Abstract
Arachidonic acid is an essential constituent of cell membranes that is esterified to the sn-2 position of glycerophospholipids and is released from selected phospholipid pools by tightly regulated phospholipase cleavage. Metabolism of the released arachidonic acid by the cytochrome P450 enzyme system (cP450) generates biologically active compounds, including epoxyeicosatrienoic acids (EETs) and hydroxyeicosatetraenoic acids. Here we report that 2-(14,15-epoxyeicosatrienoyl)glycerol (2-14,15-EG), a novel cP450 arachidonate metabolite produced in the kidney, is a potent mitogen for renal proximal tubule cells. This effect is mediated by activation of tumor necrosis factor alpha-converting enzyme (ADAM17), which cleaves membrane-bound transforming growth factor α (proTGF-α) and releases soluble TGF-α as a ligand that binds and activates epidermal growth factor receptor (EGFR). The present studies additionally demonstrate that the structurally related 14,15-EET stimulates release of soluble heparin-binding EGF-like growth factor as an EGFR ligand by activation of ADAM9, another member of the ADAM family. Thus, in addition to the characterization of 2-14,15-EG's mitogenic activity and signaling mechanism, our study provides the first example that two structurally related biologically active lipid mediators can activate different metalloproteinases and release different EGFR ligands in the same cell type to activate EGFR and stimulate cell proliferation.
In response to relevant stimulation by growth factors, cytokines, or circulating hormones, arachidonic acid is hydrolyzed from the sn-2 position of selected glycerophospholipids by activated phospholipases (20, 51). Metabolism of the released arachidonic acid by the cytochrome P450 (cP450)-dependent monooxygenase pathway produces 5,6-, 8,9-, 11,12-, and 14,15-epoxyeicosatrienoic acids (EETs) through NADPH-dependent epoxidation and forms 19- and 20-hydroxyeicosatetraenoic acids via ω/ω-1 hydroxylation (8, 47, 54). These cP450 metabolites are involved in regulation of vascular tone and salt and fluid balance (37, 41). Recent studies have suggested that EETs are endothelium-derived hyperpolarizing factors (7, 25), serve as intracellular second messengers in vasculature (26) and epithelia (16), and possess anti-inflammatory properties (52).
In the kidney, EETs are produced primarily by epoxygenases of the cP450 2C family (10), which have been localized to the renal proximal tubule (22). Direct administration of EETs inhibits amiloride-sensitive sodium transport and 86Rb uptake in cultured renal proximal tubule cells (23, 64). EETs also serve as potent mitogens for a number of cell types including renal proximal tubule cells (6, 13, 15) as well as vascular endothelial cells (49, 56) and glomerular mesangial cells (30). The mitogenic effect of EETs is mediated by activation of a tyrosine kinase cascade (13, 15, 35, 56). Further studies revealed that 14,15-EET stimulates DNA synthesis by activation of epidermal growth factor receptor (EGFR) and the extracellular signal-regulated kinase (ERK) signaling pathway by metalloproteinase activation and the consequent release of soluble heparin-binding EGF-like growth factor (HB-EGF) (12). However, the identity of 14,15-EET-activated metalloproteinase(s) has remained unknown.
The ADAM (a disintegrin and metalloproteinase) family of proteins has been implicated in the proteolytic processing of membrane-bound EGFR ligands, thus regulating EGFR signaling and EGFR-dependent functions by mediating the release of mature, soluble EGFR ligands including HB-EGF (3, 29). Of interest, ADAM17/tumor necrosis factor alpha-converting enzyme (TACE) and ADAM9 display different sensitivities to hydroxamic acid-type metalloproteinase inhibitors in vitro (58), and proHB-EGF and preproEGF also exhibit disparate sensitivities to hydroxamic acid-based metalloproteinase inhibitors, suggesting that different members of the EGFR ligand family may be cleaved by different metalloproteinases (21). However, the substrate specificity and inducibility of different members of the ADAM metalloproteinase family that are responsible for the ectodomain shedding of different EGFR ligands have not yet been clearly defined.
We have recently identified a novel group of cP450 metabolites of arachidonic acid, 2-epoxyeicosatrienoylglycerols (2-EGs) that are produced in the kidney, spleen, and brain (14). In the kidney, 2-14,15-EG is the predominant isoform of 2-EG (14). Because the renal proximal tubule epithelial cells express the highest level of cP450 enzyme in the kidney (22), in the present study we characterized the role and mechanism of action of 2-14,15-EG in the well-established renal proximal tubule epithelial cell line, LLCPKcl4 (1).
MATERIALS AND METHODS
Reagents.
EGF (receptor grade) was purchased from Collaborative Research (Bedford, MA). 2-Arachidonoylglycerol, WIN55212-2, AM251, and AM630 were from Tocris Cookson, Inc. (Ellisville, MO). Tyrphostin AG1478, phenanthroline, and TAPI-0 were obtained from EMD Biosciences (San Diego, CA). Polyclonal and monoclonal antiphosphotyrosine antibodies were purchased from Zymed (San Francisco, CA). Polyclonal anti-ERK antibodies, monoclonal antiphospho-ERK antibodies, anti-EGFR antibodies, CB1 and CB2 cannabinoid receptor antibodies, and protein A/G Agarose beads were from Santa Cruz Biotechnology (Santa Cruz, CA). [3H]thymidine, [3H]CP55940, and [α-32P]dCTP were from PerkinElmer Life and Analytical Sciences (Boston, MA). Heparin-Sepharose CL-6B column was from Amersham Biosciences Corp. (Piscataway, NJ). Monoclonal neutralizing EGFR antibody clone 528 and neutralizing transforming growth factor alpha (TGF-α) antibody were a generous gift from Robert Coffey (Vanderbilt University). Batimastat (BB-94) was from British Biotechnology (Oxford, United Kingdom). CRM197, monoclonal anti-actin antibody, and all other chemicals were from Sigma.
Synthesis of 14,15-EET and 2-14,15-EG.
14,15-EET was synthesized as we previously described (9, 15). 2-14,15-EG was synthesized as follows: 1,3-bis(triisopropylsilyl)-2-(14,15-EG, 4-dimethylaminopyridine (31 mg; 0.256 mmol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (56 mg; 0.256 mmol) were added sequentially, with stirring, to a room-temperature solution of 14,15-EET (17) (55 mg; 0.17 mmol) and 1,3-bis(triisopropylsilyl)-2-glycerol (28) (65 mg; 0.163 mmol) in dry dichloromethane (5 ml). After 24 h, the reaction mixture was diluted with dichloromethane (15 ml), washed with water (5 ml) and brine (3 ml), dried over sodium sulfate, and concentrated in vacuo. The residue was purified by column chromatography on silica gel using diethyl ether/hexane (2:98) as eluant to give 1,3-bis(triisopropylsilyl)-2-(14,15-EG (116 mg; 93%) as a colorless oil. TLC: Rf = 0.64 (20% ethylacetate [EtOAc]-hexane); 1H NMR (400 MHz, CDCl3) δ5.58 to 5.43 (m, 2H), 5.42 to 5.30 (m, 4H) 4.95 (apparent quintet, 1H, J = 5.2Hz), 3.84 (ddd, 4H, J = 15.5, 10.3, 5.2 Hz), 3.00 to 2.90 (m, 2H), 2.82 (dt, 2H, J = 14.6, 5.8Hz), 2.45 to 2.36 (m, 1H), 2.32 (t, 2H, J = 7.6Hz), 2.28 to 2.20 (m, 1H), 2.15 to 2.05 (m, 2H), 1.70 (quintet, 2H, J = 7.6Hz), 1.60 to 1.22 (m, 12H), 1.18 to 0.96 (m, 42H), 0.91 (t, 3H, J = 6.7 Hz); 13C NMR (75 MHz) δ173.30, 130.61, 129.37, 128.77, 128.71, 127.96, 124.74, 75.23, 64.10, 57.39, 56.59, 43.07, 31.94, 27.96, 26.81, 26.51, 26.48, 25.99, 25.81, 25.01, 22.81, 18.12, 14.21, 12.11.
A mixture of tetra-n-butylammonium fluoride (0.56 mmol) 560 μl of a 1 M tetrahydrofolate (THF) solution, and acetic acid (0.56 mmol) was added dropwise to a −20°C solution of the above bis-silyl ether (40 mg, 0.056 mmol) in dry THF (10 ml). After 2 h, the reaction mixture was warmed to 0°C and stirred for another 24 h; then the THF was removed under an argon stream. The residue was partitioned between water and EtOAc. After separation of the layers, the aqueous layer was extracted two more times. The combined organic fractions were washed with brine, dried over sodium sulfate, and concentrated in vacuo. The residue was purified by flash chromatography over a short column of silica gel eluted using 30 to 50% EtOAc-hexane to give pure 2-14,15-EG (20 mg; 90%). TLC: Rf = 0.12 (20% acetone/benzene); 1H NMR (400 MHz, CDCl3) δ5.56 to 5.34 (m, 6H), 4.96 (apparent quintet, 1H, J = 4.5 Hz), 3.84 (bd, 4H, J = 4.8 Hz), 2.99 to 2.93 (m, 2H), 2.86 to 2.80 (m, 4H), 2.49 to 2.36 (m, 3H), 2.24 to 2.11 (m, 4H), 1.74 (apparent quintet, 2H, J = 7.3 Hz), 1.54 to 1.29 (m, 9H), 0.91 (t, 3H, J = 6.7 Hz). High-performance liquid chromatography was performed with a Microsorb column (2.25 by 30 cm) using 7% i-PrOH-hexane and a flow rate of 9 ml/min with an Rt of 48 min for 2-(14,15-EG) and of 46 min for 1-(14,15-EG).
Cell culture.
LLCPKcl4, an established adherent proximal tubule epithelial cell line derived from porcine kidney (1), was cultured in Dulbecco's modified Eagle's medium—F-12 (DMEM-F-12) mixture; A431 cells were grown in DMEM at 37°C in a 5% CO2 cell culture incubator. All media were supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum (FBS; HyClone), and the medium was changed every 2 to 3 days.
Cell proliferation.
