Abstract
The Wilms' tumor protein Wt1 plays an essential role in mammalian urogenital development. WT1 mutations in humans lead to a variety of disorders, including Wilms' tumor, a pediatric kidney cancer, as well as Frasier and Denys-Drash syndromes. Phenotypic anomalies in Denys-Drash syndrome include pseudohermaphroditism and sex reversal in extreme cases. We have used cDNA microarray analyses on Wt1 knockout mice to identify Wt1-dependent genes involved in sexual development. The gene most dramatically affected by Wt1 inactivation was Amhr2, encoding the anti-Müllerian hormone (Amh) receptor 2. Amhr2 is an essential factor for the regression of the Müllerian duct in males, and mutations in AMHR2 lead to the persistent Müllerian duct syndrome, a rare form of male pseudohermaphroditism. Here we show that Wt1 and Amhr2 are coexpressed during urogenital development and that the Wt1 protein binds to the promoter region of the Amhr2 gene. Inactivation and overexpression of Wt1 in cell lines was followed by immediate changes of Amhr2 expression. The identification of Amhr2 as a Wt1 target provides new insights into the role of Wt1 in sexual differentiation and indicates, in addition to its function in early gonad development and sex determination, a novel function for Wt1, namely, in Müllerian duct regression.
The Wilms' tumor suppressor gene WT1 (Wt1 in the mouse) encodes an essential factor regulating mammalian urogenital development. Mutations in WT1 cause Wilms' tumor, a form of pediatric kidney cancer (11, 19), as well as a variety of human syndromes. Three of these disorders, namely, WAGR (for “Wilms tumor, aniridia, genitourinary abnormalities, mental retardation”), Denys-Drash syndrome (DDS), and Frasier syndrome, affect development of gonads and genitalia. The resulting abnormalities can range from cryptorchidism and hypospadia in male WAGR patients to streak gonads and sex reversal of internal and external genitalia in extreme DDS and Frasier cases (7, 31, 41, 47). How the reduction of WT1 levels or mutations of the gene lead to these drastic phenotypic anomalies is still unclear.
Biochemically, Wt1 is a bona fide transcription factor harboring four zinc fingers at its C terminus. Expression of Wt1 yields a protein family arising from usage of alternative translation initiation sites, RNA editing, and alternative splicing (reviewed in reference 16). The most important alternative splicing event leads to the insertion of three additional amino acids (KTS) between zinc fingers 3 and 4. Biochemical evidence suggests that the −KTS and +KTS isoforms of Wt1 play different roles (49). While Wt1(−KTS) unequivocally is a transcription factor, Wt1(+KTS) has been shown to be involved in RNA processing (10, 39).
In mice, inactivation of Wt1 results in embryonic lethality and failure of kidney and gonad development (32) as well as other organ defects (22, 55-57). With respect to the gonad, Wt1 has multiple roles. In Wt1 knockout mice, gonad development does not proceed beyond the formation of the early gonad at embryonic day 11.5 (E11.5). One gene that mediates this early function of Wt1 is Sf1, encoding steroidogenic factor 1 (58). Recently, Wt1(−KTS)- and Wt1(+KTS)-specific knockout mice have been generated (21). Regarding gonad development, the −KTS variants of Wt1 are required for the survival of the gonadal primordium, whereas the +KTS forms seem primarily involved in the male sex determination pathway.
An important step after mammalian sex determination is the differentiation of the Müllerian and the Wolffian ducts. The Müllerian duct serves as the primordium of the oviduct, uterus, and upper vagina in females, whereas in males the Wolffian duct develops into the epididymis and vas deferens. In order for male sexual development to occur, the Müllerian ducts have to be eliminated. In humans failure of Müllerian duct regression leads to the development of internal pseudohermaphrodites with uterine and oviductal tissues in otherwise normally virilized males, a condition known as persistent Müllerian duct syndrome (30).
The key factor in the regression of the Müllerian ducts is the anti-Müllerian hormone (Amh) that is secreted by Sertoli cells of the testis (for a review see reference 29). As a member of the transforming growth factor β family, Amh signals through a heterodimeric receptor complex consisting of a type I and a type II serine/threonine kinase receptor. The Amh type II receptor (Amhr2) confers the specificity and is initially expressed in the coelomic epithelium next to the Müllerian ducts in both males and females (59). Subsequently, under the influence of Amh, the Amhr2-expressing cells in males undergo an epithelial-to-mesenchymal transition and migrate into the mesenchyme surrounding the Müllerian duct. There, Amh signaling leads to degeneration of the Müllerian duct via a paracrine mechanism (1, 50). Amhr2 is also expressed in fetal and adult gonads of both sexes (4, 14, 52). The physiological function of Amhr2 in the gonad is not yet fully understood. In contrast, the observation that mutational inactivation of Amhr2 or Amh in humans and mice leads to persistent Müllerian duct syndrome (28, 36) demonstrates the biological relevance of Amhr2 for Amh signaling during Müllerian duct regression.
To gain deeper insights into the role of Wt1 during sexual development, we set out to identify Wt1 target genes mediating its role during this process. To this end we have employed Wt1 knockout mice and cDNA microarray methodology and have identified the Amhr2 gene as a novel Wt1 target. We show here that Wt1 and Amhr2 are coexpressed, that the Wt1(−KTS) protein binds to specific sites in the promoter region of the Amhr2 gene, and that inactivation and overexpression of Wt1(−KTS) are followed by immediate changes in Amhr2 expression. The identification of Amhr2 as a Wt1 target contributes to our understanding of Wt1's role in sex differentiation and indicates a novel function for Wt1 in Müllerian duct regression.
MATERIALS AND METHODS
Mouse lines.
For this study, mice carrying a Wt1 knockout allele on an Mf1 background (22) and Mf1 wild-type mice were used. Embryos were collected from timed matings, with noon of the day on which the mating plug was observed designated E0.5. Embryos from E10.5 to E12.5 were staged by counting the number of tail somites (ts), with 8 ts corresponding to E10.5 and 18 ts to E11.5 (20). Genotyping was performed as described before (58).
All mouse experiments were performed at the animal facility of the institute. The animals were housed under a cycle of 12 h of light and 12 h of darkness with free access to standard mouse chow and tap water. All experimental procedures complied with the rules of the German Animal Welfare Law and were approved by the local authorities. This is in accordance with the International Guiding Principles for Biomedical Research Involving Animals.
Cell culture.
The mesonephric mouse cell line M15 and the murine cell line of Sertoli origin TM4 (both obtained from the European Collection of Cell Cultures) were grown in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) with 10% fetal calf serum (FCS). The human osteosarcoma-derived cell lines UB27 and UD28, which harbor Wt1 alleles that can be controlled by the tet system (17), were grown in DMEM containing 10% FCS, 0.5 mg/ml G418, 1 μg/ml puromycin, and 1 μg/ml tetracycline to suppress Wt1 expression. For the induction of Wt1 expression, the cells were washed once with phosphate-buffered saline and twice with DMEM containing 10% FCS.
RNA isolation.
Total RNA from urogenital ridges was isolated and reverse transcribed as described previously (8). Total RNA from eukaryotic cell lines was isolated using an RNeasy Mini kit (QIAGEN) after homogenization with QIAshredder spin columns (QIAGEN) and eluted in a total volume of 80 μl RNase-free water. Seventeen microliters of RNA was treated with RQ1 RNase-Free DNase (Promega) before reverse transcription (RT).
Microarray analysis.
For microarray analysis, urogenital ridges (mesonephros plus gonad) of five (four female, one male) Wt1+/+ and Wt1−/− embryos with 11 to 12 tail somites were pooled before RNA isolation. The microarray analysis was performed by using CodeLink UniSet Mouse 20k microarray slides (Amersham Biosciences, now part of GE Healthcare). Both samples were measured three times. Data analysis and quality control were performed using CodeLink Expression Analysis software.
Real-time RT-PCR.
