by Western blotting analysis (Fig. 8F).
To further analyze the functional consequences of Capn6 downregulation, we examined cellular behavior during cytokinesis in control and Capn6 siRNA-transfected cells using time-lapse microscopy. After the onset of anaphase, cleavage furrow formation and progression occurred normally in Capn6-downregulated cells, as well as in control cells (Fig. 9A and B). At subsequent stages, Capn6-downregulated cells tended to form lamellipodial protrusions with ruffling and to become flat much earlier than control cells (Fig. 9A and B). When the times from anaphase onset to the first appearance of lamellipodial protrusions from round dividing cells were compared, it was significantly shorter in Capn6-downregulated cells than in control cells (Fig. 9C). In contrast, the time from anaphase onset to the completion of cell abscission was not significantly different between control and Capn6-downregulated cells (Fig. 9D).
FIG. 9.
RNAi-mediated inactivation of Capn6 affects cytoskeletal reorganization during cytokinesis. (A and B) Representative images from time-lapse recordings of control (A) and Capn6 (B) siRNA-transfected NIH 3T3 cells. Cleavage furrows were similarly formed after anaphase onset in control and Capn6 siRNA (1715)-transfected cells. At subsequent stages, Capn6 siRNA-transfected cells tended to form lamellipodial protrusions with ruffling and to become flat much earlier than control cells. Times are in h/min after anaphase onset (0:00 time point). Arrowheads, first appearance of lamellipodial protrusion; arrows, completion of cell abscission. The scale bars indicate 20 μm. (C) Comparison of the times from anaphase onset to the first appearance of lamellipodial protrusions. The time was significantly shorter in Capn6-downregulated cells (17.9 [mean] ± 4.5 [standard deviation] min; n = 30) than in control cells (34.4 ± 12.4 min; n = 30). *, P < 0.0001; Mann-Whitney nonparametric test. (D) Comparison of the times from anaphase onset to the completion of cell abscission. No significant difference was observed between control cells (118.8 ± 35.1 min; n = 25) and Capn6-downregulated cells (108.6 ± 27.6 min; n = 24).
Time-lapse microscopy also showed that cell motility was increased in Capn6-downregulated cells, in which lamellipodial ruffling was very prominent (Fig. 10A and B; see Videos S4 to S6 in the supplemental material). Changes in actin organization associated with lamellipodium formation were confirmed by staining with rhodamine-phalloidin (Fig. 10C). These observations suggest that silencing of Capn6 may facilitate dynamic instability of microtubules and actin reorganization in NIH 3T3 cells.
FIG. 10.
RNAi-mediated inactivation of Capn6 induces lamellipodium formation. (A and B) Phase-contrast micrographs of control (A) and Capn6 (B) siRNA-transfected NIH 3T3 cells. Capn6 siRNA(1715)-transfected cells demonstrated enhanced lamellipodium formation with membrane ruffling (arrowheads). (C) Double staining of control and Capn6(1715) siRNA-transfected NIH 3T3 cells with anti-tubulin and rhodamine-phalloidin. Capn6 siRNA-transfected cells demonstrate the assembly of actin filaments in lamellipodia (arrowheads). The scale bars indicate 20 μm.
DISCUSSION
In the present study, we first identified Capn6 as a downstream molecule of ET-1 signaling in pharyngeal-arch development. Subsequent analysis has demonstrated that Capn6 is a functional protein with microtubule-associated activities. Overexpression of Capn6 stimulated the formation of microtubule bundles and interrupted cytokinesis, resulting in multinucleation. Capn6 colocalized to the microtubule structures, including the central spindle and midbody, during cytokinesis. The interaction between Capn6 and microtubules was also confirmed by biochemical analysis. This interaction appeared to be mainly mediated by domain III, a C2-like domain. Finally, RNAi-mediated Capn6 inactivation caused disruption of the microtubule organization with loss of acetylated α-tubulin, affecting actin organization and cell motility.