A total of 1.5 × 104 LLCPKcl4 cells per well were seeded into six-well plates. After 24 h of growth in DMEM-F-12 mixture containing 10% FBS, the medium was changed to serum-free medium and incubated for an additional 24 h to make the cells quiescent. Cells were then exposed to 2 μM 2-14,15-EG or vehicle alone in the presence of 0.2% FBS in the medium. Cell number per well in each treatment group was counted every day. Results were plotted as the cell number/well for the indicated durations. Each experimental data point represents triplicate wells from three different experiments.
[3H]thymidine incorporation assay, immunoprecipitation, and immunoblotting.
RT-PCR.
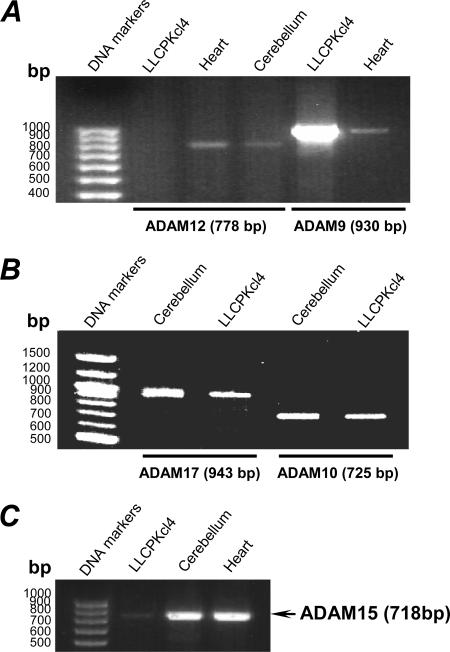
Total cellular RNA was isolated from LLCPKcl4 cells using TRIZOL Reagent (Invitrogen) and used as templates to determine whether the ADAM family members potentially involved in TGF-α and HB-EGF cleavage were expressed in LLCPKcl4 cells. One microgram of total RNA was used for reverse transcription in a 20-μl final reaction volume for all ADAM family members except for ADAM12 (up to 10 μg of total RNA from LLCPKcl4 cells was used in an attempt to detect ADAM12 expression). Our preliminary experiments indicated that the reverse transcription-PCR (RT-PCR) exponential phase determined on 15 to 30 cycles allowed semiquantitative comparisons among cDNAs derived from identical reactions. Therefore, in the reported data, each PCR regime involved a 2-min initial denaturation step at 94°C, followed by 20 cycles (for ADAM9), 25 cycles (for ADAM10 and ADAM17/TACE), 30 cycles (for ADAM15), or 20 to 40 cycles (20 cycles for ADAM12 in heart and cerebellum; 20, 30, 35, and 40 cycles were tested for ADAM12 in LLCPKcl4 cells) at 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s, with a 7-min extension at the end of the PCR using the Perkin Elmer 9600 thermal cycler (Global Medical Instrumentation, Ramsey, MN). Primer sequences were as follows: for ADAM9, 5′-CACGTCGGAGACATGACAGTGCAC-3′ (forward) and 5′-CCACACCCCAACATTTGGTGCCTC-3′ (reverse); for ADAM10, 5′-GTTAATTCTGCTCCTCTCCTGG-3′ (forward) and 5′-TGGATATCTGGGCAATCACAGC-3′ (reverse); for ADAM12 primer pair 1, 5′-GTGTTGGTAGGCGTGGAAGTGTGG-3′ (forward) and 5′-ACTCTGGGAGGTCACAGGAGTTGC-3′ (reverse); for ADAM12 primer pair 2, 5′-CTGCAAGACGGTACTGATGTCTC-3′ (forward) and 5′-TCATCCCGAAATTGTGGCCCAGC-3′ (reverse); for ADAM15, 5′-TCGATTGCCCCATGACAGTGCC-3′ (forward) and 5′-TGACTGGCACTGCTGGGCATAG-3′ (reverse); for ADAM17/TACE, 5′-GCAGTCTCTCCTATTCCTGACC-3′ (forward) and 5′-CATCTTCACATCCCAAGCATCC-3′ (reverse). The expected RT-PCR products using these primer pairs were 930 bp for ADAM9, 725 bp for ADAM10, 703 bp and 778 bp for ADAM12 primer pair 1 and 2 (respectively), 718 bp for ADAM15, and 943 bp for ADAM17/TACE.
Cloning of porcine ADAM9, ADAM10, and ADAM17/TACE cDNA fragments.
cDNA fragments of porcine ADAM9 (930 bp), ADAM10 (725 bp), and ADAM17/TACE (943 bp) were amplified from total RNA isolated from LLCPKcl4 by RT-PCR as described above and run on 1% agarose gels. The specific RT-PCR products were cut out and purified from the gels, followed by cloning into pCRII-TOPO vector using a TOPO T-A Cloning Kit (Invitrogen). These cloned cDNA fragments were sequenced at the DNA Core at Vanderbilt University.
RNA interference.
The endogenous expression of ADAM9, ADAM10, and ADAM17/TACE in LLCPKcl4 cells was silenced, respectively, using the pSUPER RNAi System from OligoEngine (Seattle, WA), according to the manufacturer's guidelines. In brief, based on the sequencing results of the porcine ADAM9, ADAM10, and ADAM17/TACE cDNA fragments that we cloned from LLCPKcl4 cells as described above, we designed the following oligonucleotides for effective silencing of these genes: ADAM9, 5′-GATCCCCTCACAGTGGAGACGTTTGCTTCAAGAGAGCAAACGTCTCCACTGTGATTTTTC-3′ (sense) and 5′-TCGAGAAAAATCACAGTGGAGACGTTTGCTCTCTTGAAGCAAACGTCTCCACTGTGAGGG-3′ (antisense); ADAM10, 5′-GATCCCCAGACATTATGAAGGATTGTTTCAAGAGAACAATCCTTCATAATGTCTTTTTTC-3′ (sense) and 5′-TCGAGAAAAAAGACATTATGAAGGATTGTTCTCTTGAAACAATCCTTCATAATGTCTGGG-3′ (antisense); ADAM17/TACE, 5′-GATCCCCGAAAAGCTTGATTCTCTGCTTCAAGAGAGCAGAGAATCAAGCTTTTCTTTTTC-3′ (sense) and 5′-TCGAGAAAAAGAAAAGCTTGATTCTCTGCTCTCTTGAAGCAGAGAATCAAGCTTTTCGGG-3′ (antisense). The sequence of each sense oligonucleotide includes the gene-specific 19-nucleotide small interfering RNA (siRNA) sequence in both sense and antisense orientation (underlined), separated by a 9-nucleotide spacer sequence. The 5′ end corresponds to the BglII site, while the 3′ end contains the T5 sequence and XhoI site. After synthesis of each pair of oligonucleotides by OligoEngine, oligonucleotides were annealed and cloned into the BglII and XhoI sites in the siRNA expression vector pSUPER.neo+gfp (OligoEngine), followed by transformation of Escherichia coli cells for amplification, sequencing verification of the cloned oligonucleotides, and transfection of these siRNA expression constructs into LLCPKcl4 cells by the Lipofectamine method (Invitrogen), as previously described (16). The construct containing irrelevant, scrambled sequence, GCGCGCTTTGTAGGATTCG, in the same vector was used as a negative siRNA control for transfection to determine the specificity of the siRNA data.
Isolation of mRNA and Northern blot analysis.
At 48 h after transfection of the pSUPER.neo+gfp cDNA construct containing scrambled sequence or an oligonucleotide specific for ADAM9, -10, or -17 siRNA into LLCPKcl4 cells, respectively, the cells were made serum free overnight; cellular mRNA was then purified by passing the total cellular RNA through columns of oligo(dT) (Sigma) and analyzed by Northern blot hybridization with [α-32P]dCTP-labeled cDNA probes specific for porcine ADAM9, ADAM10, and ADAM17/TACE, followed by stripping and reprobing with glyceraldehyde-3-phosphate dehydrogenase cDNA probes, as previously described (15).
Purification and assays of secreted mature TGF-α and HB-EGF.
Secreted mature TGF-α was partially purified by a modification of heparin affinity chromatography as described previously (60). Before exposure to vehicle or the indicated agents in serum-free medium, confluent cells were rendered quiescent and washed twice with phosphate-buffered saline. Conditioned medium (CM) was centrifuged and filtered through a 0.45-mm-pore-size filter, followed by separation of CM into two fractions, heparin-bound and -unbound. This separation was achieved by applying each sample of CM three times to a heparin-Sepharose column that was preequilibrated with 10 mM Tris-HCl (pH 7.4) containing 0.2 M NaCl and 1 mM benzamidine. The final pass-through was designated the heparin-unbound fraction, while the heparin-bound proteins were eluted with 2.0 M NaCl in 10 mM Tris-HCl (pH 7.4) after extensive washing with equilibration buffer. Both fractions were dialyzed against 10 mM Tris-HCl (pH 7.4) at 4°C overnight and lyophilized, followed by subsequent analysis of potential EGFR ligands in the heparin-bound and -unbound fractions.
Statistics.
Data are presented as means ± standard errors for at least three separate experiments (each in triplicate or duplicate). An unpaired Student's t test was used for statistical analysis, and for multiple group comparisons, analysis of variance and Bonferroni t tests were used. A P value of <0.05 compared with control was considered statistically significant.
RESULTS
2-14,15-EG is a potent mitogen for renal epithelial cells.