Quantitative real-time RT-PCR analysis was basically performed as described previously (8). The following primers were used: for Amhr2, CCCAACATCCCATCCACTT and GCTGAAAGGCAGTTCTCTGG; for AMHR2, GGACCCCTACTCAACCACAA and ACAGGAGCAGAGCCAAAGAG; for Lhx9, CAGCAGCCTTATCCACCTTC and TATCAACACCCCCATTCTCC; for Wt1, AGTTCCCCAACCATTCCTTC and TTCAAGCTGGGAGGTCATTT; for Actb, TGTTACCAACTGGGACGACA and GGGGTGTTGAAGGTCTCAAA; for ACTB, ACTGGGACGACATGGAGAAA and AGCACAGCCTGGATAGCAAC; and for Gapdh, AACTTTGGCATTGTGGAAGG and ACACATTGGGGGTAGGAACA. All primer pairs for RT-PCR covered at least one intron. PCR primers were designed using the Primer3 program (http://fokker.wi.mit.edu/cgibin/primer3/primer3_www.cgi). Expression levels were determined in one plate for all samples simultaneously and normalized to the corresponding amounts of Actb (beta-actin) cDNA measured within the same plate. Relative expression levels where calculated using the 2−ΔΔCT method (34).
RNA in situ hybridization.
In situ hybridizations on 10-μm paraffin sections and whole-mount in situ hybridizations using digoxigenin-labeled (Roche) riboprobes were performed as described previously (33). A 437-bp cDNA fragment of Amhr2 was amplified using the primers CGTTTCTCCCAGGTAATCCA and ATGGCGCATGACCTATCTTC and cloned into pCRII-TOPO with the TOPO TA Cloning kit (Invitrogen). The published plasmids containing fragments recognizing mouse Wt1 (54) as well as Lhx9 and Sf1 (58) mRNA were used.
RNA interference.
Short interfering RNAs (siRNAs) directed against Wt1 were designed using the online tool provided by the Whitehead Institute (www.whitehead.mit.edu) and synthesized by employing the Silencer siRNA Construction kit (Ambion) with the oligonucleotides AAGGATACAGCACGGTCACTTCCTGTCTC and AAAAGTGACCGTGCTGTATCCCCTGTCTC (siRNA no. 1) as well as AATGACCTCCCAGCTTGAATGCCTGTCTC and AACATTCAAGCTGGGAGGTCACCTGTCTC (siRNA no. 2) as templates. M15 cells (3 × 105) were seeded in 6-cm dishes and transfected 24 h later by applying 15 nM siRNA and 10 μl siLentFect Lipid Reagent (Bio-Rad). Medium was changed 24 h posttransfection. Cells were harvested for RNA isolation about 48 h after transfection.
Western blotting analysis.
For Western blotting, cellular lysates were extracted with 50 mM Tris, pH 7.5, 150 mM NaCl, and 0.1% sodium dodecyl sulfate (SDS). Forty micrograms of cell lysate was electrophoretically separated by SDS-10% polyacrylamide gel electrophoresis and transferred onto a polyvinyl difluoride membrane (Hybond-P; Amersham Biosciences). Proteins were detected with rabbit anti-Wt1 antibody (C-19; 1:500 dilution; Santa Cruz Biotechnology) followed by horseradish peroxidase-coupled goat anti-rabbit antibody (1:2,500 dilution; DakoCytomation) or goat anti-lactate dehydrogenase antibody (1:10,000 dilution; Chemicon International) followed by horseradish peroxidase-coupled rabbit anti-goat antibody (1:2,500 dilution; DakoCytomation), respectively, and enhanced chemiluminescence analysis (ECL Plus Western Blotting Detection System; Amersham Biosciences).
EMSA.
Electrophoretic mobility shift assay (EMSA) analysis using recombinant, bacterially expressed glutathione S-transferase (GST) fusion proteins of the zinc finger region of WT1 with or without the KTS insertion (58), a Denys-Drash mutant harboring an exchange of Arg at position 394 to a Trp residue (41), and full-length Sf1 (51) was performed as described previously (58). Amhr2 promoter fragment probes were amplified by PCR using a luciferase reporter construct containing 1.6 kb of the Amhr2 promoter as template (53) and gel purified by employing a QIAEX II gel extraction kit (QIAGEN).
DNase footprinting assay.
For footprinting analysis, a 362-bp fragment containing the Sf1 and Wt1 binding sites and a 337-bp fragment containing the Wt1 binding sites only were amplified using one 32P-labeled 5′ primer and one nonlabeled 3′ primer (GGAACCATCTTGGACAGAGC with CAGCCAAGGCTTCCTACAAA and CCCTCTCCGAGGAGAAAAAG with GLprimer2, CTTTATGTTTTTGGCGTCTTCCA, respectively) and the 1.6-kb Amhr2 promoter construct as template. DNase footprinting assays including RQ1-DNase I (Promega) treatment for 1 min were performed as described previously (58).
Luciferase reporter assay.
Murine Sf1 cDNA was amplified using primers GGGAAGCTTGAATTCTCCTTCCGTTCAGCG and GCGCTCGAGAGGCAGTGGCATCCCTGCCTA, containing a HindIII and an XhoI restriction site, respectively, and ligated into pGEM-T (Promega). An Sf1 expression construct was obtained by subcloning this cDNA into pcDNA 3.1(+) (Invitrogen) via HindIII and XhoI restriction. Sf1 expression from this construct was confirmed by Western blot analysis. Construction of Wt1(+/−KTS) expression plasmids was described previously (58).
A luciferase reporter construct containing 1.6 kb of the Amhr2 promoter sequence was kindly provided by Jose Teixeira (53). Mutagenesis constructs containing mutated Wt1 consensus-binding sites were generated using the QuikChange Site-Directed Mutagenesis kit (Stratagene). To generate the wild-type and mutated pAmhr2 F4 constructs, fragments containing a 223-bp sequence from positions −148 to +75 of the Amhr2 promoter were amplified from respective full-length constructs using the forward primer of F4 (GAAAAGATTGATTCTCTGCTCCTC) and GLprimer2 (see above), cloned into pCRII-TOPO (Invitrogen), verified by sequencing, and subcloned into the pGL3 basic reporter vector via SacI and NcoI restriction.
Transfection of murine cell lines was performed using SuperFect transfection reagent (QIAGEN) in a 24-well format. Cells were split at 5 × 104 to 8 × 104 TM4 cells or 2 × 104 to 3 × 104 M15 cells per well and transfected 24 h later with 750 ng expression construct, 200 ng promoter reporter construct, and 50 ng phRL-Tk control plasmid (Promega). Firefly and Renilla luciferase activities were measured 48 h after transfection using the Dual-Luciferase Assay kit (Promega).
ChIP.
Chromatin immunoprecipitation (ChIP) using C-19 anti-Wt1 rabbit polyclonal antibody (Santa Cruz Biotechnology), WTc8 anti-Wt1 rabbit polyclonal antibody (17), A-14 anti-c-Myc rabbit polyclonal antibody (Santa Cruz Biotechnology), and anti-acetyl-histone H3 rabbit polyclonal antibody (Upstate Biotechnology) was performed essentially as described previously (48). The purified DNA was dissolved in 100 μl water. One microliter was used for PCR analysis with 200 mM Tris, pH 8.4, 500 mM KCl, 1.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates, 0.4 μM primers, and 0.5 U Taq polymerase in a final volume of 25 μl. Cycling conditions were 94°C for 1 min and then 40 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The primer pairs used were GGTTCTCTGCTCCTCCCTTT and GGCTTCTGATCCCTCAGTCC (spanning bases −139 to −11 of the human AMHR2 promoter), AGAGGCCCACACTCAGCA and TGGCAGTGATAAATCGGACA (binding ∼2 kb downstream of the human promoter), CAGCTGGACAGCCAAGGTC and CAGCCAAGGCTTCCTACAAA (spanning bases −209 to +24 of the murine Amhr2 promoter), as well as AGATGTCTGCTCTGGGGTTG and CCTGTGGACTCTCTGGGAAA (binding ∼10 kb downstream of the murine promoter).
Microarray data accession number.
The microarray data of this work were deposited in the NCBI database GEO (accession number GSE6700).
