Abstract
Herpes simplex virus (HSV) terminase is an essential component of the molecular motor that translocates DNA through the portal vertex in the capsid during DNA packaging. The HSV terminase is believed to consist of the UL15, UL28, and UL33 gene products (pUL15, pUL28, and pUL33, respectively), whereas the HSV type 1 portal vertex is encoded by UL6. Immunoprecipitation reactions revealed that pUL15, pUL28, and pUL33 interact in cytoplasmic and nuclear lysates. Deletion of a canonical nuclear localization signal (NLS) from pUL15 generated a dominant-negative protein that, when expressed in an engineered cell line, decreased the replication of wild-type virus up to 80-fold. When engineered into the genome of recombinant HSV, this mutation did not interfere with the coimmunoprecipitation of pUL15, pUL28, and pUL33 from cytoplasmic lysates of infected cells but prevented viral replication, most nuclear import of both pUL15 and pUL28, and coimmunoprecipitation of pUL15, pUL28, and pUL33 from nuclear lysates. When the pUL15/pUL28 interaction was reduced in infected cells by the truncation of the C terminus of pUL28, pUL28 remained in the cytoplasm. Whether putative terminase components localized in the nucleus or cytoplasm, pUL6 localized in infected cell nuclei, as viewed by indirect immunofluorescence. The finding that the portal and terminase do eventually interact was supported by the observation that pUL6 coimmunoprecipitated strongly with pUL15 and weakly with pUL28 from extracts of infected cells in 1.0 M NaCl. These data are consistent with the hypothesis that the pUL15/pUL28/pUL33 complex forms in the cytoplasm and that an NLS in pUL15 is used to import the complex into the nucleus where at least pUL15 and pUL28 interact with the portal to mediate DNA packaging.
Herpesvirus procapsids and concatameric viral DNA accumulate in the nuclei of infected cells. The procapsids consist of a roughly spherical proteinaceous shell surrounding an inner protein shell or scaffold (16, 24, 36). To initiate DNA packaging, an enzyme called the terminase is believed to scan the viral DNA in search of genomic ends, cleave the concatemer into single genomes, engage the procapsid at a portal vertex designed for the passage of the DNA, and drive the genome into capsids through the hydrolysis of ATP.
Current evidence supports the hypotheses that the herpes simplex virus (HSV) terminase comprises the products of UL15, UL28, and UL33 (pUL15, pUL28, and pUL33, respectively), whereas the portal vertex consists of a dodecamer of the UL6 protein (pUL6). These hypotheses are supported by the observations that (i) pUL6, pUL15, pUL28, and pUL33 are each essential for DNA packaging (2, 5, 25, 26, 34, 44); (ii) epitopes of these proteins are present on the external surface of viral capsids, and at least pUL15 and pUL28 are associated with procapsids (23, 31, 41); (iii) pUL15 interacts with the pUL28 moiety of a pUL28/pUL33 complex in infected cells (9, 18, 19, 43); (iv) pUL15 contains an ATPase-like motif that is essential for viral replication (13, 45); (v) pUL28 binds DNA sequences known to be required for the formation of normal DNA termini (1, 17); and (iv) pUL6 forms a dodecameric ring in vitro with a size and conformation that match the dimensions of capsid vertices and portal vertices of some bacteriophages (23, 37).
The main focus of the current study concerns a key question that distinguishes two models of DNA packaging: specifically, whether the terminase engages the portal vertex in the cytoplasm or in the nucleus. If the terminase were to engage the portal in the cytoplasm, it follows that portal assembly into the procapsid would also incorporate the bound terminase. This would imply that the entire procapsid, with incorporated terminase, would then scan viral DNA in search of genomic ends. On the other hand, if the terminase were imported into the nucleus separately from the portal, it would be free to scan the DNA independently of the procapsid and, once bound to target DNA sequences, could engage the portal vertex for eventual DNA cleavage and translocation into the capsid. The latter mechanism is similar to that used by many bacteriophage terminases (4, 6, 11).