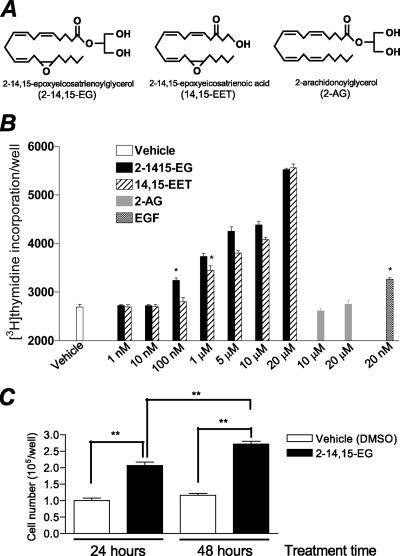
Renal proximal tubule cells are susceptible to injury by ischemia or reperfusion and toxins, but these cells also have a remarkable capacity for repair, characterized by hyperplasia and recovery of the damaged epithelial cells lining the tubules. These tubular cells express the highest levels of cP450 within the kidney (4, 22). We have previously found that 14,15-EET is a potent mitogen for the renal proximal tubule cell line LLCPKcl4, which was cloned from the parental cell line LLC-PK1 and selected for its proximal tubule characteristics (1). The mitogenic effect of 14,15-EET is mediated by initiation of a tyrosine kinase phosphorylation cascade that activates the p44/p42 ERKs and phosphatidylinositol 3-kinase (15). Our further studies demonstrated that EGFR transactivation is an essential event in the mitogenic signaling pathway of 14,15-EET and that EGFR is upstream of ERK activation (12). We also determined that soluble HB-EGF released by increased metalloproteinase activity in LLCPKcl4 cells in response to 14,15-EET is the ligand for EGFR activation (12). Utilizing liquid chromatography-electrospray ionization-tandem mass spectrometry analysis, we have recently identified endogenous production of a new cP450 metabolite of arachidonic acid, 2-14,15-EG, in the kidney (14). Figure 1A shows the structure of 2-14,15-EG, in comparison with 14,15-EET and 2-arachidonoylglycerol (2-AG, a structurally related and previously described endocannabinoid [45, 66]). When we examined the effect of 2-14,15-EG in LLCPKcl4 cells, we found that 2-14,15-EG had no mitogenic effect when its concentration was ≤10 nM, while 14,15-EET was not effective at a concentration of ≤100 nM in stimulating mitogenesis in these cells. However, both 2-14,15-EG (within the tested concentration range of 100 nM through 20 μM) and 14,15-EET (within the concentration range of 1 μM through 20 μM) concentration-dependently increased [3H]thymidine incorporation; in contrast, 2-AG had no effect (Fig. 1B). EGF (20 nM) was included as a positive control for the assays. 2-14,15-EG also stimulated cell proliferation within 24 h, as indicated by increased cell numbers (Fig. 1C). These data indicate that 2-14,15-EG is a potent mitogen for renal epithelial cells.
FIG. 1.
2-14,15-EG is a potent mitogen for renal epithelial cells. (A) Chemical structures of 2-14,15-EG, 14,15-EET, and 2-AG. (B) Increased DNA synthesis in LLCPKcl4 cells in response to increasing concentrations of 2-14,15-EG and 14,15-EET, but not to 2-AG, indicated by [3H]thymidine incorporation assays as described in Materials and Methods. EGF (20 nM) was included as a positive control (n = 4; *, P < 0.001, each compared to vehicle alone). (C) Stimulation of cell proliferation in the absence of fetal calf serum with the addition of 1 μM 2-14,15-EG (n = 6; **, P < 0.001). DMSO, dimethyl sulfoxide.
2-14,15-EG-induced EGFR and ERK activation is not mediated by cannabinoid receptors.
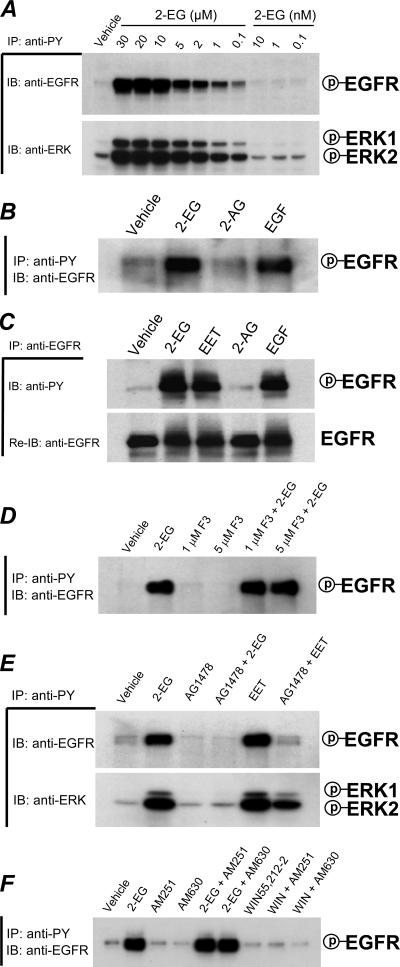
When we examined the early signaling in response to 2-14,15-EG by immunoprecipitation with anti-phosphotyrosine antibodies (anti-PY), followed by immunoblotting with either anti-EGFR or anti-ERK antibodies, we found that 2-14,15-EG concentration-dependently induced tyrosine phosphorylation of EGFR in LLCPKcl4 cells within 5 min (Fig. 2A, top); 2-14,15-EG also induced tyrosine phosphorylation of ERK1 and ERK2 (Fig. 2A). In contrast, 2-AG did not activate EGFR (Fig. 2B). To confirm these findings, we performed additional experiments by immunoprecipitation with an EGFR antibody, followed by immunoblotting analysis with anti-PY to assess tyrosine-phosphorylated EGFR levels (Fig. 2C, top). The same blots were then stripped and reprobed with anti-EGFR antibodies to ensure that equivalent amounts of total EGFR had been immunoprecipitated (Fig. 2C, bottom). Our results revealed increased tyrosine-phosphorylated EGFR levels in LLCPKcl4 cells in response to 2-14,15-EG, 14,15-EET, or EGF but not to 2-AG (Fig. 2C). These experiments confirmed that 2-14,15-EG can transactivate EGFR in LLCPKcl4 cells.
FIG. 2.
2-14,15-EG activates the EGFR-ERK signaling pathway in renal epithelial cells. (A) 2-14,15-EG induced EGFR and ERK tyrosine phosphorylation in LLCPKcl4 cells. Cells were made quiescent by serum deprivation for 24 h and treated for 5 min with either vehicle alone or increasing concentrations of 2-14,15-EG (2-EG) as indicated. Cell lysates were subjected to immunoprecipitation with anti-PY antibodies and immunoblotting with antibodies to EGFR (anti-EGFR) or p44/42 ERKs (anti-ERK) to determine the tyrosine phosphorylation levels of EGFR and ERKs. (B) The structurally similar endocannabinoid compound, 2-AG (1 μM), did not induce EGFR activation. (C) Activation of EGFR in response to 2-14,15-EG (1 μM), 14,15-EET (1 μM), or EGF (100 nM), but not to 2-AG (1 μM), was further confirmed by additional experiments performing immunoprecipitation with an EGFR antibody, followed by immunoblotting analysis with anti-PY to assess tyrosine-phosphorylated EGFR levels. The same plots were then stripped and reprobed (Re-IB) with anti-EGFR antibodies to ensure that an equivalent amount of total EGFR had been immunoprecipitated. (D) The potent pan-lipase inhibitor AA-cF3 did not inhibit 2-14,15-EG-induced EGFR activation. Quiescent LLCPKcl4 cells were pretreated with or without the pan-lipase inhibitor AA-cF3 (1 or 5 μM), followed by treatment with or without 2-14,15-EG (1 μM). Cell lysates were subjected to immunoprecipitation and immunoblotting analysis with the indicated antibodies. (E) The specific EGFR tyrosine kinase inhibitor AG1478 blocked 2-14,15-EG-induced EGFR activation. Quiescent cells were pretreated with AG1478 (100 nM) for 30 min before exposure to 2-14,15-EG (1 μM) for 5 min. Cell lysates were analyzed by immunoprecipitation and immunoblotting with the indicated antibodies. (F) The selective CB1 antagonist AM251 or CB2 antagonist AM630 did not affect the tyrosine phosphorylation levels of EGFR increased by 2-14,15-EG, and the cannabinoid receptor agonist, WIN55212-2, did not activate EGFR. Quiescent LLCPKcl4 cells were pretreated with or without 1 μM AM251 or AM630 for 20 min before treatment with or without 1 μM 2-14,15-EG or WIN55212-2 for 10 min. Cell lysates were analyzed as described in the legend of Fig. 1A. Shown are representative blots of three separate experiments with similar results for each set studied. IP, immunoprecipitation; IB, immunoblotting; P, phosphorylated.
Because 14,15-EET also activates EGFR in these cells (12), we examined whether EGFR activation by 2-14,15-EG was the result of conversion of 2-14,15-EG to 14,15-EET by lipase cleavage following administration of 2-14,15-EG to the cells. We therefore preincubated the cells with the potent pan-lipase inhibitor AA-cF3 at two different concentrations (1 and 5 μM) before treating the cells with or without 2-14,15-EG. Even at a high concentration (5 μM), AA-cF3 did not alter 2-14,15-EG-induced EGFR activation (Fig. 2D) and ERK tyrosine phosphorylation (data not shown), indicating that the increases in EGFR and ERK tyrosine phosphorylation in response to the administration of 2-14,15-EG were not the result of conversion of 14,15-EET to 2-14,15-EG.
Similar to 14,15-EET, 2-14,15-EG-induced EGFR activation was completely abolished by pretreatment of the cells with the specific EGFR tyrosine kinase inhibitor, AG1478 (Fig. 2E, top). Of interest, inhibition of EGFR by AG1478 also prevented 2-14,15-EG-induced ERK activation, indicating that EGFR is upstream of ERK activation in the early signaling events elicited by 2-14,15-EG (Fig. 2E, bottom).
Because G-protein-coupled receptors have been shown to mediate transactivation of EGFR (57) and we have demonstrated that 2-14,15-EG can bind and activate both cannabinoid receptor subtypes, CB1 and CB2 (14), we next examined whether 2-14,15-EG activation of EGFR was mediated through cannabinoid receptors, which belong to the G-protein-coupled receptor superfamily (43, 48). As shown in Fig. 2F, pretreatment with either the selective CB1 antagonist AM251 or CB2 antagonist AM630 did not affect 2-14,15-EG-induced EGFR activation (Fig. 2F). Similar to 2-AG, another cannabinoid receptor agonist, WIN55212-2, did not activate EGFR in LLCPKcl4 cells (Fig. 2F). Moreover, immunoblotting with antibodies against either CB1 or CB2 failed to detect expression of either cannabinoid receptor in LLCPKcl4 cells, although these two antibodies detected CB1 and CB2 protein expression in the brain and spleen, respectively (data not shown). Furthermore, there was no specific binding in LLCPKcl4 cells of [3H]CP55940, a ligand with high affinity for both CB1 and CB2 receptors.