Microtubules are filamentous polymers constituted of α- and β-tubulin that are engaged in many cellular functions, including cell division, cell shape integration, and organelle transport. The dynamic instability involving polymerization-depolymerization cycles is regulated by microtubule-associated proteins (MAPs), such as MAP1, MAP2, and tau, in neural cells and is critical for the function of microtubules. Many previous reports have demonstrated that the classical calpains can cleave tubulin and MAPs under in vitro conditions (11). However, little is known about the physiological role of the calpain system in the regulation of microtubule dynamics. Recently, several studies have implicated the calpain-induced proteolysis of MAPs in the pathogenesis of neurodegenerative disorders, including Alzheimer's disease (12), and the role of the calpain system in microtubule organization is now an issue of growing concern. In this context, the present findings may provide new insight into this issue by demonstrating that the protease-deficient calpain can associate with and stabilize microtubules.
Preferential distribution to the microtubule network is highly characteristic of Capn6 among the calpain family members. Capn1, for example, is homogeneously distributed in the cytoplasm and is translocated to the plasma membrane upon an increase in cytosolic Ca2+ (8). Lane et al. reported that Capn1 is distributed through the cytoplasm in a fibrillar form, reminiscent of the cytoskeletal architecture (24). In our present study, however, Capn1 was detected in the 0.1% NP-40-soluble fraction and distributed in a rather granular form in NIH 3T3 cells. Capn2, another classical calpain, also localizes in the cytoplasm in a diffuse or fine granular form (24). No other calpains have been shown to be associated with microtubules.
The interaction between Capn6 and microtubules appears to be mediated mainly by domain III. In Capn1 and Capn2, domain III constitutes a pair of four-stranded antiparallel β-sheets (15, 30, 37), like the C2 domain, a Ca2+- and phospholipid-binding module (33). Although the function of this domain is unclear, previous reports have indicated its roles in the regulation of Ca2+ sensitivity of the enzyme (14, 15) and in Ca2+-dependent translocation to the cell membrane (8). Domain III of Capn6 (amino acids 327 to 503) shows similarity to that of Capn2, with only 28% amino acid identity, as revealed by a BLAST search (data not shown), suggesting limited conservation of the domain structure. In addition, domain III of Capn6 lacks sequences corresponding to the conserved acidic loop to form interdomain salt bridges important for Ca2+ sensitivity (13). Thus, this domain may be unusual in structure and function in the calpain family.
In addition to colocalization to microtubules, Capn6 causes the formation of microtubule bundles. Increased acetylated α-tubulin contents in Capn6-induced microtubule bundles and resistance to nocodazole suggest that Capn6 has a microtubule-stabilizing property. Furthermore, disruption of microtubule bundles and decrease in acetylated α-tubulin levels by RNAi-mediated Capn6 inactivation indicate the role of endogenous Capn6 in the maintenance of stable microtubule architecture.
The Capn6-induced disruption of cytokinesis may be a consequence of its stabilizing effect on microtubules. In Capn6-overexpressing cells, the cleavage furrow was created along with chromosomal separation with an expected time course, but furrow ingression was retarded thereafter, suggesting that Capn6 functioned at the late stage of cytokinesis. During the process of cytokinesis, overexpressed Capn6 colocalized to the central spindle of the midzone and to the whole midbody, where Capn6 may affect the progression and completion of cytokinesis. Cytokinesis is elaborately controlled at several steps involving various molecules concentrating at the midbody (10). Changes in microtubule stability might interrupt such cytokinetic machinery, leading to disrupted cytokinesis in Capn6-overexpressing cells. Another possible explanation is that stabilization of microtubules by Capn6 may prevent the cytoskeletal reorganization required for cell division, although the molecular network underlying this process remains largely unknown. Correspondingly, Capn6 siRNA transfection resulted in the early appearance of lamellipodial protrusions during cytokinesis, indicating that cytoskeletal reorganization may be facilitated by Capn6 inactivation.
The observation that siRNA-mediated Capn6 inactivation enhanced the formation of lamellipodia with membrane ruffling is also consistent with the involvement of Capn6 in microtubule stability. Previous reports have demonstrated that changes in microtubule stability affect cell motility through microtubule-actin interactions (34). For example, Hubbert et al. have shown that deacetylation of α-tubulin by HDAC6 promotes chemotactic cell migration (17). Waterman-Storer et al. have provided a model in which the growth phase of microtubule dynamic instability drives actin polymerization and lamellipodial protrusion via Rac1 activation (39). Taken together with these findings, the present results suggest that Capn6 may be involved in the control of microtubule dynamic instability and actin organization.