Where the HSV terminase forms in the cell and where the portal and terminase interact have been addressed previously using transient expression assays. For example, transiently expressed pseudorabies virus pUL28 localizes in the cytoplasm unless coexpressed with HSV type 1 (HSV-1) pUL15, suggesting that pUL15 is responsible for the import of the terminase complex (20). On the other hand, pUL6 was also shown to import pUL28 into the nucleus when the proteins were coexpressed, and mutations that precluded the nuclear importation of pUL6 caused coexpressed pUL15 to remain in the cytoplasm, despite the fact that pUL15 localizes in infected cell nuclei by 12 h after infection (5, 40, 45). The transient expression assays argue that a terminase/portal complex forms in the cytoplasm and is then incorporated into the nucleus, where it would then presumably form a nidus for procapsid formation. In studies using infected cells, however, pUL6 was not found to coimmunoprecipitate with pUL28, pUL15, or pUL33 (9, 43). Because the lysis conditions were mild in these studies, it was suggested that the terminase formed in the cytoplasm but that the portal and terminase interacted elsewhere. These interpretations were complicated by (i) the possibility that antibodies can interfere with interactions between proteins and (ii) uncertainty as to whether the lysates in which the interactions occurred were truly derived from the cytoplasm.
The current study clarifies previously described immunoprecipitation results to indicate that the putative terminase complex of pUL15, pUL28, and pUL33 can be efficiently coimmunoprecipitated from lysates derived from either the cytoplasm or the nuclei of infected cells. Although pUL6 could be coimmunoprecipitated with pUL15 and pUL28, these interactions were not detected unless cells were extracted using 1 M NaCl. We also show that pUL15 contains a simian virus 40 T-antigen-like nuclear localization signal (NLS) required for the normal nuclear import of pUL15 and pUL28 in infected cells. This NLS was entirely dispensable for the nuclear import of the UL6-encoded portal protein, further suggesting that the two complexes, the portal and the terminase, are imported into the nucleus independently of one another.
MATERIALS AND METHODS
Cells and virus.
Hep2 cell lines, Vero cell lines, and CV1 cell lines were obtained from the American Type Culture Collection (ATCC) and were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% newborn calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Flp-In-CV1 cell lines were purchased from Invitrogen and maintained in DMEM supplemented with 10% fetal bovine serum, 100 U of penicillin per ml, 100 μg/ml streptomycin, and 100 μg/ml zeocin. CV15 and CV15M cell lines described herein were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 200 μg/ml hygromycin. The UL15 deletion mutant was derived from HSV F strain [HSV-1(F)] (3, 15). The UL6 null mutant was derived from HSV-1 strain 17 (25). The UL28 null mutant, UL28 insertion mutant 74li, and UL28 truncation mutant 741s were derived from strain HSV-1(Kos) (18, 34). Recombinant viruses vJB12, vJB13, vJB15, vJB16, and vJB19 are described below and in Tables 1 and 2.
TABLE 1.