2-14,15-EG induced-EGFR activation is not mediated by release of HB-EGF.
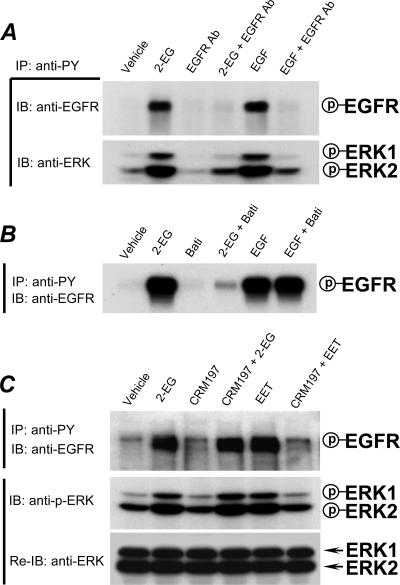
When we pretreated the cells with a specific neutralizing EGFR antibody, we found that the effect of 2-14,15-EG as well as that of exogenous EGF was almost completely blocked by the neutralizing antibody, as shown in Fig. 3A, suggesting that 2-14,15-EG activation of EGFR was mediated by an EGFR ligand. The blockade of 2-14,15-EG-induced ERK activation by the specific neutralizing EGFR antibody further confirmed that ERK was downstream of EGFR activation by 2-14,15-EG (Fig. 3A). Pretreatment with the metalloproteinase inhibitor Batimastat inhibited EGFR activation by 2-14,15-EG but had no effect on EGFR activation by administration of exogenous EGF (Fig. 3B). Similar effects were seen with ERK tyrosine phosphorylation (data not shown). These data suggested that similar to its parental 14,15-EET (12), 2-14,15-EG induced activation of EGFR-ERK signaling by releasing an EGFR ligand through activation of metalloproteinase activity. Our previous studies indicated that the parental 14,15-EET activated cleavage of membrane-bound proHB-EGF to release a soluble HB-EGF as a ligand to activate EGFR (12). To determine whether 2-14,15-EG activated an identical signaling pathway, cells were pretreated with or without the specific HB-EGF inhibitor CRM197 before exposure to 2-14,15-EG. As revealed in Fig. 3C, CRM197 had no effect on 2-14,15-EG-stimulated tyrosine phosphorylation of EGFR, although it completely blocked the effect of 14,15-EET. Similar effects were seen with the phosphorylation of p44/p42 ERKs (Fig. 3C). These data indicated that, unlike its parental 14,15-EET, 2-14,15-EG-induced activation of the EGFR-ERK signaling pathway was not dependent on HB-EGF.
FIG. 3.
Release of an EGFR ligand mediates the activation of EGFR-ERK signaling in response to 2-14,15-EG. (A) Neutralizing EGFR antibodies blocked 2-14,15-EG-induced tyrosine phosphorylation of EGFR and ERKs. LLCPKcl4 cells were made quiescent by serum deprivation for 24 h and preincubated with a monoclonal neutralizing EGFR antibody (EGFR Ab; 20 μg/ml), followed by treatment with or without 2-14,15-EG (1 μM) or EGF (100 nM). The tyrosine phosphorylation levels of EGFR and ERKs were detected by immunoprecipitation with anti-PY and immunoblotting with antibodies to EGFR and ERKs, respectively. (B) Inhibition of metalloproteinase activity abolished 2-14,15-EG-induced EGFR activation. Quiescent LLCPKcl4 cells were pretreated with or without the metalloproteinase inhibitor Batimastat (5 μM) for 30 min before treatment with or without 2-14,15-EG (1 μM) or EGF (100 nM) for 5 min. Cell lysates were subjected to immunoprecipitation with anti-PY and immunoblotting with an antibody to EGFR. (C) Inhibition of HB-EGF prevented 14,15-EET-induced phosphorylation of EGFR and ERK but had no effect on 2-14,15-EG-induced EGFR and ERK phosphorylation. Quiescent LLCPKcl4 cells were pretreated with or without the specific HB-EGF inhibitor CRM197 (10 μg/ml) for 30 min, followed by treatment with 2-14,15-EG or 14,15-EET for 5 min. Cells were washed twice with ice-cold Ca2+/Mg2+-free PBS and lysed as described previously (15). Cell lysates were subjected to immunoprecipitation with anti-PY and immunoblotting with an anti-EGFR antibody or directly subjected to immunoblotting with an antibody that recognizes only Thr202- and Tyr204-phosphorylated ERK1/2 (anti-p-ERK); the same blot was then stripped and reprobed (Re-IB) with an antibody recognizing total ERK1 and ERK2 (anti-ERK) to ensure equal loading. Shown is a representative blot of three separate experiments with similar results. IP, immunoprecipitation; IB, immunoblotting; P, phosphorylated.
TGF-α release mediates EGFR activation in response to 2-14,15-EG.
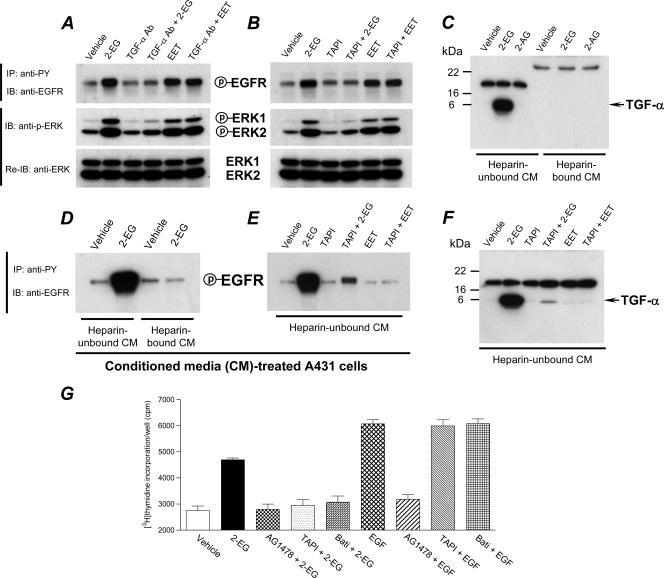
We found that neutralizing TGF-α antibodies blocked the effect of 2-14,15-EG; in contrast, the effect of 14,15-EET was not affected (Fig. 4A), suggesting that TGF-α was the ligand released by 2-14,15-EG. In this regard, ADAM17/TACE has been suggested to be the metalloproteinase that cleaves and releases TGF-α. In cells pretreated with the ADAM17/TACE inhibitor, TAPI, 2-14,15-EG-mediated EGFR and ERK activation was abolished, while there was no effect on 14,15-EET-induced EGFR and ERK phosphorylation (Fig. 4B). CM from LLCPKcl4 cells treated with or without 2-14,15-EG was separated into two fractions, the heparin-bound and -unbound fractions, respectively, by passing them through a heparin-Sepharose column. As demonstrated in Fig. 4C, 2-14,15-EG induced significant release of the 6-kDa soluble TGF-α into the heparin-unbound fraction of the CM; no TGF-α was detected in the heparin-bound fraction. In contrast, the structurally related 2-AG did not induce release of TGF-α (Fig. 4C).
FIG. 4.
Identification of active TGF-α as the EGFR ligand released in response to 2-14,15-EG. (A and B) Neutralization of TGF-α or inhibition of ADAM17/TACE activity blocked 2-14,15-EG-induced activation of EGFR-ERK signaling. Quiescent LLCPKcl4 cells were pretreated with or without either a monoclonal neutralizing TGF-α antibody (TGF-α Ab; 20 μg/ml) (A) or the ADAM17/TACE inhibitor, TAPI (5 μM) (B) before treatment with or without 2-14,15-EG (2-EG) or 14,15-EET (EET). Cells were lysed, and cell lysates were subjected to analysis of EGFR tyrosine phosphorylation and ERK phosphorylation at Thr202/Tyr204 as described in the legend of Fig. 3C. (C to F) 2-14,15-EG, but neither 2-AG nor 14,15-EET, induced release of an active TGF-α species into the heparin-unbound fraction of the CM. Quiescent LLCPKcl4 cells were treated with or without 2-14,15-EG or 2-AG and the CM was collected and passed through the heparin-Sepharose columns. The pass-through (heparin-unbound) fraction and heparin-bound fraction were analyzed by immunoblotting with a monoclonal antibody against mature TGF-α (C and F). The heparin-unbound fraction of the CM that contains a mature TGF-α species derived from 2-14,15-EG-treated LLCPKcl4 cells stimulated EGFR phosphorylation in A431 cells (D); this effect was inhibited by pretreatment of LLCPKcl4 cells with the ADAM17/TACE inhibitor, TAPI, before collection and separation of the CM for testing in A431 cells (E). TAPI pretreatment inhibited the release of the mature TGF-α species into the heparin-unbound fraction of the CM from LLCPKcl4 cells treated with 2-14,15-EG (F). (G) 2-14,15-EG-stimulated DNA synthesis in LLCPKcl4 cells was inhibited by pretreatment with the specific EGFR tyrosine kinase inhibitor AG1478 (100 nM), the metalloproteinase inhibitor Batimastat (Bati; 5 μM), or the ADAM17/TACE inhibitor TAPI (5 μM); in contrast, the increased DNA synthesis in response to exogenously added EGF was inhibited only by AG1478 but not by Batimastat or TAPI. IP, immunoprecipitation; IB, immunoblotting; P, phosphorylated.