How can Capn6 induce microtubule stabilization? It is unlikely that Capn6 has a concealed protease activity by which it may modulate microtubule dynamics, because calpain inhibitors did not inhibit Capn6-induced microtubule bundling. Rather, Capn6 may exert its effect by antagonizing other calpains as a nonproteolytic family member. Indeed, the stability of microtubules is enhanced by MAPs (6, 18), which are in vitro calpain substrates. Rho GTPase, another possible calpain substrate (20), and its effector, mDia, also have microtubule-stabilizing properties (3, 31). It would be worthwhile to test whether Capn6 may protect these microtubule-stabilizing factors from proteolysis in the restricted cytoskeletal regions. It is also possible that Capn6 may stabilize microtubules through an as-yet-unknown function independent of protease activity. For example, the pattern of microtubule bundling may suggest possible interactions between Capn6 and other signaling pathways. Capn6-transfected cells exhibited stable microtubule bundling predominantly in the perinuclear region. Remarkably, a similar pattern of microtubule bundling is induced by certain members of sterile 20 (STE20)-like kinases. Xenopus p21-activated kinase 5 (X-PAK5), a Cdc42/Rac effector, colocalizes to microtubules and promotes the formation of stable microtubule bundles around the nucleus (1). Prostate-derived STE20-like kinase, a member of the germinal-center kinase-like kinase subfamily, also produces perinuclear microtubule bundles (27). These kinases are also involved in the regulation of actin organization, suggesting that they may play roles in the cross talk between microtubules and the actin cytoskeleton. Although the role of Capn6 in the regulation of actin organization has not yet been determined, the enhanced lamellipodium formation induced by Capn6 silencing may suggest the possible involvement of Capn6 in this cytoskeletal cross talk.
Is Capn6 involved in embryogenesis through its microtubule-stabilizing effect? The answer to this question remains unknown, although Capn6 was identified as a molecule in the genetic hierarchy controlling pharyngeal-arch development. In this respect, it is noteworthy that cytoskeletal remodeling, including microtubules, is involved in various processes during animal development. For example, Rho family GTPase-regulating proteins required for cytokinesis have also emerged as modulators of cytoskeletal reorganization in transitions between the epithelium and mesenchyme in either direction (23). It is possible that Capn6, expressed in the pharyngeal-arch epithelium and mesenchyme, participates in developmental processes involving cytoskeletal reorganization in cooperation with these proteins. Further investigations using a gene-targeting approach are expected to clarify the link between the molecular functions of Capn6 and developmental processes.
Supplementary Material
Acknowledgments
We thank Hideyuki Saya (Kumamoto University), Yoshimitsu Kanai, and Yasuko Noda (The University of Tokyo) for technical advice and helpful discussion. K.T. is a Research Fellow of the Japan Society for the Promotion of Science (DC1).
This work was supported by grants from the Japan Society for the Promotion of Science Research for the Future Program; grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology, Japan; and a Research Grant from the Uehara Memorial Foundation.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Cau, J., S. Faure, M. Comps, C. Delsert, and N. Morin. 2001. A novel p21-activated kinase binds the actin and microtubule networks and induces microtubule organization. J. Cell Biol. 155:1029-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouthier, D. E., K. Hosoda, J. A. Richardson, S. C. Williams, H. Yanagisawa, T. Kuwaki, M. Kumada, R. E. Hammer, and M. Yanagisawa. 1998. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 125:813-824. [DOI] [PubMed] [Google Scholar]