RESULTS
Amhr2 is not expressed in urogenital ridges of Wt1−/− mouse embryos.
To identify targets of Wt1 in sexual development, we compared levels of gene expression in urogenital ridges of Wt1+/+ and Wt1−/− mouse embryos using a microarray that covered 20,000 unique genes. The embryos had 11 to 12 tail somites (∼E11.0), a stage immediately before the beginning of sex differentiation. Of the 12,043 genes that fulfilled the quality criteria, 142 showed differential expression at a level higher than 3-fold and 15 showed differential expression at a level higher than 10-fold. In most cases (e.g., 100 out of 142), expression of the respective gene was reduced in Wt1−/− mutant urogenital ridges (for details, see http://www.fli-leibniz.de/www_molgen/supp-mat/MCB2007SupplTable.pdf). The gene which showed the greatest difference was the gene encoding the anti-Müllerian hormone receptor Amhr2, with 64-times-higher signal in wild-type versus knockout urogenital ridges. For comparison, the second and third most differentially expressed genes showed factors of 36 and 23, respectively.
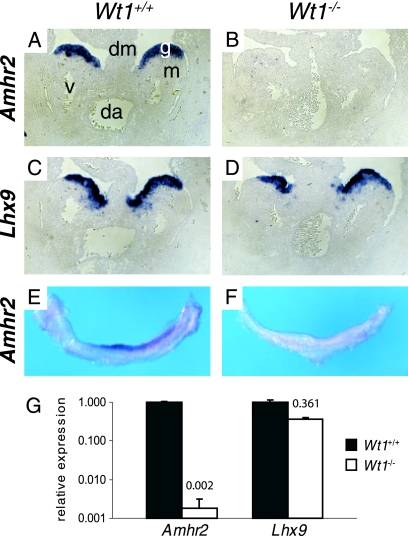
To verify the expression difference and to obtain information about the localization of Amhr2 mRNA in the urogenital ridge, RNA in situ hybridization was performed. We observed Amhr2 expression in the gonadal part of the urogenital ridge of wild-type embryos starting from about E10.5, but not in knockout embryos (Fig. 1A, B, E, and F). In the latter, expression of Lhx9, a gene encoding another transcription factor involved in early gonad development (9), was diminished but could be clearly observed, as previously described (58) (Fig. 1C and D). The persistence of Lhx9 expression demonstrates that the gonadal part of the urogenital ridge still exists in E11.5 knockout embryos. Differential expression of Amhr2 was also confirmed by real-time PCR analysis (Fig. 1G). With this method we determined a 500-fold reduction in Amhr2 expression in Wt1−/− gonads from embryos with 12 tail somites compared to the respective wild-type tissue. Reduction of Lhx9 was 2.8-fold.
FIG. 1.
Amhr2 expression is lost in gonads of Wt1−/− embryos. Expression of Amhr2 (A, B, E, and F) and Lhx9 (C and D) in urogenital ridges of Wt1+/+ (A, C, and E) and Wt1−/− (B, D, and F) embryos with 12 (A to D), 15 (E), or 16 (F) tail somites (E11.0 to E11.5) was analyzed by RNA in situ hybridization on paraffin sections (A to D) and whole urogenital ridges (E and F). (G) Quantitative real-time RT-PCR analysis of Amhr2 and Lhx9 expression in Wt1−/− urogenital ridges of the 12-tail-somite stage compared to wild-type tissue. Expression levels were normalized to Actb. For comparison, expression in the wild-type urogenital ridge was set as 1. In order to make the value for Amhr2 expression in Wt1−/− tissue visible, the scale of the y axis is logarithmic. Numbers above white bars indicate the ratio between mutant and wild-type expression levels. Data represent means and standard deviations of one representative experiment, measured in triplicate. da, dorsal aorta; dm, dorsal mesentery; g, gonad; m, mesonephros; v, subcardial vein.
Amhr2 and Wt1 show overlapping expression patterns.
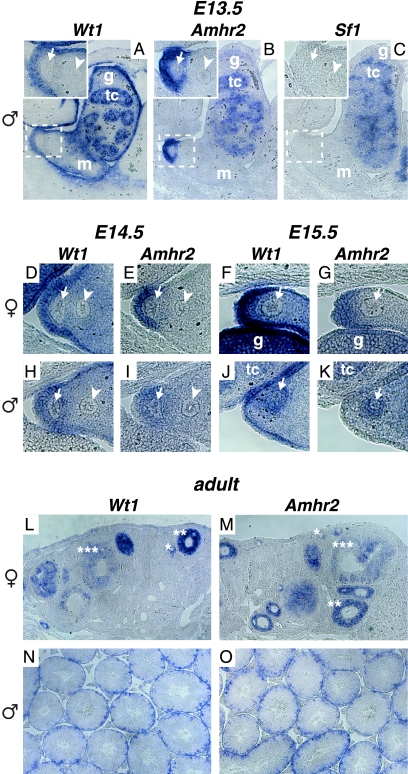
Previous studies have identified the nuclear hormone receptor Sf1 as a potential regulator of Amhr2 expression (6, 53). As Sf1 itself is regulated by Wt1 during early gonad development (58), the loss of Amhr2 expression in Wt1−/− gonads could be indirectly caused by the loss of Sf1. We therefore analyzed the expression of all three genes in the urogenital ridge region from embryonic stage 13.5, a time point when Amhr2 expression can predominantly be observed in the cells of the coelomic epithelium in proximity to the Müllerian duct (59). Our analysis showed that at this time point Wt1 was expressed in testis cords of male embryos, the mesonephros, and throughout the coelomic epithelium (Fig. 2A), conforming to its published expression pattern (2, 44). Amhr2 was expressed in testis cords of male embryos and in the coelomic epithelium next to the Müllerian duct, thus overlapping with the Wt1 expression domain (Fig. 2B and insets in A and B). In contrast to Wt1, however, Sf1 was not expressed in all regions of Amhr2 expression in the developing embryo. We did not detect any expression of Sf1 in cells surrounding the Müllerian duct and only weak expression within testis cords at E13.5 (Fig. 2C). As published by others, we found strong Sf1 expression in interstitial cells of the gonad (27).
FIG. 2.
Wt1 and Amhr2 show overlapping expression patterns in the region of the developing Müllerian duct as well as in embryonic and adult gonads. Expression of Wt1 (A, D, F, H, and J), Amhr2 (B, E, G, I, and K), and Sf1 (C) in the urogenital region of E13.5 (A to C), E14.5 (D, E, H, and I), and E15.5 (F, G, J, and K) wild-type male (A to C and H to K) and female (D to G) embryos was investigated by RNA in situ hybridization on paraffin sections. Insets in A, B, and C show areas marked by dashed boxes in higher magnification. Expression of Wt1 (L and N) and Amhr2 (M and O) was also analyzed in adult ovary (L and M) and testis (N and O). tc, testis cord; g, gonad; m, mesonephros; arrowhead, Wolffian duct; arrow, Müllerian duct; *, primary follicle; **, secondary follicle; ***, tertiary follicle.
When we examined Amhr2 and Wt1 expression in female urogenital ridges of later stages, we found that both genes were expressed in the coelomic epithelium but not around the Müllerian duct (Fig. 2D to G). In contrast, in male urogenital ridges both Amhr2 and Wt1 expression became weaker in the coelomic epithelium at E14.5 than at E13.5, and Amhr2- as well as Wt1-expressing cells became visible between the Müllerian and the Wolffian duct (Fig. 2H and I). One day later both genes were expressed around the degenerating Müllerian duct (Fig. 2J and K). Thus, the expression of Wt1 mirrors the dynamic and sexually dimorphic expression pattern of Amhr2 in the urogenital ridge. Of note, Wt1 and Amhr2 were both expressed in developing female gonads as well as in testis cords at all time points analyzed (Fig. 2F, G, J, and K and data not shown).