PCR primers used for constructing recombinant viruses
| Primer purpose | Sequencea | Recombinant virus |
|---|---|---|
| To delete NLS of UL15 forward | TCCTTCCGGGCCTCCAGC | vJB12, vJB16 |
| CTCACGGAGACCACGGGC | ||
| GTGGACGTGGCCACCCAC | ||
| TAGGGATAACAGGGTAATCGATTT | ||
| To delete NLS of UL15 reverse | CGTGCCGTACGTCCGGCC | |
| GTGGGTGGCCACGTCCAC | ||
| GCCCGTGGTCTCCGTGAG | ||
| GCCAGTGTTACAACCAATTAACC | ||
| To fuse FLAG tag to UL15 C terminus forward | GGACCTCCGCACACATTCGCTCCT | vJB13 |
| ATCACACGCGTCTCG | ||
| GATTACAAGGATGACGACGATAAG | ||
| TAGGGATAACAGGGTAATCGATTT | ||
| To fuse FLAG tag to UL15 C terminus reverse | GTGCGTAGCATACCTGGGTGTGTTTATTGGGCGCTCA | |
| CTTATCGTCGTCATCCTTGTAATC | ||
| CGAGACGCGTGTGAT | ||
| GCCAGTGTTACAACCAATTAACC | ||
| To repair NLS to vJB12 forward | TCCTTCCGGGCCTCCAGC | vJB15, vJB19 |
| CTCACGGAGACCACGGGC | ||
| CCACCGAAGAAACGGGCCAAG | ||
| TAGGGATAACAGGGTAATCGATTT | ||
| To repair NLS to vJB12 reverse | CGTGCCGTACGTCCGGCCGTGGGTGGCCACGTCCAC | |
| CTTGGCCCGTTTCTTCGGTGG | ||
| GCCCGTGGTCTCCGTGAG | ||
| GCCAGTGTTACTACCAATTAACC |
Underlined sequences are identical to the PCR template pEPkan-S; sequences in normal type, double-underlined type, underlined boldface type, double-underlined boldface type, and italic type are different UL15 sequences. Sequences in boldface type encode either FLAG (construct vJB13) or the UL15 NLS (constructs vJB15 and vJB19). Sequences in the same type are complementary within primer pairs and were designed according to methods described previously by Tischer et al. (35).
TABLE 2.
Virus replication assay
| Virus | Genotype of of UL15 | Cell line infected/cell line used to determine yielda | Titerb |
|---|---|---|---|
| HSV-1(F) | WT | Vero/Vero | (2.0 ± 0.55) × 107 |
| HSV-1(F) | WT | CV15/Vero | (2.0 ± 0.4) × 107 |
| HSV-1(F) | WT | CV15M/Vero | (2.5 ± 0.6) × 105 |
| 15 null | Δ exon IIc | CV15/CV15 | (1.0 ± 0.5) × 107 |
| 15 null | Δ exon II | CV15M/CV15 | <102 |
| vJB12 | ΔNLS | CV15/CV15 | (6.1 ± 1.5) × 105 |
| vJB12 | ΔNLS | Vero/CV15 | <102 |
| vJB13 | FLAG tag at C terminus | Vero/Vero | (2.0 ± 0.2) × 107 |
| vJB15 | NLS repaired (derived from vJB12) | Vero/Vero | (2.0 ± 1.5) × 107 |
| vJB16 | FLAG tag at C terminus, ΔNLS (derived from vJB13) | Vero/CV15 | <102 |
| vJB19 | FLAG tag at C terminus, NLS repaired (derived from vJB16) | Vero/Vero | (2.2 ± 1.5) × 107 |
The first listed cell line was infected at 0.01 PFU/cell, and at 24 hpi, the yield of infectious virus produced from pooled extracellular and intracellular fractions was determined on the second listed cell line. CV15 is an engineered complementing cell line expressing wild-type (WT) pUL15, whereas CV15M was engineered to express pUL15 lacking the NLS by deletion of codons 182 to 189.
Virus titers were determined as described in Materials and Methods. The data represent means ± standard deviations for three independent experiments.
Delta (Δ) indicates a deletion of those sequences.
Antibodies.
Separate polyclonal rabbit antisera recognizing the first 35 amino acids of pUL15 (anti-pUL15N), the C terminus of pUL15 (anti-pUL15C), and the entire pUL28, pUL33, and pUL6 proteins were described previously (5, 9, 28, 29, 33). Anti-FLAG mouse monoclonal antibodies were purchased from Sigma. Anti-lamin A/C mouse monoclonal antibodies were obtained from Santa Cruz Biotechnology. Rabbit polyclonal antibody directed against superoxide dismutase 4 (a cytoplasmic marker protein of 23 kDa) was purchased from Abcam. Anti-ICP35 mouse monoclonal antibody (MCA406) was purchased from Serotec. Goat anti-rabbit immunoglobulin G (IgG) conjugated to Alexa 488 (green) and goat anti-mouse IgG conjugated to Alexa 568 (red) were obtained from Molecular Probes.
Plasmids.