When the heparin-unbound fraction of the CM collected from 2-14,15-EG-treated LLCPKcl4 cells was added to the human epidermoid carcinoma cell line A431, which expresses high levels of EGFR, a marked stimulation of EGFR tyrosine phosphorylation was seen, while the heparin-bound fraction did not activate EGFR in A431 cells (Fig. 4D). These data indicated that the 2-14,15-EG-released TGF-α was active. This activity in the CM was markedly diminished when LLCPKcl4 cells were pretreated with the TACE inhibitor TAPI before exposure to 2-14,15-EG for collecting CM (Fig. 4E). In contrast, the heparin-unbound fraction of the CM collected from 14,15-EET-treated LLCPKcl4 cells had no effect on EGFR tyrosine phosphorylation in A431 cells (Fig. 4E). Immunoblotting analysis revealed that TAPI pretreatment almost completely blocked 2-14,15-EG-induced TGF-α release into the CM of LLCPKcl4 cells (Fig. 4F), while 14,15-EET did not induce the release of TGF-α (Fig. 4E and F). 2-14,15-EG-stimulated DNA synthesis in LLCPKcl4 cells was inhibited by pretreatment with the specific EGFR tyrosine kinase inhibitor AG1478, the metalloproteinase inhibitor Batimastat, or the ADAM17/TACE inhibitor TAPI (Fig. 4G). In contrast, DNA synthesis in response to exogenously added EGF was inhibited only by AG1478, but not by Batimastat or TAPI (Fig. 4G). These pharmacological data suggested that 2-14,15-EG exerted its mitogenic effect by transactivating EGFR through activation of ADAM17/TACE, which cleaved and released the EGFR ligand, TGF-α.
ADAM17/TACE siRNA inhibits the release of TGF-α in response to 2-14,15-EG, while ADAM9 siRNA inhibits the release of HB-EGF in response to 14,15-EET.
Increasing evidence suggests that the release of EGFR ligands from the cell membrane is primarily mediated by multiple metalloproteinases of the ADAM family (3, 29). To determine the identity of the metalloproteinase(s) responsible for the release of TGF-α in LLCPKcl4 cells in response to 2-14,15-EG, we utilized RT-PCR to examine which ADAM family members were potentially involved in TGF-α and HB-EGF cleavage in LLCPKcl4 cells. Because the porcine cDNA sequences of these ADAMs were not available, we designed RT-PCR primer pairs based on the consensus cDNA sequences among human, rat, and mouse that are specific for ADAM9, ADAM10, ADAM12, ADAM15, and ADAM17/TACE, respectively. LLCPKcl4 cells expressed high levels of ADAM9 (Fig. 5A), ADAM10 (Fig. 5B), and ADAM17/TACE (Fig. 5B), with minimal ADAM15 expression (Fig. 5C). Although ADAM12 has been suggested to cleave EGFR ligands in certain cell types (2, 39), the expression of ADAM12 was not detected in LLCPKcl4 cells, even when increased amounts (5 to 10 μg) of total RNA from LLCPKcl4 cells and increasing PCR cycle numbers (30, 35, and 40 cycles) were used; in contrast, using the same primer pairs and under the same conditions, we detected ADAM12 expression in the presence of only 1 μg of total RNA isolated from either heart or cerebellum, two tissues known to express ADAM12, as indicated in Fig. 5A. The lack of ADAM12 expression in LLCPKcl4 cells was further confirmed by using two different RT-PCR primer pairs specific for ADAM12 that readily detected ADAM12 expression in both heart and cerebellum (data not shown).
FIG. 5.
RT-PCR detection of metalloproteinases in LLCPKcl4 cells. Preliminary experiments had determined that the RT-PCR exponential phase was 15 to 30 cycles to allow semiquantitative comparisons among cDNAs derived from identical reactions. (A) Detection of ADAM12 and ADAM9 expression. Using TRIZOL reagent (Invitrogen), total RNA was isolated from LLCPKcl4 cells as well as heart and cerebellum, two tissues known to express high levels of multiple ADAMs and thus used as positive controls. One microgram of total RNA from heart or cerebellum was used for RT, followed by PCR detection of ADAM12 and ADAM9 using their specific primer pairs, as detailed in Materials and Methods. Each PCR regime involved a 2-min initial denaturation step at 94°C, followed by 20 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 90 s, with a 7-min extension at the end of the PCR. Since no ADAM12 expression signal was detected in LLCPKcl4 cells when 1 μg of RNA was used for RT-PCR, additional experiments increased the amount of cellular total RNA up to 5 and 10 μg for RT with increased PCR cycle numbers (30, 35, and 40 cycles); however, no ADAM12 expression was detected under any conditions (data not shown). (B) Detection of ADAM17/TACE and ADAM10 expression. RT-PCR was conducted as described in panel A except that 25 cycles were used for the PCR amplification. (C) Detection of ADAM15 expression. RT-PCR was performed as described in panel A except for the 30 cycles of PCR amplification for ADAM15.
Further experiments examined whether siRNA-mediated downregulation of the highly expressed ADAM family members (ADAM9, ADAM10, and ADAM17/TACE) could inhibit the effect of 2-14,15-EG in LLCPKcl4 cells. To ensure adequate silencing of expression of these ADAM genes in LLCPKcl4 cells and also to confirm the authenticity of the RT-PCR products shown in Fig. 5A to C, these cDNA fragments (930-bp ADAM9, 725-bp ADAM10, and 943-bp ADAM17/TACE) derived from LLCPKcl4 cells were sequenced. The 930-bp ADAM9, 725-bp ADAM10, and 943-bp ADAM17/TACE of porcine origin are 92%, 95%, and 89% homologous to the corresponding human ADAMs and 85%, 91%, and 86% homologous to the corresponding mouse ADAMs (see Fig. S1 in the supplemental material). These fragments contain sequences that are predicted to be suitable for siRNA targeting (indicated in bold type in red in the figure); in addition, these sequences have been reported to be effectively targeted for silencing the gene expression of ADAM9 (24), ADAM10 (50), or TACE (50), respectively, in other species.
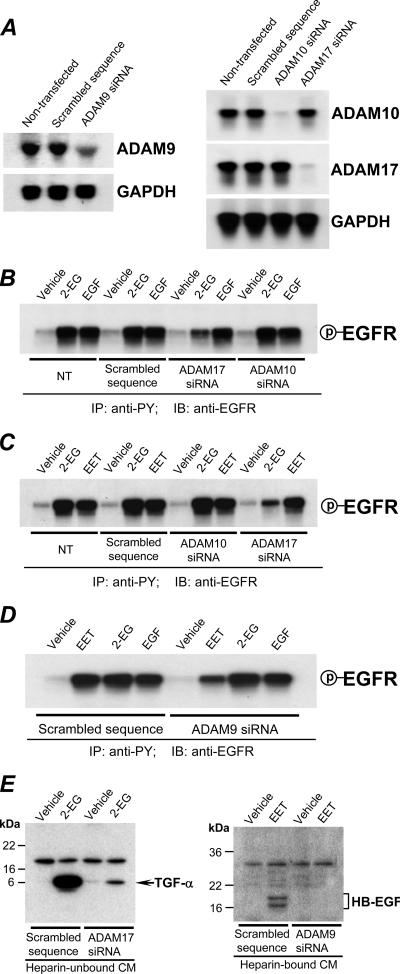
Utilizing the above sequence-confirmed 930-bp ADAM9, 725-bp ADAM10, and 943-bp ADAM17/TACE cDNA fragments as specific probes for Northern blotting analysis, we demonstrated that the expression of ADAM9, ADAM 10, and ADAM17/TACE in LLCPKcl4 cells was markedly inhibited by their respective siRNAs (Fig. 6A), while the same vector containing an irrelevant, scrambled sequence, GCGCGCTTTGTAGGATTCG, used as a negative siRNA control, was without effect (Fig. 6A). In nontransfected and control siRNA-transfected LLCPKcl4 cells, 2-14,15-EG stimulated EGFR tyrosine phosphorylation to equivalent levels; this effect was inhibited by ADAM17 siRNA but not by ADAM9 or ADAM10 siRNA (Fig. 6B to D). Neither ADAM10 siRNA nor ADAM17 siRNA had any effect on EGFR activation in response to exogenously added EGF (Fig. 6B). Since 2-14,15-EG-stimulated release of TGF-α was inhibited by the ADAM17 inhibitor, TAPI (Fig. 4F), we performed additional experiments to measure TGF-α release into the heparin-unbound fraction of the CM collected from scrambled control siRNA- or ADAM17 siRNA-transfected LLCPKcl4 cells treated with either vehicle (dimethyl sulfoxide) alone or 2-14,15-EG. Our results revealed that 2-14,15-EG-induced release of TGF-α was indeed inhibited by ADAM17 siRNA but not by scrambled control siRNA (Fig. 6E, left). These siRNA data confirmed the finding with the specific inhibitor of ADAM17/TACE shown in Fig. 4A to F that ADAM17/TACE is the metalloproteinase that cleaves and releases TGF-α, which serves as a natural ligand to bind and activate EGFR in response to 2-14,15-EG.
FIG. 6.