As Wt1 and Amhr2 have also been reported to be expressed in adult gonads, we examined the expression patterns of both genes in the gonads of adult mice. In ovaries, both genes showed expression in granulosa cells during follicle maturation, with the highest expression in secondary follicles (Fig. 2L and M). This is in line with previous analyses of rat ovaries (3, 26). In testis, expression of both genes was detected in Sertoli cells (Fig. 2N and O), as described by others (4, 42). However, with the method used here, we were not able to detect the reported weak Amhr2 expression in Leydig cells (43).
These results show a significant coexpression of Wt1 and Amhr2 in developing as well as in adult tissue and are compatible with a direct regulation of Amhr2 by Wt1. The data also rule out regulation of Amhr2 by Sf1 during Müllerian duct regression.
Endogenous Amhr2 expression is altered after Wt1 knockdown or Wt1 induction in mammalian cell lines.
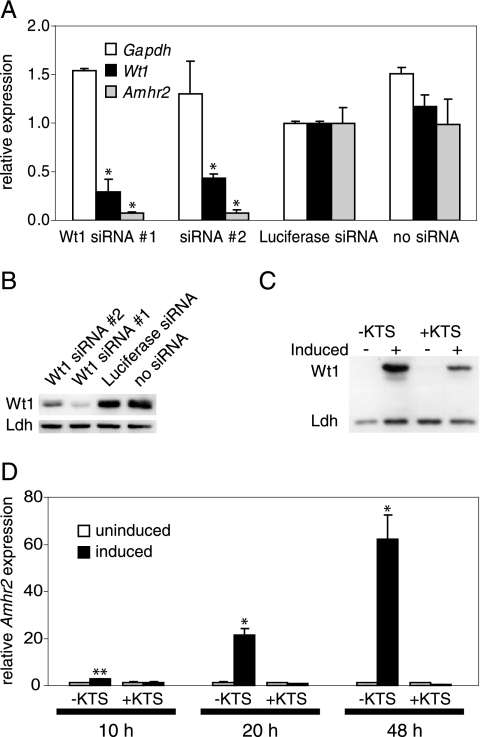
To analyze the short-term effect of Wt1 reduction on Amhr2 expression, we chose RNA interference and established two different Wt1-directed siRNAs. Both siRNAs led to a specific knockdown of Wt1 mRNA and protein with slightly different efficiencies (Fig. 3A and B). M15 mesonephric mouse cells treated with siRNA directed against Wt1 mRNA showed a drastic reduction of Amhr2 expression (Fig. 3A). The fact that Amhr2 expression is more drastically reduced upon Wt1 siRNA treatment than Wt1 itself suggests that a threshold level of Wt1 is necessary to achieve proper Amhr2 activation.
FIG. 3.
Amhr2 expression is altered by Wt1 knockdown and Wt1 induction. (A) M15 cells were treated with two different siRNAs directed against Wt1, an siRNA directed against luciferase, or transfection reagent only. Expression of Amhr2, Wt1, and Gapdh was measured 48 h after transfection by real-time RT-PCR and normalized to Actb expression. Expression levels in luciferase siRNA-treated cells were set as 1. Data represent the means and standard deviations of two independent experiments. Asterisks indicate statistically significant differences (P < 0.05; Student's t test) from expression in both controls (luciferase siRNA and cells not treated with siRNA). (B) Western blot analysis of Wt1 protein levels in siRNA-treated cells 48 h after transfection. Lactate dehydrogenase (Ldh) served as a loading control. (C) Western blot analysis of cells harboring an inducible allele of Wt1(−KTS) or Wt1(+KTS) 24 h after induction. (D) AMHR2 expression in uninduced and induced cells was measured by real-time RT-PCR 10 h, 20 h, and 48 h after induction of Wt1(−KTS) or Wt1(+KTS) in UB27 and UD28 cells. Expression levels were normalized to ACTB, and expression levels in uninduced cells were set as 1. Data represent the means and standard deviations of two independent experiments. Asterisks indicate statistically significant differences (*, P < 0.05; **, P < 0.01; Student's t test) from expression in uninduced cells.
After having shown that Amhr2 expression is decreased by inactivation of Wt1 in gonads and M15 cells, we studied the effect of Wt1 overexpression on Amhr2 expression. We employed the human osteosarcoma cell lines UB27 and UD28, which express Wt1(−KTS) and Wt1(+KTS), respectively, under the control of a tetracycline-regulatable promoter (17) (Fig. 3C). AMHR2 was upregulated in a time-dependent manner after induction of Wt1(−KTS) but not Wt1(+KTS). Ten hours after Wt1 induction, AMHR2 expression was about three times higher than that in uninduced cells. AMHR2 mRNA levels were increased more than 20-fold after 20 h and approximately 60-fold 48 h after Wt1(−KTS) induction (Fig. 3D). Thus, expression of endogenous murine and human Amhr2 showed an immediate response upon changes in Wt1 concentration in the respective cell lines, suggesting a direct regulation of Amhr2 by Wt1(−KTS).
The Amhr2 promoter contains potential Wt1 binding sites.
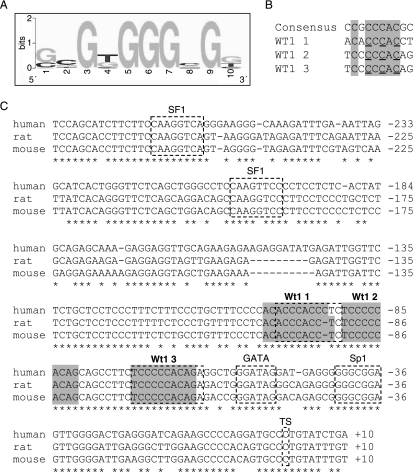
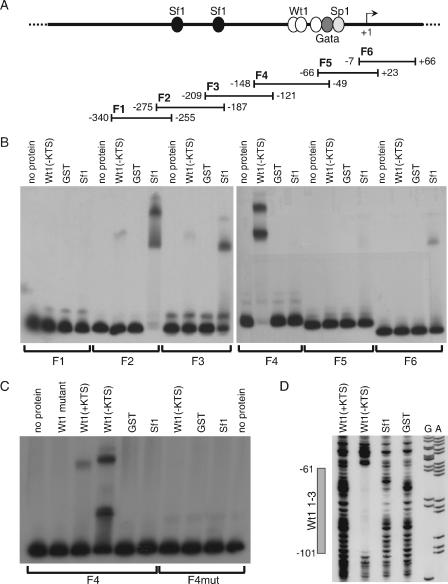
On the basis of an optimized binding site for Wt1(−KTS), called WTE (38), and considering several naturally occurring Wt1 binding sites that have recently been characterized, we have generated a refined Wt1 consensus binding motif (Fig. 4A and B; see also http://www.fli-leibniz.de/www_molgen/supp-mat/MCB2007SupplFig.pdf). Using this motif we have found three potential Wt1 binding sites in the published 1.6-kb murine Amhr2 promoter sequence (53). This promoter fragment had been shown to activate a reporter gene in the rat Leydig cell tumor line R2C but not in other cell lines without endogenous Amhr2 expression, and it harbors Gata, Sp1, and Sf1 consensus binding sites (Fig. 4C). The latter were protected in a DNase footprinting assay using extracts from R2C cells (53). In addition to these sites, two more sites were protected, for which the binding factor was not identified. These two sites overlap with the first and third Wt1 binding motifs (Fig. 4C). The three Wt1 binding motifs are highly conserved between mouse, human, and rat (Fig. 4C). The same is true for the transcriptional start site, the Gata binding site, the Sp1 binding site, and the first of the two Sf1 binding sites.
FIG. 4.
Amhr2 promoter contains three conserved Wt1(−KTS) consensus binding sites. (A) Graphical representation of the more G-rich strand of the Wt1(−KTS) consensus binding site designed with http://weblogo.berkeley.edu. (B) Alignment of the consensus sequence with the three Wt1(−KTS) consensus binding sites found in the murine Amhr2 promoter. In all cases the more C-rich strand is shown in the 5′ to 3′ direction. Conserved positions are boxed in gray. Positions mutated for EMSA and reporter gene assays (C to A) are underlined. (C) The mouse Amhr2 promoter sequence (accession number AF092445) was aligned with homologous human and rat sequences acquired from http://genome.ucsc.edu/index.html (human assembly, May 2004; rat assembly, November 2004) using the T-coffee program (http://igs-server.cnrs-mrs.fr/Tcoffee/tcoffee_cgi/index.cgi). Conserved transcription factor binding sites for Sf1, Gata, Sp1, and two unknown factors as well as the transcriptional start site (TS) (53) are indicated by dashed boxes. All sequences contain three conserved Wt1(−KTS) consensus binding sites (gray shaded boxes) overlapping with the two described binding sites for unknown factors (53).