Plasmids pBAD-I-SceI, containing the gene encoding the yeast I-SceI endonuclease, and pEPkan-S, containing aphAI (encoding kanamycin [Kan] resistance) adjacent to an I-SceI restriction site, were gifts from N. Osterrieder, Cornell University. Plasmid pCAGGS-nlsCre, expressing Cre recombinase, was a gift from Michael Kotlikoff, Cornell University. pJB112, containing UL28 downstream of the human cytomegalovirus (CMV) early promoter/enhancer in pCDNA3, was described previously (43).
To construct pJB437, a PCR amplicon from HSV-1(F) DNA containing the entire UL6 coding sequence was cloned into pCDNA3 at the BamHI and EcoRI sites such that the gene was under the transcriptional control of the CMV promoter/enhancer. UL15 genes fused to DNA encoding the FLAG epitope and deletion mutants of the fused gene are indicated in Fig. 2 and were amplified by PCR using pRB4120 (containing a cDNA of the UL15 gene) as a template, followed by cloning into pCDNA3 at the HindIII and EcoRV sites. The deletions were confirmed by DNA sequencing.
FIG. 2.
Schematic colinear diagram of UL15 expression plasmids used in the experiment shown in Fig. 3. (A) DNA encoding the indicated codons of UL15 was fused to a FLAG epitopic tag ( ). A boldface line indicates colinear codons within each expression plasmid, whereas the numbers above each line indicate the first and last codons of each segment. Each construct was cloned into the mammalian expression vector pcDNA3.
). A boldface line indicates colinear codons within each expression plasmid, whereas the numbers above each line indicate the first and last codons of each segment. Each construct was cloned into the mammalian expression vector pcDNA3.
To transfer UL15 cDNA into a defined site within CV1 cells, a shuttle plasmid (designated pJB414) was constructed by producing a PCR amplicon (using pRB4120 as a template) that contained a His6 tag fused to the C terminus of UL15 cDNA. This amplicon was then inserted into the polylinker of the pCDNA5/FRT vector (Invitrogen) at the HindIII and EcoRV sites.
To construct a cell line expressing UL15 lacking an NLS [pUL15-NLS(−)], another shuttle plasmid, pJB509, was constructed. Thus, pJB502 [containing UL15-NLS(−), as indicated in Fig. 2] was digested with HindIII and EcoRV, and the UL15-containing DNA fragment was gel purified and cloned into pCDNA5/FRT at the HindIII and EcoRV sites. In all cases, the constructs were confirmed by DNA sequencing and immunoblotting with appropriate antibodies.
A plasmid encoding the UL15 NLS fused to enhanced green fluorescent protein (EGFP) was constructed by annealing oligonucleotides 5′-AGC TTA TGG GCC CAC CTA AGA AAC GGG CCA AGG TGC GG-3′ and 5′-GAT CCC GCA CCT TGG CCC GTT TCTT AGG TGG GCC CAT A-3′ (encoding an NLS) and cloning them into the HindIII and BamHI sites in the pEGFP-N1 vector (Clontech) such that they were in frame with the N terminus of EGFP. The genotype of the construct was confirmed by DNA sequencing.
Constructions of recombinant viruses.
Recombinant viruses were constructed by en passant mutagenesis, a two-step Red-mediated recombination system described previously by Tischer et al. (35). The Red recombination system was induced by heating log-phase Escherichia coli strain EL250 cells harboring the entire HSV-1(F) genome in a bacterial artificial chromosome (BAC) (32) for 15 min at 42°C, followed by chilling and preparation for electroporation. A Kan resistance gene (aphAI) was amplified by PCR using plasmid pEPkan-S as a template and the primer combinations listed in Table 1. The resulting PCR products were flanked by sequences homologous to the target gene. The PCR products were then treated with DpnI to digest the template DNA and gel purified on agarose gels prior to transformation into electrocompetent EL250 bacteria. After electroporation, the competent cells were resuspended in 1.0 ml of LB broth, incubated at 32°C with shaking for 2 h, and then plated onto LB plates containing 50 μg/ml Kan and 30 μg/ml chloramphenicol (Cmp). After 24 h incubation at 32°C, the Kanr Cmpr colonies were screened by restriction fragment length polymorphism of BAC DNA for insertion of the Kan resistance cassette into the proper genomic locus.