Effects of siRNA-mediated downregulation of ADAMs on release of EGFR ligands and transactivation of EGFR. (A) Downregulation of ADAM9, -10, and -17 expression by transfection of their respective siRNA expression constructs into LLCPKcl4 cells. Cells were transfected with or without pSUPER.neo+gfp cDNA constructs carrying scrambled sequence (GCGCGCTTTGTAGGATTCG) or oligonucleotide specific for ADAM9, -10, or -17 siRNA, respectively, as detailed in Materials and Methods. Forty-eight hours after transfection, cells were serum deprived overnight, cellular mRNA was purified, and 2 μg of mRNA was used for Northern blot analysis as detailed in Materials and Methods. (B to D) siRNA-mediated downregulation of ADAM17 inhibited 2-14,15-EG-induced EGFR tyrosine phosphorylation, while siRNA-mediated downregulation of ADAM9 inhibited 14,15-EET-induced EGFR transactivation. LLCPKcl4 cells were transfected with or without pSUPER.neo+gfp cDNA constructs carrying a scrambled sequence or oligonucleotide specific for ADAM17 and ADAM10 (B and C) or ADAM9 (D). Forty-eight hours after transfection, cells were serum deprived overnight and treated with vehicle alone, 2-14,15-EG (2-EG), EGF, or 14,15-EET (EET) for 5 min. Cells were lysed, and cell lysates were subjected to immunoprecipitation with anti-PY antibodies and immunoblotting with antibodies to EGFR (anti-EGFR) to determine the tyrosine phosphorylation levels of EGFR, as indicated. (E) ADAM17 siRNA inhibited 2-14,15-EG-induced release of TGF-α, while ADAM9 siRNA inhibited 14,15-EET-induced release of HB-EGF; in contrast, scrambled control siRNA had no effect. IP, immunoprecipitation; IB, immunoblotting; P, phosphorylated; NT, nontransfected; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
We had previously determined that 2-14,15-EG's parental compound, 14,15-EET, transactivated EGFR by releasing soluble HB-EGF through activation of an unidentified metalloproteinase(s) in these cells (12). In other systems, several members of the ADAM family including ADAM9 (24, 38), ADAM10 (40), ADAM12 (2, 39), and ADAM17 (67) have been suggested to release soluble HB-EGF. We therefore examined whether ADAM10 and ADAM17 were also responsible for the release of soluble HB-EGF in response to 14,15-EET. As shown in Fig. 6C, siRNA-mediated downregulation of either ADAM10 or ADAM17 (Fig. 6A) had no effect on the increased EGFR tyrosine phosphorylation in response to 14,15-EET. Additional experiments revealed that siRNA-mediated downregulation of ADAM9 (Fig. 6A) inhibited EGFR transactivation in response to 14,15-EET (Fig. 6D), although ADAM9 downregulation did not alter EGFR activation by either 2-14,15-EG or exogenous EGF (Fig. 6D). Our previous studies have determined that release of soluble HB-EGF is detected only in the heparin-bound, but not the heparin-unbound, fraction of the CM in response to 14,15-EET (12). We therefore examined whether ADAM9 siRNA could block the HB-EGF release induced by 14,15-EET. Our results demonstrated that siRNA-mediated downregulation of ADAM9 (Fig. 6A) inhibited 14,15-EET-induced release of HB-EGF (Fig. 6E); in contrast, scrambled control siRNA had no effect on the release of HB-EGF.
DISCUSSION
The primary goal of the present study was to characterize the potential roles and mechanisms of action of the newly identified cP450-dependent metabolite of arachidonic acid, 2-14,15-EG, using the well-established renal proximal tubule epithelial cell line, LLCPKcl4 (1), because the renal proximal tubule epithelial cells express the highest level of cP450 enzyme in the kidney but minimal levels of cyclooxygenase or lipoxygenase activity (4, 22). We initially determined that 2-14,15-EG is a potent mitogen for LLCPKcl4 cells, indicated by increased DNA synthesis and cell proliferation in response to 2-14,15-EG. We then attempted to characterize the mitogenic signaling mechanism of this new lipid mediator. Interestingly, we found that 2-14,15-EG exerts mitogenic effects by activating an EGFR-ERK signaling pathway. These effects were inhibited by the specific EGFR tyrosine kinase inhibitor AG1478, neutralizing EGFR antibodies, or the metalloproteinase inhibitor Batimastat, suggesting that similar to 14,15-EET (12), 2-14,15-EG also transactivates EGFR by activating a metalloproteinase and releasing an EGFR ligand. However, further experiments unexpectedly revealed that, unlike 14,15-EET (12), 2-14,15-EG-induced EGFR transactivation is not dependent on HB-EGF, suggesting that 2-14,15-EG transactivates EGFR through a signaling mechanism distinct from that of the structurally related 14,15-EET in the same cell type. In searching for the EGFR ligand released by 2-14,15-EG stimulation, we found that both the specific ADAM17/TACE inhibitor TAPI and neutralizing TGF-α antibodies inhibited EGFR transactivation and downstream ERK activation by 2-14,15-EG, suggesting that TGF-α might be the EGFR ligand released in response to 2-14,15-EG. This was confirmed by further experiments that detected the release of a mature 6-kDa TGF-α into the CM of LLCPKcl4 cells after treatment with 2-14,15-EG. The released 6-kDa TGF-α is indeed active, as confirmed further by its ability to activate EGFR in another cell line, A431 cells, which express high levels of EGFR. When ADAM17 was inactivated by its inhibitor TAPI or was knocked down by its siRNA, 2-14,15-EG-induced TGF-α release as well as EGFR activation was prevented. These data further confirmed that ADAM17 is the metalloproteinase that mediates 2-14,15-EG-induced release of TGF-α and activation of EGFR. In addition, our siRNA experiments also identified ADAM9 as the metalloproteinase responsible for the release of soluble HB-EGF and activation of EGFR in renal epithelial cells in response to 14,15-EET.
Of interest, the expression of HB-EGF as well as EGFR in the kidney is acutely upregulated in response to acute renal injury, and the levels of TGF-α also increase at a later stage during the recovery period; in contrast, renal expression of other EGFR ligands such as betacellulin and amphiregulin does not increase after acute injury (34, 36). Our data suggest that lipid mediators such as 14,15-EET and 2-14,15-EG activate different members of the ADAM metalloproteinase family and induce release of different EGFR ligands, specifically HB-EGF and TGF-α, which may play important roles in different stages of the renal recovery from injury. It will be important in future studies to determine the levels of these mitogenic lipid mediators and the enzymatic activities of both cP450 epoxygenases and the metalloproteinases of ADAM family in the kidney in response to acute renal insults such as ischemic and toxin-mediated epithelial cell damage.
In mammalian cells, the ligand-mediated EGFR activation involves EGF (11), TGF-α (18), HB-EGF (31), amphiregulin (63), betacellulin (62), epiregulin (69), or epigen (65). These EGFR ligands are initially synthesized as integral membrane precursor proteins consisting of an N-terminal extension, EGF-like domain, transmembrane region, and a cytoplasmic tail. The EGF-like motif, shared by all EGFR ligands, contains six conserved cysteine residues and forms the three-loop structure essential for EGFR binding (27). Biologically active soluble EGFR ligands are released from the cell surface by proteolytic cleavage in response to cell stimulation. These released ligands act as autocrine or paracrine growth factors for EGFR-expressing cells, although the membrane-bound forms of some EGFR ligands may also possess juxtacrine activity, directly interacting and activating EGFR on neighboring cells (5, 29, 32, 42, 70).
Metalloproteinases are responsible for proteolytic cleavage and release of membrane-bound EGFR ligands, thus regulating EGFR signaling and EGFR-dependent functions (3, 29). Although there have been studies suggesting a role for matrix metalloproteinases (68), recent evidence implicates the involvement of the ADAM family of proteins in the release of these ligands (3, 29). The ADAM family belongs to the metzincin protease superfamily. Although 40 ADAM family members have been identified from protozoans to mammals (J. M. White and T. G. Wolfsberg, http://people.virginia.edu/∼jw7g/Table_of_the_ADAMs.html), primarily ADAM9, -10, -12, -15, and -17 have been reported to cleave EGFR ligands in mammalian cells (3, 53). To date, however, the detailed molecular mechanisms underlying the substrate specificity and activation of different ADAM family members that are responsible for the ectodomain shedding of different EGFR ligands remain unclear (3, 53, 61). Protein kinase C has been suggested to mediate HB-EGF shedding by phorbol myristate acetate through its interaction with ADAM9 (38), while intracellular Ca2+-increasing agents have been shown to activate HB-EGF processing independently of protein kinase C (19). Src kinase activity has been reported to be essential for the release of HB-EGF in COS-7 cells (55); in contrast, Src kinase(s) does not appear to be involved in TGF-α shedding in colonic epithelial cells (44). Activation of phospholipase C also seems to mediate ADAM activation and HB-EGF shedding in response to angiotensin II (46). Activation of the p38 mitogen-activated protein kinase signaling pathway has been reported to mediate activation of ADAM-dependent EGFR ligand release in response to environmental stress such as hyperosmolarity and oxidative stress (24). While more than one proteolytic enzyme has been reported to process a single EGFR ligand and a single ADAM family member has been shown to process several EGFR ligands in certain cell types (3, 53, 61), recent studies have also suggested the intrinsic substrate specificity in ectodomain shedding of EGFR ligands between different ADAM members (33, 59). Furthermore, phosphorylation of ADAMs through a protein kinase(s) may modulate the activity of ADAMs (53). Finally, protein-protein interactions through several specific protein interaction domains such as PXXP motif in the cytoplasmic domain of ADAMs with Src homology 3 domain-containing proteins may also be involved in ADAM activation (53). Given the diversity of ADAM-activating signals and the reported mediators or pathways, it is possible that the ADAM-dependent shedding of EGFR ligands is controlled by multiple regulatory mechanisms. In this regard, it is noteworthy that 2-14,15-EG is a more polar molecule than 14,15-EET and may thereby activate distinct signaling pathways. Further studies will investigate signaling mechanisms of ADAM9-dependent release of HB-EGF and ADAM17-dependent release of TGF-α.
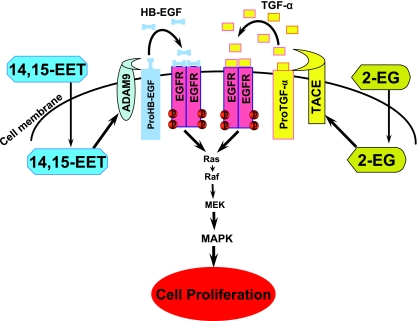
In summary, the present study documents for the first time that 2-14,15-EG is a potent mitogen for renal proximal tubule epithelial cells and characterizes the underlying signaling mechanism. The mitogenic effect of this new cP450 arachidonate metabolite is mediated by EGFR transactivation through a molecular mechanism distinct from that of its parental compound, 14,15-EET (10, 12). Specifically, 2-14,15-EG induces ADAM17 activation, which releases mature TGF-α, while 14,15-EET activates ADAM9 and releases soluble HB-EGF, both of which are natural ligands for EGFR that activate the downstream mitogen-activated protein kinase signaling pathway, leading to cell proliferation, as depicted in Fig. 7. These results represent the first evidence that two structurally related biologically active lipid mediators of cP450 metabolites activate different metalloproteinases and release different EGFR ligands to activate EGFR in the same cell type, although the detailed mechanisms underlying the activation and the substrate specificity of these ADAMs require further studies.
FIG. 7.