Wt1(−KTS) binds to the Amhr2 promoter in vitro.
We used electrophoretic mobility shift assays (EMSA) to investigate whether Wt1 actually binds to the identified consensus binding motives in the Amhr2 promoter. For EMSA, the Amhr2 promoter was divided into six overlapping fragments (F1 to F6) (Fig. 5A). As expected, the most efficient binding by Wt1(−KTS) was observed for F4, which contained the three Wt1 consensus binding sequences (Fig. 5B). Sf1 bound most efficiently to F2 and F3, the fragments containing the published Sf1 binding sites. Additionally, much weaker binding of Wt1(−KTS) to F2 and F3 as well as of Sf1 to F5 and F6 was observed. Subsequently, Wt1 binding of F4 was analyzed in more detail. In contrast to wild-type Wt1(−KTS), a mutated form, which contained the most common Denys-Drash syndrome-causing mutation in zinc finger III (R394W), did not bind to this fragment at all, while weak binding of Wt1(+KTS) could be observed (Fig. 5C, left). This is in line with previously published weak binding of Wt1(+KTS) to Wt1(−KTS) binding sites (46). When all potential Wt1 binding sites were mutated (see Fig. 4B), Wt1 binding to F4 was completely lost (Fig. 5C, right).
FIG. 5.
Wt1(−KTS) and Sf1 bind to the murine Amhr2 promoter in vitro. (A) Schematic representation of the Amhr2 promoter, containing potential transcription factor binding sites, and the fragments used for EMSA. (B) For EMSA analysis, labeled fragments F1 to F6 were incubated without protein and with recombinant GST, GST-Wt1(−KTS), and GST-Sf1. (C) Wild-type and mutated F4 were additionally analyzed by adding GST-Wt1(+KTS) and a mutant form of GST-Wt1(−KTS). F4 was mutated by replacing three of the five conserved cytosines with adenines in all three Wt1 binding sites. (D) Binding of Wt1 to the consensus binding sites was also shown by DNase footprinting analysis using the same recombinant proteins and a 5′-labeled PCR fragment. Sequencing reactions for A and G bases of the same fragment are shown. The gray box indicates the region protected by Wt1(−KTS) spanning all three consensus binding sites. Numbers in panels A and D refer to the murine Amhr2 promoter sequence (53).
To confirm the EMSA results and to determine the position of Wt1 binding in more detail, we performed a DNase footprinting assay. This assay corroborated specific binding of Wt1(−KTS) to the identified consensus binding sites (Fig. 5D). A region spanning all three consensus binding sites was protected by Wt1(−KTS) but not by Wt1(+KTS), Sf1, and GST. As shown in Fig. 5B, the bandshift assay showed weak binding of Wt1 to F2 and F3, two fragments without perfect Wt1 consensus binding sites. However, these fragments contain several G- or C-rich sites with some similarity to the Wt1(−KTS) consensus. DNase footprinting assays revealed less pronounced protection of sequences within F2 and F3 by Wt1(−KTS) (data not shown).
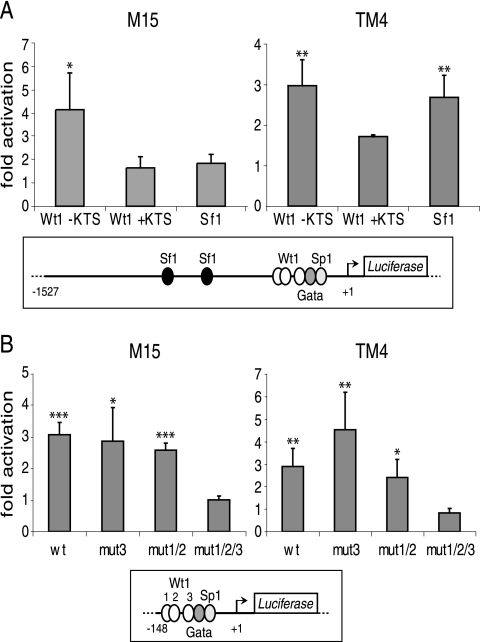
Wt1 transactivates the Amhr2 promoter in a reporter gene assay.
After having shown that Wt1 binds the Amhr2 promoter in vitro, we wanted to examine whether Wt1 was also able to transactivate this promoter. Using the 1.6-kb Amhr2 promoter fragment in the context of a luciferase reporter plasmid, we observed transactivation by Wt1(−KTS) but not Wt1(+KTS) in the mesonephric cell line M15 and the Sertoli-like cell line TM4, both from mouse (Fig. 6A). Transactivation by Sf1 was only observed in TM4 cells. There was no synergism between the effects of Wt1 and Sf1 on the Amhr2 promoter (data not shown). To address the specificity of the interaction, we generated a shorter Amhr2 promoter construct by deleting sequences 5′ of F4 (see Fig. 5A). This construct, which contains no Sf1 binding site, still showed transactivation by Wt1 in both cell lines (Fig. 6B). When all three potential Wt1 binding sites were mutated (see Fig. 4B), however, the resulting promoter fragment could not be activated by cotransfection with a Wt1(−KTS) expression construct (Fig. 6B). Mutation of one or two binding sites was not enough to abolish transactivation by Wt1.
FIG. 6.
Wt1(−KTS) transactivates the Amhr2 promoter. (A) M15 and TM4 cells were transfected with the 1.6-kb Amhr2 promoter construct together with expression constructs for Wt1(−KTS), Wt1(+KTS), and Sf1. Subsequently, luciferase reporter assays were performed. (B) Constructs harboring a shorter fragment and mutated forms of this fragment with mutations in binding site 3 (mut3), 1 and 2 (mut1/2), or all three sites together (mut1/2/3) were cotransfected with Wt1(−KTS). The respective Amhr2 promoter fragments are indicated schematically below the diagrams. Results are given as relative activation of the reporter by the expression constructs compared to the empty vector. Data represent the means and standard deviations of three (A) and four (B) independent experiments. Asterisks indicate statistically significant differences of activation compared to cells transfected with an empty expression construct (*, P < 0.05; **, P < 0.01; ***, P < 0.001; analysis of variance).
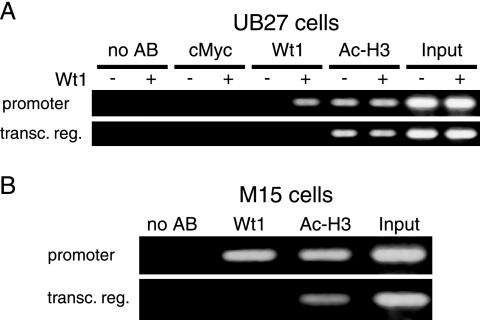
Wt1(−KTS) binds to the Amhr2 promoter in living cells.
To examine whether Wt1(−KTS) binds the Amhr2 promoter also in living cells, ChIP was employed. We used human UB27 cells, harboring an inducible Wt1(−KTS) allele (Fig. 7A), and murine M15 cells, expressing significant levels of endogenous Wt1 (Fig. 7B). After the ChIP procedure the DNA was tested for the presence of an Amhr2 promoter fragment by PCR with specific primer pairs spanning the identified Wt1 consensus binding sites. As controls, primer pairs binding within the transcribed region of the human and murine Amhr2 gene were used. DNA isolated directly from the formaldehyde-treated cells served as positive controls for PCR (Fig. 7, input). As a positive control for ChIP we used an antibody against acetylated histone H3, while an antibody directed against the transcription factor c-Myc and samples with no antibody added served as negative controls. Significant enrichment of Amhr2 promoter fragments could be observed when an anti-Wt1 antibody was combined with material from cells containing Wt1, either expressed from the transgene (Fig. 7A) or expressed endogenously (Fig. 7B). However, when uninduced UB27 cells were used, no PCR product was obtained. No or background signals were observed for genomic fragments located within the transcribed region of Amhr2. Thus, when Wt1 is present in UB27 or M15 cells, it specifically binds to the human and murine Amhr2 promoters.