For the second Red recombination event to simultaneously remove the Kanr cassette and insert the desired mutation, pBAD-I-SceI, encoding ampicillin (Amp) resistance and the SceI restriction endonuclease, was electroporated into bacteria bearing the BAC with the proper Kanr insertion. Cmpr Ampr Kanr colonies were selected and grown to early log phase, and 1% arabinose was added to induce the expression of the SceI restriction endonuclease. After incubation for 1 h at 32°C with shaking, the Red recombination system was induced by incubation in a 42°C water bath with shaking for 30 min, followed by a further incubation for 1 h at 32°C. The pBAD-I-SceI-containing bacteria were then plated onto LB plates containing Cmp, Amp, and 1% arabinose and incubated overnight at 32°C. Individual colonies were replica plated to identify Camr/Kans colonies, and the BAC DNA in these colonies was again characterized by restriction fragment length polymorphism, PCR, and DNA sequencing. BAC DNA with the proper mutation was cotransfected with a Cre expression plasmid, pCAGGS-nelsCre, into CV15 cells (a UL15 complementing cell line) or rabbit skin cells (a cell line lacking UL15) using Superfect reagents (QIAGEN) according to the manufacturer's protocol. It was anticipated that the expression of Cre recombinase would cause a deletion of the BAC vector sequences inasmuch as they were flanked by appropriate recombination sites (32). Each recombinant virus was further subjected to two rounds of plaque purification. The genotypes of the recombinant viruses as indicated in Tables 1 and 2 were confirmed by Southern blot and PCR to ensure that the BAC was removed and the target gene was mutated as designed.
Constructions of cell lines.
UL15-expressing cell lines were constructed by using the Flp-In-CV1 system (Invitrogen) according to the manufacturer's protocol and as described previously (21). Briefly, either pJB414 or pJB509 was cotransfected with a plasmid (pOG44) containing Flp recombinase under the control of a constitutive CMV promoter/enhancer into an engineered cell line (Flp-CV1). After recombination, cells resistant to hygromycin were selected by growth in DMEM supplemented with 10% fetal bovine serum and 200 μg/ml hygromycin B.
Transfections and immunofluorescence microscopy.
Glass coverslips in six-well plates were seeded with 2.5 × 10 5 cells/well Hep2 cells and were incubated overnight in growth medium. A transfection mixture containing 4.0 μg of plasmid and 10 μl of Lipofectamine 2000 was prepared according to the manufacturer's instructions. Briefly, transfection reagent was diluted into 250 μl of OptiMEM, incubated for 5 min at room temperature, and mixed gently with plasmids in an additional 250 μl OptiMEM. The transfection mixture was incubated for 20 min at room temperature and added to a 2.0-ml overlay of normal growth medium without antibiotics. Incubation was continued at 37°C in a humidified 5% CO2 incubator for a further 24 h before the cells were fixed and examined by fluorescence or prepared for indirect immunofluorescence.
Preliminary indirect immunofluorescence assays indicated that it was necessary to reduce background staining of the pUL28 antisera described previously (9). To preadosorb the UL28-specific antisera against proteins other than pUL28, one 850-cm2 roller bottle containing approximately 2 × 108 Hep2 cells was infected with a UL28 deletion virus (gCB) at a multiplicity of infection (MOI) of 5.0 PFU/cell. At 18 h postinfection (hpi), cells were harvested and washed extensively with phosphate-buffered saline (PBS). An acetone powder of the infected cells was prepared according to the protocol described previously by Sambrook et al. (30). The powder was added to anti-UL28 serum and PBS, making the final antiserum concentration 1%. The mixture was then mixed on a rotator at 4°C overnight, debris was removed by centrifugation at 10,000 × g for 10 min at 4°C, and the diluted supernatant was used for indirect immunofluorescence as detailed below. The anti-pUL6 rabbit polyclonal antiserum was preadsorbed in a similar fashion except that cells used for the preadsorption were previously infected with a UL6 null mutant (25).