The mitogenic effect and signaling mechanism of 2-14,15-EG, a novel cP450 arachidonate metabolite. 2-14,15-EG acts as a potent mitogen for renal proximal tubule epithelial cells. This effect is mediated by transactivation of EGFR through a molecular mechanism distinct from that of its parental compound, 14,15-EET (10, 12). Specifically, 2-14,15-EG induces ADAM17/TACE activation, which releases mature TGF-α, while 14,15-EET activates ADAM9 and releases soluble HB-EGF, both of which are natural ligands for EGFR activation, which activates downstream mitogen-activated protein kinase (MAPK) signaling pathways, leading to cell proliferation.
Supplementary Material
Acknowledgments
This work was supported by funds from National Institutes of Health Grant DK38226 (J.-K.C., J.R.F., J.H.C., and R.C.H.), DK51265 (R.C.H.), the Department of Veterans Affairs (R.C.H.), and Robert A. Welch Foundation (J.R.F.).
Footnotes
Published ahead of print on 5 February 2007.
Supplemental material for this article may be found at http//:mcb.asm.org/.
REFERENCES
- 1.Amsler, K., and J. S. Cook. 1982. Development of Na+-dependent hexose transport in a cultured line of porcine kidney cells. Am. J. Physiol. 242:C94-C101. [DOI] [PubMed] [Google Scholar]
- 2.Asakura, M., M. Kitakaze, S. Takashima, Y. Liao, F. Ishikura, T. Yoshinaka, H. Ohmoto, K. Node, K. Yoshino, H. Ishiguro, H. Asanuma, S. Sanada, Y. Matsumura, H. Takeda, S. Beppu, M. Tada, M. Hori, and S. Higashiyama. 2002. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nat. Med. 8:35-40. [DOI] [PubMed] [Google Scholar]
- 3.Blobel, C. P. 2005. ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell. Biol. 6:32-43. [DOI] [PubMed] [Google Scholar]
- 4.Bonvalet, J. P., P. Pradelles, and N. Farman. 1987. Segmental synthesis and actions of prostaglandins along the nephron. Am. J. Physiol. 253:F377-F387. [DOI] [PubMed] [Google Scholar]
- 5.Brachmann, R., P. B. Lindquist, M. Nagashima, W. Kohr, T. Lipari, M. Napier, and R. Derynck. 1989. Transmembrane TGF-alpha precursors activate EGF/TGF-alpha receptors. Cell 56:691-700. [DOI] [PubMed] [Google Scholar]
- 6.Burns, K. D., J. Capdevila, S. Wei, M. D. Breyer, T. Homma, and R. C. Harris. 1995. Role of cytochrome P-450 epoxygenase metabolites in EGF signaling in renal proximal tubule. Am. J. Physiol. 269:C831—C340. [DOI] [PubMed] [Google Scholar]
- 7.Campbell, W. B., D. Gebremedhin, P. F. Pratt, and D. R. Harder. 1996. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ. Res. 78:415-423. [DOI] [PubMed] [Google Scholar]
- 8.Capdevila, J., N. Chacos, J. Werringloer, R. A. Prough, and R. W. Estabrook. 1981. Liver microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Proc. Natl. Acad. Sci. USA 78:5362-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capdevila, J. H., J. R. Falck, E. Dishman, and A. Karara. 1990. Cytochrome P-450 arachidonate oxygenase. Methods Enzymol. 187:385-394. [DOI] [PubMed] [Google Scholar]
- 10.Capdevila, J. H., J. R. Falck, and R. C. Harris. 2000. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 41:163-181. [PubMed] [Google Scholar]
- 11.Carpenter, G., and S. Cohen. 1990. Epidermal growth factor. J. Biol. Chem. 265:7709-7712. [PubMed] [Google Scholar]
- 12.Chen, J. K., J. Capdevila, and R. C. Harris. 2002. Heparin-binding EGF-like growth factor mediates the biological effects of P450 arachidonate epoxygenase metabolites in epithelial cells. Proc. Natl. Acad. Sci. USA 99:6029-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, J. K., J. Capdevila, and R. C. Harris. 2000. Overexpression of C-terminal Src kinase blocks 14, 15-epoxyeicosatrienoic acid-induced tyrosine phosphorylation and mitogenesis. J. Biol. Chem. 275:13789-13792. [DOI] [PubMed] [Google Scholar]
- 14.Chen, J. K., J. Chen, J. D. Imig, S. Wei, D. L. Hachey, S. G. Jagadeesh, J. R. Falck, J. H. Capdevila, and R. C. Harris. Identification of novel endogenous cytochrome P450 arachidonate metabolites with high affinity for cannabinoid receptors, in press. [DOI] [PMC free article] [PubMed]
- 15.Chen, J. K., J. R. Falck, K. M. Reddy, J. Capdevila, and R. C. Harris. 1998. Epoxyeicosatrienoic acids and their sulfonimide derivatives stimulate tyrosine phosphorylation and induce mitogenesis in renal epithelial cells. J. Biol. Chem. 273:29254-29261. [DOI] [PubMed] [Google Scholar]
- 16.Chen, J. K., D. W. Wang, J. R. Falck, J. Capdevila, and R. C. Harris. 1999. Transfection of an active cytochrome P450 arachidonic acid epoxygenase indicates that 14,15-epoxyeicosatrienoic acid functions as an intracellular second messenger in response to epidermal growth factor. J. Biol. Chem. 274:4764-4769. [DOI] [PubMed] [Google Scholar]
- 17.Corey, E. J., N. Haruki, and J. R. Falck. 1979. Selective epoxidation of eicosa-cis-5,8,11,14-tetraenoic (arachidonic) acid and eicosa-cis-8,11,14-trienoic acid. J. Am. Chem. Soc. 101:1586-1587. [Google Scholar]
- 18.Derynck, R. 1992. The physiology of transforming growth factor-alpha. Adv. Cancer Res. 58:27-52. [DOI] [PubMed] [Google Scholar]
- 19.Dethlefsen, S. M., G. Raab, M. A. Moses, R. M. Adam, M. Klagsbrun, and M. R. Freeman. 1998. Extracellular calcium influx stimulates metalloproteinase cleavage and secretion of heparin-binding EGF-like growth factor independently of protein kinase C. J. Cell Biochem. 69:143-153. [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo, V. 1995. Arachidonic acid and eicosanoids as targets and effectors in second messenger interactions. Prostaglandins Leukot. Essent. Fatty Acids 53:239-254. [DOI] [PubMed] [Google Scholar]
- 21.Dong, J., and H. S. Wiley. 2000. Trafficking and proteolytic release of epidermal growth factor receptor ligands are modulated by their membrane-anchoring domains. J. Biol. Chem. 275:557-564. [DOI] [PubMed] [Google Scholar]
- 22.Endou, H. 1983. Cytochrome P-450 monooxygenase system in the rabbit kidney: its intranephron localization and its induction. Jpn. J. Pharmacol. 33:423-433. [DOI] [PubMed] [Google Scholar]
- 23.Escalante, B. A., R. Staudinger, M. Schwartzman, and N. Abraham. 1995. Amiloride-sensitive ion transport inhibition by epoxyeicosatrienoic acids in renal epithelial cells. Adv. Prostaglandin Thromboxane Leukot. Res. 23:207-209. [PubMed] [Google Scholar]
- 24.Fischer, O. M., S. Hart, A. Gschwind, N. Prenzel, and A. Ullrich. 2004. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol. Cell. Biol. 24:5172-5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisslthaler, B., R. Popp, L. Kiss, M. Potente, D. R. Harder, I. Fleming, and R. Busse. 1999. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 401:493-497. [DOI] [PubMed] [Google Scholar]
- 26.Graier, W. F., S. Simecek, and M. Sturek. 1995. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J. Physiol. 482:259-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groenen, L. C., E. C. Nice, and A. W. Burgess. 1994. Structure-function relationships for the EGF/TGF-alpha family of mitogens. Growth Factors 11:235-257. [DOI] [PubMed] [Google Scholar]
- 28.Han, L., and R. K. Razdan. 1999. Total synthesis of 2-arachidonylglycerol (2-ara-Gl). Tetrahedron Lett. 40:1631-1634. [Google Scholar]
- 29.Harris, R. C., E. Chung, and R. J. Coffey. 2003. EGF receptor ligands. Exp. Cell Res. 284:2-13. [DOI] [PubMed] [Google Scholar]
- 30.Harris, R. C., T. Homma, H. R. Jacobson, and J. Capdevila. 1990. Epoxyeicosatrienoic acids activate Na+/H+ exchange and are mitogenic in cultured rat glomerular mesangial cells. J. Cell Physiol. 144:429-437. [DOI] [PubMed] [Google Scholar]
- 31.Higashiyama, S., J. A. Abraham, J. Miller, J. C. Fiddes, and M. Klagsbrun. 1991. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science 251:936-939. [DOI] [PubMed] [Google Scholar]
- 32.Higashiyama, S., R. Iwamoto, K. Goishi, G. Raab, N. Taniguchi, M. Klagsbrun, and E. Mekada. 1995. The membrane protein CD9/DRAP 27 potentiates the juxtacrine growth factor activity of the membrane-anchored heparin-binding EGF-like growth factor. J. Cell Biol. 128:929-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinkle, C. L., S. W. Sunnarborg, D. Loiselle, C. E. Parker, M. Stevenson, W. E. Russell, and D. C. Lee. 2004. Selective roles for tumor necrosis factor alpha-converting enzyme/ADAM17 in the shedding of the epidermal growth factor receptor ligand family: the juxtamembrane stalk determines cleavage efficiency. J. Biol. Chem. 279:24179-24188. [DOI] [PubMed] [Google Scholar]
- 34.Hise, M. K., M. Salmanullah, L. Liu, C. I. Drachenberg, J. C. Papadimitriou, and R. M. Rohan. 2001. Control of the epidermal growth factor receptor and its ligands during renal injury. Nephron 88:71-79. [DOI] [PubMed] [Google Scholar]