FIG. 7.
Wt1(−KTS) binds the Amhr2 promoter in living cells. PCR analysis of DNA purified after chromatin immunoprecipitation with (A) uninduced and induced UB27 cells and (B) M15 cells. Two different antibodies against Wt1 were used, Wt1 C-19 (A) and WTc8 (B). Immunoprecipitation using an antibody against c-Myc or without antibody served as negative controls, while antibody against acetylated histone H3 and input DNA served as positive controls. Primer pairs binding to the transcribed region (transc. reg.) of human and murine Amrh2, 2 and 10 kb downstream of the transcriptional start site, respectively, were used as controls for PCR.
DISCUSSION
The different splice forms of the Wilms' tumor protein Wt1 have been implicated in several aspects of mammalian gonad development and sex determination. Wt1, particularly the −KTS splice form, is required for development and survival of the gonad primordium (21). However, Wt1 also has a role in the process of sex determination, a role that is demonstrated by reduction of Sry expression in Wt1(+KTS) knockout mice (21). Recently, a role of Wt1 after the initial phase of sex differentiation, namely, in the maintenance of testis architecture, has been reported (18).
In this report we provide evidence for another late role for Wt1 in the process of sex development, namely, in regression of the Müllerian duct, a process that is essential for normal male development to occur. This process is initiated by binding of the anti-Müllerian hormone Amh to its cognate receptor, Amhr2. Our data suggest that Wt1 is required for cells to gain the competence for Amhr2 expression. As is the case for Amhr2 (59), Wt1 is initially expressed in the coelomic epithelium. Subsequently, in male urogenital ridges the expression domains for both genes migrate towards the Müllerian duct and eventually surround the regressing ductal epithelium. This dynamic expression pattern involves cell migration as well as epithelial-to-mesenchymal transition, a process in which Wt1 has been implicated (23). However, not all Wt1-expressing cells show Amhr2 activity. This becomes obvious especially in the coelomic epithelium and suggests the involvement of additional factors in Amhr2 regulation. Initially, only cells of the coelomic epithelium that directly neighbor the Müllerian duct express Amhr2, while Wt1 is expressed throughout the coelomic epithelium. Thus, a second determinant is necessary to define those cells that express Amhr2. One possible candidate is Wnt7a, a secreted signaling molecule originating from the Müllerian duct (40). It has been shown that Wnt7a knockout mice of both sexes lack Amhr2 expression in the Müllerian duct region, leading to persistent Müllerian ducts in males. In support of these data, Amhr2 has been shown to be a target of β-catenin, a downstream component of the canonical Wnt signaling pathway (25). Sf1, another factor that had been implicated in Amhr2 activation (6, 25, 53), was not expressed in the cells that mediate Müllerian duct regression. This excludes Sf1 as a decisive factor during Müllerian duct regression but is still compatible with Sf1-mediated Amhr2 activation in cells that do express Sf1 and Amhr2 but not Wt1, for example, in Leydig cells, as previously suggested (53).
In addition to the region surrounding the Müllerian duct, we have observed coexpression of Wt1 and Amhr2 in developing gonads of both sexes and in Sertoli cells of adult males as well as throughout development of the ovarian follicle. Although our knowledge about the role of Wt1 in adult tissues is still limited, there is evidence to suggest that Wt1 and Amh signaling affect the same processes in adult gonad biology. Regarding the testis, Amhr2 knockout mice show Leydig cell hyperplasia and vacuolization of Sertoli cells (36). The latter phenotype has also been reported for mice with adult Sertoli cell-specific Wt1 inactivation (45). In ovaries, both Wt1 and Amh signaling have been suggested to function as inhibitors of early follicular growth (12, 15).
One prediction from our finding that Wt1 is an activator of Amhr2 is that males with Wt1 mutations should show signs of persisting derivatives of the Müllerian ducts. Because of the degeneration of urogenital ridges in Wt1 mutant embryos after E11.5, we could not analyze Müllerian duct regression in those mice. There are, however, cases where remnants of the Müllerian ducts have been reported to occur in human Denys-Drash syndrome patients. This syndrome comprises the triad of partial gonadal dysgenesis, congenital or infantile nephropathy, and Wilms' tumor. All components of the syndrome appear to be caused by dominant loss-of-function mutations of the WT1 gene. In most cases, DDS mutations are point mutations that alter DNA contact sites within the Wt1 zinc fingers (41). We have used the most frequent DDS mutant protein (R394W) and shown that it is not able to bind to the Amhr2 promoter. One of the initial publications about Denys-Drash syndrome described a patient who possessed internal genitalia that were very reminiscent of persistent Müllerian duct syndrome (13). In addition to a normally developed vagina and uterus, two pairs of ducts as well as one histologically normal testis and a vas deferens were present. In a similar case, a 4-month-old patient with a 46 XY karyotype was presented who developed a Wilms' tumor and had a vagina, uterus, testes, epididymis, and vas deferens (5). Further cases have been reported since then (35).
The existence of male internal genitalia in these patients demonstrates normal testis development, including the generation of Sertoli and Leydig cells as well as the respective hormones without which structures like the epididymis and vas deferens would not form. Thus, the persistence of Müllerian duct structures cannot be explained by the activation of a female default pathway caused by the failure of male sex determination to occur but rather by missing execution of a genetic program that normally leads to regression of the Müllerian duct. While we provide evidence for Amhr2 as a target of Wt1, the latter has also been implicated in the activation of the Amh gene encoding the Amhr2 ligand (24, 37). In our microarray analysis, which was performed using RNA from mouse embryos of stage E11.0, a time point when Amh starts to be expressed, Amh levels were low but detectable and were not different between wild-type and Wt1 knockout embryos. It is still possible that XY Denys-Drash syndrome patients like the ones indicated above show reduced Amh expression, although testis differentiation seems to proceed normally, and that these reduced Amh levels might also contribute to the Denys-Drash phenotype. The regulation of two components within one pathway, namely, the Amh ligand and its receptor, by Wt1 could explain why Müllerian duct regression seems to be more drastically affected in the mentioned Denys-Drash syndrome patients than other processes depending on Wt1, namely, early gonad development and testis differentiation.
In summary, we have identified the Amhr2 gene as a physiologically relevant target of the Wilms' tumor protein Wt1. This provides new insights into the role of Wt1 in sexual development and indicates a novel function for Wt1 in sex differentiation, in addition to its known function in early gonad development and sex determination.
Acknowledgments
We thank Helen Morrison, Frank Bollig, and Falk Weih for discussions and for critically reading and improving the manuscript. We also thank Susanne Spahr and Debra Weih as well as the students Nicole Borth and Christian Linke for technical support, Dagmar Wilhelm for fruitful discussions and the pGex-Sf1 construct, and Jose Teixeira for providing the Amhr2 promoter constructs and his readiness to reannotate the corresponding sequence. The help from Richard Münch in generating a Wt1 consensus binding sequence is gratefully appreciated.