Either 24 h after transfection or 18 h after infection, cells on glass coverslips were washed with PBS, fixed with 3% (wt/vol) paraformaldehyde (in PBS) for 10 min at room temperature, and then permeabilized with 0.2% Triton X-100 in PBS for 5 min. The coverslips were blocked with 10% human serum in PBS for 2 h at room temperature. Cells were reacted with the following primary antibodies diluted in 10% human serum and PBS at room temperature for 60 min: anti-FLAG monoclonal antibody (M2; Sigma) diluted 1:100, preadsorbed anti-pUL28 diluted 1:80 for transiently transfected cells and 1:40 for virus-infected cells, anti-ICP35 (MCA406; Serotec) diluted 1:250, and preadsorbed anti-pUL6 diluted 1:100. After extensive washing in PBS, the cells were incubated for 60 min at room temperature with secondary antibodies including goat anti-mouse IgG conjugated to Alexa Fluor 488 (green), goat anti-rabbit IgG conjugated to Alexa Fluor 488, or goat anti-mouse IgG conjugated to Alexa Fluor 568 (red) diluted 1:200 in 10% human serum and PBS. The cells were counterstained with Hoechst stain to detect nuclei, mounted in Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and visualized using a Nikon Eclipse E600 fluorescence microscope, with a 60× Plan Apo objective. Images were captured with a cooled SensiCam charge-coupled-device camera. Image files were exported in TIF format for processing with Adobe Photoshop 6.0. Each experiment was repeated at least three times.
Cell fractionation.
Approximately 5.0 × 107 CV1 cells were mock infected or infected with the indicated viruses at an MOI of 5 PFU/cell. Eighteen hours after infection, cells were harvested and washed with cold PBS, resuspended in 3.0 ml of buffer A (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 5 mM dithiothreitol, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml), incubated on ice for 1 h, and Dounce homogenized (25 to 30 strokes), and the fractions were observed microscopically to ensure adequate cell lysis. The nuclei were pelleted by centrifugation at 1,000 × g for 5 min, and the supernatant was stored as the cytoplasmic fraction. The pellets were then washed with excess cold buffer A twice, and 3.0 ml ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA) containing a cocktail of protease inhibitors (as described above) was added. The mixture was incubated on ice for 30 min to extract nuclear proteins. The nuclear fraction was clarified by centrifugation at 14,000 rpm for 15 min in a microcentrifuge. Separately, equal amounts of 2× RIPA buffer were added to the cytoplasmic fraction to ensure that immunoprecipitations from nuclear and cytoplasmic fractions were performed under similar conditions. The cytoplasmic and nuclear extracts and nuclear pellets were denatured by boiling in sodium dodecyl sulfate (SDS) and mercaptoethanol and electrophoretically separated on separate lanes of 12% denaturing polyacrylamide gels, or the extracts were divided into three equal aliquots and subjected to immunoprecipitation with either anti-pUL15N, anti-pUL28, or anti-pUL33 antibody.
Immunoprecipitation and immunoblotting.
Immunoprecipitation and immunoblotting were performed as previously described (43). In the case of pUL6 immunoprecipitation and immunoblotting, the virus-infected cells were treated with RIPA buffer that was unsupplemented or supplemented with various concentrations of NaCl as indicated in Results. The antisera used for immunoprecipitation were diluted 1:100. The primary antibodies for immunoblotting were diluted in PBS plus 2% bovine serum albumin as follows: anti-pUL6 was diluted 1:1,000, anti-pUL15C was diluted 1:1,000, anti-pUL28 was diluted 1:1,000, anti-pUL33 was diluted 1:400, anti-FLAG (designated M2) was diluted 1:2,000, anti-lamin A/C mouse monoclonal antibodies were diluted 1:200, and anti-superoxide dismutase 4 rabbit polyclonal antibodies were diluted 1:1,000. Horseradish peroxidase-conjugated anti-mouse IgG or anti-rabbit IgG diluted 1:5,000 in PBS plus 2% bovine serum albumin was reacted with the primary antibodies. Bound immunoglobulins were revealed by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech).