- 3.Cook, T. A., T. Nagasaki, and G. G. Gundersen. 1998. Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. J. Cell Biol. 141:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dear, N., K. Matena, M. Vingron, and T. Boehm. 1997. A new subfamily of vertebrate calpains lacking a calmodulin-like domain: implications for calpain regulation and evolution. Genomics 45:175-184. [DOI] [PubMed] [Google Scholar]
- 5.Dear, T. N., and T. Boehm. 1999. Diverse mRNA expression patterns of the mouse calpain genes Capn5, Capn6 and Capn11 during development. Mech. Dev. 89:201-209. [DOI] [PubMed] [Google Scholar]
- 6.Drewes, G., A. Ebneth, and E.-M. Mandelkow. 1998. MAPs, MARKs and microtubule dynamics. Trends Biochem. Sci. 23:307-311. [DOI] [PubMed] [Google Scholar]
- 7.Fukuhara, S., Y. Kurihara, Y. Arima, N. Yamada, and H. Kurihara. 2004. Temporal requirement of signaling cascade involving endothelin-1/endothelin A receptor in branchial arch development. Mech. Dev. 121:1223-1233. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Parrado, S., O. Popp, T. A. Knoch, S. Zahler, F. Bestvater, M. Felgentrager, A. Holloschi, A. Fernandez-Montalvan, E. A. Auerswald, H. Fritz, P. Fuentes-Prior, W. Machleidt, and E. Spiess. 2003. Subcellular localization and in vivo subunit interactions of ubiquitous μ-calpain. J. Biol. Chem. 278:16336-16346. [DOI] [PubMed] [Google Scholar]
- 9.Glading, A., D. A. Lauffenburger, and A. Wells. 2002. Cutting to the chase: calpain proteases in cell motility. Trends Cell Biol. 12:46-54. [DOI] [PubMed] [Google Scholar]
- 10.Glotzer, M. 2005. The molecular requirements for cytokinesis. Science 307:1735-1739. [DOI] [PubMed] [Google Scholar]
- 11.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi, M., N. Iwata, and T. C. Saido. 2005. Understanding molecular mechanisms of proteolysis in Alzheimer's disease: progress toward therapeutic interventions. Biochim. Biophys. Acta 1751:60-67. [DOI] [PubMed] [Google Scholar]
- 13.Hosfield, C. M., J. S. Elce, P. L. Davies, and Z. Jia. 1999. Crystal structure of calpain reveals the structural basis for Ca2+-dependent protease activity and a novel mode of enzyme activation. EMBO J. 18:6880-6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosfield, C. M., T. Moldoveanu, P. L. Davies, J. S. Elce, and Z. Jia. 2001. Calpain mutants with increased Ca2+ sensitivity and implications for the role of the C2-like domain. J. Biol. Chem. 276:7404-7407. [DOI] [PubMed] [Google Scholar]
- 15.Hosfield, C. M, J. S. Elce, and Z. Jia. 2004. Activation of calpain by Ca2+: roles of the large subunit N-terminal and domain III-IV linker peptides. J. Mol. Biol. 343:1049-1053. [DOI] [PubMed] [Google Scholar]
- 16.Huang, Y., and K. K. Wang. 2001. The calpain family and human disease. Trends Mol. Med. 7:355-362. [DOI] [PubMed] [Google Scholar]
- 17.Hubbert, C., A. Guardiola, R. Shao, Y. Kawaguchi, A. Ito, A. Nixon, M. Yoshida, X. F. Wang, and T. P. Yao. 2002. HDAC6 is a microtubule-associated deacetylase. Nature 417:455-458. [DOI] [PubMed] [Google Scholar]
- 18.Kaech, S., B. Ludin, and A. Matus. 1996. Cytoskeletal plasticity in cells expressing neuronal microtubule-associated proteins. Neuron 17:1189-1199. [DOI] [PubMed] [Google Scholar]
- 19.Kimmel, C. B., B. Ullmann, M. Walker, C. T. Miller, and J. G. Crump. 2003. Endothelin 1-mediated regulation of branchial bone development in zebrafish. Development 130:1339-1351. [DOI] [PubMed] [Google Scholar]
- 20.Kulkarni, S., D. E. Goll, and J. E. Fox. 2002. Calpain cleaves RhoA generating a dominant-negative form that inhibits integrin-induced actin filament assembly and cell spreading. J. Biol. Chem. 277:24435-24441. [DOI] [PubMed] [Google Scholar]
- 21.Kurihara, Y., H. Kurihara, H. Suzuki, T. Kodama, K. Maemura, R. Nagai, H. Oda, T. Kuwaki, W.-H. Cao, N. Kamada, K. Jishage, Y. Ouchi, S. Azuma, Y. Toyoda, T. Ishikawa, M. Kumada, and Y. Yazaki. 1994. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature 368:703-710. [DOI] [PubMed] [Google Scholar]