This work was supported by grants En280/6-1 and En280/6-2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Allard, S., P. Adin, L. Gouedard, N. di Clemente, N. Josso, M. C. Orgebin-Crist, J. Y. Picard, and F. Xavier. 2000. Molecular mechanisms of hormone-mediated Mullerian duct regression: involvement of beta-catenin. Development 127:3349-3360. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. F., K. Pritchard-Jones, W. A. Bickmore, N. D. Hastie, and J. B. Bard. 1993. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 40:85-97. [DOI] [PubMed] [Google Scholar]
- 3.Baarends, W. M., J. T. Uilenbroek, P. Kramer, J. W. Hoogerbrugge, E. C. van Leeuwen, A. P. Themmen, and J. A. Grootegoed. 1995. Anti-Mullerian hormone and anti-Mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136:4951-4962. [DOI] [PubMed] [Google Scholar]
- 4.Baarends, W. M., M. J. van Helmond, M. Post, P. J. van der Schoot, J. W. Hoogerbrugge, J. P. de Winter, J. T. Uilenbroek, B. Karels, L. G. Wilming, J. H. Meijers, et al. 1994. A novel member of the transmembrane serine/threonine kinase receptor family is specifically expressed in the gonads and in mesenchymal cells adjacent to the Mullerian duct. Development 120:189-197. [DOI] [PubMed] [Google Scholar]
- 5.Barakat, A. Y., Z. L. Papadopoulou, R. S. Chandra, C. E. Hollerman, and P. L. Calcagno. 1974. Pseudohermaphroditism, nephron disorder and Wilms' tumor: a unifying concept. Pediatrics 54:366-369. [PubMed] [Google Scholar]
- 6.Barbara, P. S., B. Moniot, F. Poulat, B. Boizet, and P. Berta. 1998. Steroidogenic factor-1 regulates transcription of the human anti-Mullerian hormone receptor. J. Biol. Chem. 273:29654-29660. [DOI] [PubMed] [Google Scholar]
- 7.Barbaux, S., P. Niaudet, M. C. Gubler, J. P. Grunfeld, F. Jaubert, F. Kuttenn, C. N. Fekete, N. Souleyreau-Therville, E. Thibaud, M. Fellous, and K. McElreavey. 1997. Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat. Genet. 17:467-470. [DOI] [PubMed] [Google Scholar]
- 8.Barrionuevo, F., S. Bagheri-Fam, J. Klattig, R. Kist, M. M. Taketo, C. Englert, and G. Scherer. 2006. Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol. Reprod. 74:195-201. [DOI] [PubMed] [Google Scholar]
- 9.Birk, O. S., D. E. Casiano, C. A. Wassif, T. Cogliati, L. Zhao, Y. Zhao, A. Grinberg, S. Huang, J. A. Kreidberg, K. L. Parker, F. D. Porter, and H. Westphal. 2000. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature 403:909-913. [DOI] [PubMed] [Google Scholar]
- 10.Bor, Y., J. Swartz, A. Morrison, D. Rekosh, M. Ladomery, and M. L. Hammarskjold. 2006. The Wilms' tumor 1 (WT1) gene (+KTS isoform) functions with a CTE to enhance translation from an unspliced RNA with a retained intron. Genes Dev. 20:1597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Call, K. M., T. Glaser, C. Y. Ito, A. J. Buckler, J. Pelletier, D. A. Haber, E. A. Rose, A. Kral, H. Yeger, W. H. Lewis, et al. 1990. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 60:509-520. [DOI] [PubMed] [Google Scholar]
- 12.Chun, S. Y., E. A. McGee, S. Y. Hsu, S. Minami, P. S. LaPolt, H. H. Yao, J. M. Bahr, A. Gougeon, D. W. Schomberg, and A. J. Hsueh. 1999. Restricted expression of WT1 messenger ribonucleic acid in immature ovarian follicles: uniformity in mammalian and avian species and maintenance during reproductive senescence. Biol. Reprod. 60:365-373. [DOI] [PubMed] [Google Scholar]
- 13.Denys, P., P. Malvaux, H. Van Den Berghe, W. Tanghe, and W. Proesmans. 1967. Association of an anatomo-pathological syndrome of male pseudohermaphroditism, Wilms' tumor, parenchymatous nephropathy and XX/XY mosaicism. Arch. Fr. Pediatr. 24:729-739. [PubMed] [Google Scholar]
- 14.di Clemente, N., C. Wilson, E. Faure, L. Boussin, P. Carmillo, R. Tizard, J. Y. Picard, B. Vigier, N. Josso, and R. Cate. 1994. Cloning, expression, and alternative splicing of the receptor for anti-Mullerian hormone. Mol. Endocrinol. 8:1006-1020. [DOI] [PubMed] [Google Scholar]
- 15.Durlinger, A. L., P. Kramer, B. Karels, F. H. de Jong, J. T. Uilenbroek, J. A. Grootegoed, and A. P. Themmen. 1999. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789-5796. [DOI] [PubMed] [Google Scholar]
- 16.Englert, C. 1998. WT1—more than a transcription factor. Trends Biochem. Sci. 23:389-393. [DOI] [PubMed] [Google Scholar]
- 17.Englert, C., X. Hou, S. Maheswaran, P. Bennett, C. Ngwu, G. G. Re, A. J. Garvin, M. R. Rosner, and D. A. Haber. 1995. WT1 suppresses synthesis of the epidermal growth factor receptor and induces apoptosis. EMBO J. 14:4662-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao, F., S. Maiti, N. Alam, Z. Zhang, J. M. Deng, R. R. Behringer, C. Lecureuil, F. Guillou, and V. Huff. 2006. The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc. Natl. Acad. Sci. USA 103:11987-11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gessler, M., A. Poustka, W. Cavenee, R. L. Neve, S. H. Orkin, and G. A. Bruns. 1990. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature 343:774-778. [DOI] [PubMed] [Google Scholar]
- 20.Hacker, A., B. Capel, P. Goodfellow, and R. Lovell-Badge. 1995. Expression of Sry, the mouse sex determining gene. Development 121:1603-1614. [DOI] [PubMed] [Google Scholar]
- 21.Hammes, A., J. K. Guo, G. Lutsch, J. R. Leheste, D. Landrock, U. Ziegler, M. C. Gubler, and A. Schedl. 2001. Two splice variants of the Wilms' tumor 1 gene have distinct functions during sex determination and nephron formation. Cell 106:319-329. [DOI] [PubMed] [Google Scholar]
- 22.Herzer, U., A. Crocoll, D. Barton, N. Howells, and C. Englert. 1999. The Wilms tumor suppressor gene wt1 is required for development of the spleen. Curr. Biol. 9:837-840. [DOI] [PubMed] [Google Scholar]
- 23.Hohenstein, P., and N. D. Hastie. 2006. The many facets of the Wilms' tumour gene, WT1. Hum. Mol. Genet. 15(Spec. No. 2):R196-R201. [DOI] [PubMed] [Google Scholar]
- 24.Hossain, A., and G. F. Saunders. 2003. Role of Wilms tumor 1 (WT1) in the transcriptional regulation of the Mullerian-inhibiting substance promoter. Biol. Reprod. 69:1808-1814. [DOI] [PubMed] [Google Scholar]
- 25.Hossain, A., and G. F. Saunders. 2003. Synergistic cooperation between the beta-catenin signaling pathway and steroidogenic factor 1 in the activation of the Mullerian inhibiting substance type II receptor. J. Biol. Chem. 278:26511-26516. [DOI] [PubMed] [Google Scholar]
- 26.Hsu, S. Y., M. Kubo, S. Y. Chun, F. G. Haluska, D. E. Housman, and A. J. Hsueh. 1995. Wilms' tumor protein WT1 as an ovarian transcription factor: decreases in expression during follicle development and repression of inhibin-alpha gene promoter. Mol. Endocrinol. 9:1356-1366. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda, Y., W. H. Shen, H. A. Ingraham, and K. L. Parker. 1994. Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol. Endocrinol. 8:654-662. [DOI] [PubMed] [Google Scholar]
- 28.Imbeaud, S., E. Faure, I. Lamarre, M. G. Mattei, N. di Clemente, R. Tizard, D. Carre-Eusebe, C. Belville, L. Tragethon, C. Tonkin, J. Nelson, M. McAuliffe, J. M. Bidart, A. Lababidi, N. Josso, R. L. Cate, and J. Y. Picard. 1995. Insensitivity to anti-Mullerian hormone due to a mutation in the human anti-Mullerian hormone receptor. Nat. Genet. 11:382-388. [DOI] [PubMed] [Google Scholar]