Virus replication assay.
Cells in 25-cm2 flasks were infected with viruses (indicated in Table 2) at an MOI of 0.01 PFU/cell. After adsorption for 2 h at 37°C with shaking, the inoculum was removed, and the cells were washed with CBS buffer (40 mM citric acid, 10 mM KCl, 135 mM NaCl [pH 3.0]) to remove residual infectivity. The cells were washed sequentially with excess PBS and medium 199 supplemented with 1% bovine serum and overlaid with 5 ml DMEM supplemented with 5% newborn calf serum and antibiotics. Twenty-four hours after infection, virus was harvested by three cycles of freezing and thawing. Infectivity was determined by plaque assay on monolayers of cells as indicated in Table 2. Each experiment was repeated three times.
RESULTS
pUL15, pUL28, and pUL33 form a complex in the cytoplasm and nucleus.
Previously, we reported that pUL15, pUL28, and pUL33 of HSV-1(F) form a complex in transiently transfected cells and virus-infected cells (43). To determine whether the complex forms initially in the cytoplasm or nucleus, CV1 cells were mock infected or infected with HSV-1(F), and cytoplasmic and nuclear lysates were prepared at 18 hpi. (Fig. 1). Immunoblots of the lysates were then probed with antibodies directed against superoxide dismutase 4, an exclusively cytoplasmic protein, and anti-lamin A/C, an exclusively nuclear protein.
FIG. 1.
Immunoblots of the pUL15/pUL28/pUL33 complex immunoprecipitated from cytoplasmic or nuclear lysates of virus-infected cells. CV1 cells were mock infected (M) or infected with HSV-1(F) (designated F) at an MOI of 5.0 PFU/cell. Eighteen hours after infection, cells were lysed and separated into cytoplasmic and nuclear fractions. (A and B) Immunoblots of cytoplasmic (designated C above each lane) or nuclear (N) lysates were denatured, electrophoretically separated, transferred onto nitrocellulose, and probed with antibody directed against superoxide dismutase 4 (a cytoplasmic marker) (A) or lamin A/C (a nuclear marker) (B). After extensive washing, bound immunoglobulin was revealed by using appropriately conjugated secondary antibodies, followed by ECL and exposure to X-ray film. (C) Immunoblot to detect pUL15 in immunoprecipitations. Proteins from the cytoplasmic (lanes 1 to 4) or nuclear (lanes 5 to 8) lysates shown in A and B were immunoprecipitated with rabbit antisera directed against the N terminus of pUL15, full-length pUL28, or pUL33. The presence of pUL15 in the immunoprecipitated material was determined by elution, electrophoretic separation, and immunoblotting with antibodies to the C terminus of pUL15. Bound IgG was revealed using horseradish peroxidase-conjugated anti-rabbit antibodies and ECL. The positions of pUL15 and the IgG heavy chain are indicated.
Figure 1A shows that superoxide dismutase 4, with an apparent Mr of 23,000, was readily detected in the cytoplasmic lysate of mock-infected cells and virus-infected cells but was not detectable in nuclear lysates. In contrast, immunoblotting with lamin A/C antibody (Fig. 1B) showed that lamin A/C was present exclusively in the nuclear fractions of mock- and HSV-1(F)-infected cells (Fig. 1, lanes marked M/N and F/N, respectively). Similar results were obtained with lysates prepared at 12 h after infection (data not shown). These data indicate that the cytoplasmic and nuclear fractions were appropriate for subsequent analyses to determine the cytoplasmic or nuclear distribution of the UL15-, UL28-, and UL33-encoded protein complex.