- 35.Hoebel, B. G., E. Steyrer, and W. F. Graier. 1998. Origin and function of epoxyeicosatrienoic acids in vascular endothelial cells: more than just endothelium-derived hyperpolarizing factor? Clin. Exp. Pharmacol. Physiol. 25:826-830. [DOI] [PubMed] [Google Scholar]
- 36.Homma, T., M. Sakai, H. F. Cheng, T. Yasuda, R. J. Coffey, Jr., and R. C. Harris. 1995. Induction of heparin-binding epidermal growth factor-like growth factor mRNA in rat kidney after acute injury. J. Clin. Investig. 96:1018-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Imig, J. D. 2000. Epoxygenase metabolites. Epithelial and vascular actions. Mol. Biotechnol. 16:233-251. [DOI] [PubMed] [Google Scholar]
- 38.Izumi, Y., M. Hirata, H. Hasuwa, R. Iwamoto, T. Umata, K. Miyado, Y. Tamai, T. Kurisaki, A. Sehara-Fujisawa, S. Ohno, and E. Mekada. 1998. A metalloprotease-disintegrin, MDC9/meltrin-gamma/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J. 17:7260-7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurisaki, T., A. Masuda, K. Sudo, J. Sakagami, S. Higashiyama, Y. Matsuda, A. Nagabukuro, A. Tsuji, Y. Nabeshima, M. Asano, Y. Iwakura, and A. Sehara-Fujisawa. 2003. Phenotypic analysis of Meltrin α (ADAM12)-deficient mice: involvement of Meltrin α in adipogenesis and myogenesis. Mol. Cell. Biol. 23:55-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemjabbar, H., and C. Basbaum. 2002. Platelet-activating factor receptor and ADAM10 mediate responses to Staphylococcus aureus in epithelial cells. Nat. Med. 8:41-46. [DOI] [PubMed] [Google Scholar]
- 41.Makita, K., J. R. Falck, and J. H. Capdevila. 1996. Cytochrome P450, the arachidonic acid cascade, and hypertension: new vistas for an old enzyme system. FASEB J. 10:1456-1463. [DOI] [PubMed] [Google Scholar]
- 42.Massague, J., and A. Pandiella. 1993. Membrane-anchored growth factors. Annu. Rev. Biochem. 62:515-541. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda, L. A., S. J. Lolait, M. J. Brownstein, A. C. Young, and T. I. Bonner. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561-564. [DOI] [PubMed] [Google Scholar]
- 44.McCole, D. F., S. J. Keely, R. J. Coffey, and K. E. Barrett. 2002. Transactivation of the epidermal growth factor receptor in colonic epithelial cells by carbachol requires extracellular release of transforming growth factor-alpha. J. Biol. Chem. 277:42603-42612. [DOI] [PubMed] [Google Scholar]
- 45.Mechoulam, R., S. Ben-Shabat, L. Hanus, M. Ligumsky, N. E. Kaminski, A. R. Schatz, A. Gopher, S. Almog, B. R. Martin, D. R. Compton, and et al. 1995. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem. Pharmacol. 50:83-90. [DOI] [PubMed] [Google Scholar]
- 46.Mifune, M., H. Ohtsu, H. Suzuki, H. Nakashima, E. Brailoiu, N. J. Dun, G. D. Frank, T. Inagami, S. Higashiyama, W. G. Thomas, A. D. Eckhart, P. J. Dempsey, and S. Eguchi. 2005. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin-binding epidermal growth factor shedding through angiotensin II type-1 receptor. J. Biol. Chem. 280:26592-26599. [DOI] [PubMed] [Google Scholar]
- 47.Morrison, A. R., and N. Pascoe. 1981. Metabolism of arachidonate through NADPH-dependent oxygenase of renal cortex. Proc. Natl. Acad. Sci. USA 78:7375-7378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munro, S., K. L. Thomas, and M. Abu-Shaar. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61-65. [DOI] [PubMed] [Google Scholar]
- 49.Munzenmaier, D. H., and D. R. Harder. 2000. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am. J. Physiol. Heart Circ. Physiol. 278:H1163-H1167. [DOI] [PubMed] [Google Scholar]
- 50.Murai, T., Y. Miyazaki, H. Nishinakamura, K. N. Sugahara, T. Miyauchi, Y. Sako, T. Yanagida, and M. Miyasaka. 2004. Engagement of CD44 promotes Rac activation and CD44 cleavage during tumor cell migration. J. Biol. Chem. 279:4541-4550. [DOI] [PubMed] [Google Scholar]
- 51.Needleman, P., J. Turk, B. A. Jakschik, A. R. Morrison, and J. B. Lefkowith. 1986. Arachidonic acid metabolism. Annu. Rev. Biochem. 55:69-102. [DOI] [PubMed] [Google Scholar]
- 52.Node, K., Y. Huo, X. Ruan, B. Yang, M. Spiecker, K. Ley, D. C. Zeldin, and J. K. Liao. 1999. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285:1276-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohtsu, H., P. J. Dempsey, and S. Eguchi. 2006. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. Am. J. Physiol. Cell Physiol. 291:C1-10. [DOI] [PubMed] [Google Scholar]
- 54.Oliw, E. H., J. A. Lawson, A. R. Brash, and J. A. Oates. 1981. Arachidonic acid metabolism in rabbit renal cortex. Formation of two novel dihydroxyeicosatrienoic acids. J. Biol. Chem. 256:9924-9931. [PubMed] [Google Scholar]
- 55.Pierce, K. L., A. Tohgo, S. Ahn, M. E. Field, L. M. Luttrell, and R. J. Lefkowitz. 2001. Epidermal growth factor (EGF) receptor-dependent ERK activation by G protein-coupled receptors: a co-culture system for identifying intermediates upstream and downstream of heparin-binding EGF shedding. J. Biol. Chem. 276:23155-23160. [DOI] [PubMed] [Google Scholar]
- 56.Potente, M., U. R. Michaelis, B. Fisslthaler, R. Busse, and I. Fleming. 2002. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J. Biol. Chem. 277:15671-15676. [DOI] [PubMed] [Google Scholar]
- 57.Prenzel, N., E. Zwick, H. Daub, M. Leserer, R. Abraham, C. Wallasch, and A. Ullrich. 1999. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402:884-888. [DOI] [PubMed] [Google Scholar]
- 58.Roghani, M., J. D. Becherer, M. L. Moss, R. E. Atherton, H. Erdjument-Bromage, J. Arribas, R. K. Blackburn, G. Weskamp, P. Tempst, and C. P. Blobel. 1999. Metalloprotease-disintegrin MDC9: intracellular maturation and catalytic activity. J. Biol. Chem. 274:3531-3540. [DOI] [PubMed] [Google Scholar]
- 59.Sahin, U., G. Weskamp, K. Kelly, H. M. Zhou, S. Higashiyama, J. Peschon, D. Hartmann, P. Saftig, and C. P. Blobel. 2004. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164:769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakai, M., M. Zhang, T. Homma, B. Garrick, J. A. Abraham, J. A. McKanna, and R. C. Harris. 1997. Production of heparin binding epidermal growth factor-like growth factor in the early phase of regeneration after acute renal injury. Isolation and localization of bioactive molecules. J. Clin. Investig. 99:2128-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seals, D. F., and S. A. Courtneidge. 2003. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17:7-30. [DOI] [PubMed] [Google Scholar]
- 62.Shing, Y., G. Christofori, D. Hanahan, Y. Ono, R. Sasada, K. Igarashi, and J. Folkman. 1993. Betacellulin: a mitogen from pancreatic beta cell tumors. Science 259:1604-1607. [DOI] [PubMed] [Google Scholar]
- 63.Shoyab, M., G. D. Plowman, V. L. McDonald, J. G. Bradley, and G. J. Todaro. 1989. Structure and function of human amphiregulin: a member of the epidermal growth factor family. Science 243:1074-1076. [DOI] [PubMed] [Google Scholar]
- 64.Staudinger, R., B. Escalante, M. L. Schwartzman, and N. G. Abraham. 1994. Effects of epoxyeicosatrienoic acids on 86Rb uptake in renal epithelial cells. J. Cell Physiol. 160:69-74. [DOI] [PubMed] [Google Scholar]
- 65.Strachan, L., J. G. Murison, R. L. Prestidge, M. A. Sleeman, J. D. Watson, and K. D. Kumble. 2001. Cloning and biological activity of epigen, a novel member of the epidermal growth factor superfamily. J. Biol. Chem. 276:18265-18271. [DOI] [PubMed] [Google Scholar]
- 66.Sugiura, T., S. Kondo, A. Sukagawa, S. Nakane, A. Shinoda, K. Itoh, A. Yamashita, and K. Waku. 1995. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem. Biophys. Res. Commun. 215:89-97. [DOI] [PubMed] [Google Scholar]
- 67.Sunnarborg, S. W., C. L. Hinkle, M. Stevenson, W. E. Russell, C. S. Raska, J. J. Peschon, B. J. Castner, M. J. Gerhart, R. J. Paxton, R. A. Black, and D. C. Lee. 2002. Tumor necrosis factor-alpha converting enzyme (TACE) regulates epidermal growth factor receptor ligand availability. J. Biol. Chem. 277:12838-12845. [DOI] [PubMed] [Google Scholar]
- 68.Suzuki, M., G. Raab, M. A. Moses, C. A. Fernandez, and M. Klagsbrun. 1997. Matrix metalloproteinase-3 releases active heparin-binding EGF-like growth factor by cleavage at a specific juxtamembrane site. J. Biol. Chem. 272:31730-31737. [DOI] [PubMed] [Google Scholar]
- 69.Toyoda, H., T. Komurasaki, D. Uchida, Y. Takayama, T. Isobe, T. Okuyama, and K. Hanada. 1995. Epiregulin: a novel epidermal growth factor with mitogenic activity for rat primary hepatocytes. J. Biol. Chem. 270:7495-7500. [DOI] [PubMed] [Google Scholar]
- 70.Wong, S. T., L. F. Winchell, B. K. McCune, H. S. Earp, J. Teixido, J. Massague, B. Herman, and D. C. Lee. 1989. The TGF-alpha precursor expressed on the cell surface binds to the EGF receptor on adjacent cells, leading to signal transduction. Cell 56:495-506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.