- 22.Kurihara, Y., H. Kurihara, H. Oda, K. Maemura, R. Nagai, T. Ishikawa, and Y. Yazaki. 1995. Aortic arch malformations and ventricular septal defect in mice deficient in endothelin-1. J. Clin. Investig. 96:293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labouesse, M. 2004. Epithelium-mesenchyme: a balancing act of RhoGAP and RhoGEF. Curr. Biol. 14:R508-R510. [DOI] [PubMed] [Google Scholar]
- 24.Lane, R. D., D. M. Allan, and R. L. Mellgren. 1992. A comparison of the intracellular distribution of μ-calpain, m-calpain, and calpastatin in proliferating human A431 cells. Exp. Cell Res. 203:5-16. [DOI] [PubMed] [Google Scholar]
- 25.Masaki, T. 2004. Historical review: endothelin. Trends Pharmacol. Sci. 25:219-224. [DOI] [PubMed] [Google Scholar]
- 26.Matena, K., T. Boehm, and N. Dear. 1998. Genomic organization of mouse Capn5 and Capn6 genes confirms that they are a distinct calpain subfamily. Genomics 48:117-120. [DOI] [PubMed] [Google Scholar]
- 27.Mitsopoulos, C., C. Zihni, R. Garg, A. J. Ridley, and J. D. H. Morris. 2003. The prostate-derived sterile 20-like kinase (PSK) regulates microtubule organization and stability. J. Biol. Chem. 278:18085-18091. [DOI] [PubMed] [Google Scholar]
- 28.Nakaseko, Y., K. Nabeshima, K. Kinoshita, and M. Yanagida. 1996. Dissection of fission yeast microtubule associating protein p93Dis1: regions implicated in regulated localization and microtubule interaction. Genes Cells 1:633-644. [DOI] [PubMed] [Google Scholar]
- 29.Ozeki, H., Y. Kurihara, K. Tonami, S. Watatani, and H. Kurihara. 2004. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech. Dev. 121:387-395. [DOI] [PubMed] [Google Scholar]
- 30.Pal, G. P., T. De Veyra, J. S. Elce, and Z. Jia. 2003. Crystal structure of a micro-like calpain reveals a partially activated conformation with low Ca2+ requirement. Structure 11:1521-1526. [DOI] [PubMed] [Google Scholar]
- 31.Palazzo, A. F., T. A. Cook, A. S. Alberts, and G. G. Gundersen. 2001. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3:723-729. [DOI] [PubMed] [Google Scholar]
- 32.Piperno, G., M. LeDizet, and X. J. Chang. 1987. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J. Cell Biol. 104:289-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizo, J., and T. C. Südhof. 1998. C2-domains, structure and function of a universal Ca2+-binding domain. J. Biol. Chem. 273:15879-15882. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez, O. C., A. W. Schaefer, C. A. Mandato, P. Forscher, W. M. Bement, and C. M. Waterman-Storer. 2003. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat. Cell Biol. 5:599-609. [DOI] [PubMed] [Google Scholar]
- 35.Sorimachi, H., S. Ishiura, and K. Suzuki. 1997. Structure and physiological function of calpains. Biochem. J. 328:721-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorimachi, H., and K. Suzuki. 2001. The structure of calpain. J. Biochem. (Tokyo) 129:653-664. [DOI] [PubMed] [Google Scholar]
- 37.Strobl, S., C. Fernandez-Catalan, M. Braun, R. Huber, H. Masumoto, K. Nakagawa, A. Irie, H. Sorimachi, G. Bourenkow, H. Bartunik, K. Suzuki, and W. Bode. 2000. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc. Natl. Acad. Sci. USA 97:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki, K., and H. Sorimachi. 1998. A novel aspect of calpain activation. FEBS Lett. 433:1-4. [DOI] [PubMed] [Google Scholar]
- 39.Waterman-Storer, C. M., R. A. Worthylake, B. P. Liu, K. Burridge, and E. D. Salmon. 1999. Microtubule growth activates Rac1 to promote lamellipodial protrusion in fibroblasts. Nat. Cell Biol. 1:45-50. [DOI] [PubMed] [Google Scholar]
- 40.Webster, D. R., and J. M. Bratcher. 2006. Developmental regulation of cardiac MAP4 protein expression. Cell Motil. Cytoskeleton 63:512-522. [DOI] [PubMed] [Google Scholar]
- 41.Wilkinson, D. 1992. In situ hybridisation: a practical approach. IRL Press, Oxford, United Kingdom.
- 42.Yanagisawa, M., H. Kurihara, S. Kimura, Y. Tomobe, M. Kobayashi, Y. Mitsui, Y. Yazaki, K. Goto, and T. Masaki. 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332:411-415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.