- 29.Josso, N., N. di Clemente, and L. Gouedard. 2001. Anti-Mullerian hormone and its receptors. Mol. Cell Endocrinol. 179:25-32. [DOI] [PubMed] [Google Scholar]
- 30.Josso, N., J. Y. Picard, S. Imbeaud, N. di Clemente, and R. Rey. 1997. Clinical aspects and molecular genetics of the persistent mullerian duct syndrome. Clin. Endocrinol. (Oxford) 47:137-144. [DOI] [PubMed] [Google Scholar]
- 31.Klamt, B., A. Koziell, F. Poulat, P. Wieacker, P. Scambler, P. Berta, and M. Gessler. 1998. Frasier syndrome is caused by defective alternative splicing of WT1 leading to an altered ratio of WT1 +/−KTS splice isoforms. Hum. Mol. Genet. 7:709-714. [DOI] [PubMed] [Google Scholar]
- 32.Kreidberg, J. A., H. Sariola, J. M. Loring, M. Maeda, J. Pelletier, D. Housman, and R. Jaenisch. 1993. WT-1 is required for early kidney development. Cell 74:679-691. [DOI] [PubMed] [Google Scholar]
- 33.Leimeister, C., A. Bach, and M. Gessler. 1998. Developmental expression patterns of mouse sFRP genes encoding members of the secreted frizzled related protein family. Mech. Dev. 75:29-42. [DOI] [PubMed] [Google Scholar]
- 34.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔC(T)) method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 35.Manivel, J. C., R. K. Sibley, and L. P. Dehner. 1987. Complete and incomplete Drash syndrome: a clinicopathologic study of five cases of a dysontogenetic-neoplastic complex. Hum. Pathol. 18:80-89. [DOI] [PubMed] [Google Scholar]
- 36.Mishina, Y., R. Rey, M. J. Finegold, M. M. Matzuk, N. Josso, R. L. Cate, and R. R. Behringer. 1996. Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 10:2577-2587. [DOI] [PubMed] [Google Scholar]
- 37.Nachtigal, M. W., Y. Hirokawa, D. L. Enyeart-VanHouten, J. N. Flanagan, G. D. Hammer, and H. A. Ingraham. 1998. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93:445-454. [DOI] [PubMed] [Google Scholar]
- 38.Nakagama, H., G. Heinrich, J. Pelletier, and D. E. Housman. 1995. Sequence and structural requirements for high-affinity DNA binding by the WT1 gene product. Mol. Cell. Biol. 15:1489-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niksic, M., J. Slight, J. R. Sanford, J. F. Caceres, and N. D. Hastie. 2004. The Wilms' tumour protein (WT1) shuttles between nucleus and cytoplasm and is present in functional polysomes. Hum. Mol. Genet. 13:463-471. [DOI] [PubMed] [Google Scholar]
- 40.Parr, B. A., and A. P. McMahon. 1998. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707-710. [DOI] [PubMed] [Google Scholar]
- 41.Pelletier, J., W. Bruening, C. E. Kashtan, S. M. Mauer, J. C. Manivel, J. E. Striegel, D. C. Houghton, C. Junien, R. Habib, L. Fouser, et al. 1991. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67:437-447. [DOI] [PubMed] [Google Scholar]
- 42.Pelletier, J., M. Schalling, A. J. Buckler, A. Rogers, D. A. Haber, and D. Housman. 1991. Expression of the Wilms' tumor gene WT1 in the murine urogenital system. Genes Dev. 5:1345-1356. [DOI] [PubMed] [Google Scholar]
- 43.Racine, C., R. Rey, M. G. Forest, F. Louis, A. Ferre, I. Huhtaniemi, N. Josso, and N. di Clemente. 1998. Receptors for anti-Mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl. Acad. Sci. USA 95:594-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rackley, R. R., A. M. Flenniken, N. P. Kuriyan, P. M. Kessler, M. H. Stoler, and B. R. Williams. 1993. Expression of the Wilms' tumor suppressor gene WT1 during mouse embryogenesis. Cell Growth Differ. 4:1023-1031. [PubMed] [Google Scholar]
- 45.Rao, M. K., J. Pham, J. S. Imam, J. A. MacLean, D. Murali, Y. Furuta, A. P. Sinha-Hikim, and M. F. Wilkinson. 2006. Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis. Genes Dev. 20:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rauscher, F. J., III, J. F. Morris, O. E. Tournay, D. M. Cook, and T. Curran. 1990. Binding of the Wilms' tumor locus zinc finger protein to the EGR-1 consensus sequence. Science 250:1259-1262. [DOI] [PubMed] [Google Scholar]
- 47.Riccardi, V. M., E. Sujansky, A. C. Smith, and U. Francke. 1978. Chromosomal imbalance in the Aniridia-Wilms' tumor association: 11p interstitial deletion. Pediatrics 61:604-610. [PubMed] [Google Scholar]
- 48.Riemann, M., R. Endres, S. Liptay, K. Pfeffer, and R. M. Schmid. 2005. The IkappaB protein Bcl-3 negatively regulates transcription of the IL-10 gene in macrophages. J. Immunol. 175:3560-3568. [DOI] [PubMed] [Google Scholar]
- 49.Rivera, M. N., and D. A. Haber. 2005. Wilms' tumour: connecting tumorigenesis and organ development in the kidney. Nat. Rev. Cancer 5:699-712. [DOI] [PubMed] [Google Scholar]
- 50.Roberts, L. M., Y. Hirokawa, M. W. Nachtigal, and H. A. Ingraham. 1999. Paracrine-mediated apoptosis in reproductive tract development. Dev. Biol. 208:110-122. [DOI] [PubMed] [Google Scholar]
- 51.Schepers, G., M. Wilson, D. Wilhelm, and P. Koopman. 2003. SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J. Biol. Chem. 278:28101-28108. [DOI] [PubMed] [Google Scholar]
- 52.Teixeira, J., W. W. He, P. C. Shah, N. Morikawa, M. M. Lee, E. A. Catlin, P. L. Hudson, J. Wing, D. T. Maclaughlin, and P. K. Donahoe. 1996. Developmental expression of a candidate mullerian inhibiting substance type II receptor. Endocrinology 137:160-165. [DOI] [PubMed] [Google Scholar]
- 53.Teixeira, J., D. J. Kehas, R. Antun, and P. K. Donahoe. 1999. Transcriptional regulation of the rat Mullerian inhibiting substance type II receptor in rodent Leydig cells. Proc. Natl. Acad. Sci. USA 96:13831-13838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Veith, A. M., J. Klattig, A. Dettai, C. Schmidt, C. Englert, and J. N. Volff. 2006. Male-biased expression of X-chromosomal DM domain-less Dmrt8 genes in the mouse. Genomics 88:185-195. [DOI] [PubMed] [Google Scholar]
- 55.Wagner, K. D., N. Wagner, V. P. Vidal, G. Schley, D. Wilhelm, A. Schedl, C. Englert, and H. Scholz. 2002. The Wilms' tumor gene Wt1 is required for normal development of the retina. EMBO J. 21:1398-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner, N., K. D. Wagner, A. Hammes, K. M. Kirschner, V. P. Vidal, A. Schedl, and H. Scholz. 2005. A splice variant of the Wilms' tumour suppressor Wt1 is required for normal development of the olfactory system. Development 132:1327-1336. [DOI] [PubMed] [Google Scholar]
- 57.Wagner, N., K. D. Wagner, H. Theres, C. Englert, A. Schedl, and H. Scholz. 2005. Coronary vessel development requires activation of the TrkB neurotrophin receptor by the Wilms' tumor transcription factor Wt1. Genes Dev. 19:2631-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilhelm, D., and C. Englert. 2002. The Wilms tumor suppressor WT1 regulates early gonad development by activation of Sf1. Genes Dev. 16:1839-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhan, Y., A. Fujino, D. T. MacLaughlin, T. F. Manganaro, P. P. Szotek, N. A. Arango, J. Teixeira, and P. K. Donahoe. 2006. Mullerian inhibiting substance regulates its receptor/SMAD signaling and causes mesenchymal transition of the coelomic epithelial cells early in Mullerian duct regression. Development 133:2359-2369. [DOI] [PubMed] [Google Scholar]