The nuclear and cytoplasmic fractions were subjected to immunoprecipitation with antibodies directed against either pUL15, pUL28, or pUL33, and the presence of pUL15 in the immunoprecipitated material was determined by immunoblotting. Figure 1C shows that pUL15 was immunoprecipitated by antiserum directed against the N-terminal 35 amino acids of pUL15 in roughly equal amounts from both the cytoplasmic and nuclear fractions (lanes 2 and 6). Moreover, pUL15 was coimmunoprecipitated with pUL28 or pUL33 antibody from both the cytoplasmic and nuclear fractions (Fig. 1C, lanes 3 and 4 and lanes 7 and 8, respectively). In contrast, no pUL15 was detected in mock-infected cells (Fig. 1C, lanes 1 and 5). That all three proteins were coimmunoprecipitated was also determined by immunoblotting with all three antibodies (not shown). These results indicate that the complex of pUL15, pUL28, and pUL33 is detectable in the cytoplasm and nuclei of infected cells.
Identification of a functional NLS in pUL15.
The simplest explanation for the results obtained thus far is that the pUL15/pUL28/pUL33 complex forms initially in the cytoplasm and is imported into the nucleus. Because we could not formally exclude the possibility that some nuclear protein leaked into the cytoplasmic extracts during preparation, additional experiments were performed to confirm the conclusion that the putative terminase complex forms in the cytoplasm. We reasoned that if one of the proteins was responsible for nuclear import of the complex and the complex formed in the cytoplasm, then ablation of the NLS in that protein should preclude the nuclear import of the entire complex. Therefore, the amino acid sequences of pUL15, pUL28, and pUL33 were scanned in silico for putative NLSs using PSORT II (http://psort.nibb.ac.jp). With the caveat that many NLSs are not detectable by such programs (12), the results of this analysis indicated that only pUL15 contained a canonical NLS at amino acids 183 to 189 (PKKRAKV [pattern 7]). This region was similar to the NLS of polyomavirus large T antigen and matched the consensus sequence K(K/R)X(K/R).
A series of experiments was then undertaken to determine if this sequence could function as an NLS and, more importantly, if it was necessary for the nuclear import of pUL15 and the putative terminase complex. As a first step in this regard, a series of pUL15 deletion mutants fused to an N- or C-terminal FLAG epitope was constructed (diagrammed in Fig. 2) and verified by DNA sequencing and immunoblotting (data not shown). The various UL15 expression plasmids were then transfected into Hep2 cells, and the intracellular distribution of pUL15 was determined by indirect immunofluorescence microscopy using an anti-FLAG monoclonal antibody.
As shown in Fig. 3, full-length pUL15 localized exclusively in the nuclei of transfected cells, indicating the presence of an NLS somewhere within the protein. Similarly, mutants lacking amino acids 1 to 68 and 233 to 735 localized within the nucleus. In contrast, mutants lacking amino acids 1 to 212, 1 to 343, 1 to 442, and 181 to 735 localized exclusively within the cytoplasm. These data indicated that amino acids between positions 180 and 212 were necessary for the nuclear localization of pUL15, consistent with the potential NLS (identified above) located at amino acids 183 to 189. The finding that this was a likely NLS was confirmed inasmuch as a deletion of only amino acids 182 to 189 caused pUL15 to remain in the cytoplasm (Fig. 3, bottom).
FIG. 3.
Conventional indirect immunofluorescence showing localization of pUL15-FLAG expressed in Hep2 cells. Hep2 cells were transiently transfected with the expression plasmids shown in Fig. 2, encoding FLAG-tagged full-length pUL15 or various deletion mutants thereof. Twenty-four hours posttransfection, the cells were fixed in paraformaldehyde, permeabilized, and reacted with mouse anti-FLAG antibody, followed by reaction with Alexa Fluor 488-labeled goat anti-mouse IgG. Nuclei were identified by Hoechst staining. The missing codons in each construct and summary of the localization of each protein are indicated to the right (C, cytoplasmic; N, nuclear). The images are representative of three independent assays. WT, wild type; aa, amino acids.
To determine whether 183-PKKRAKV-189 was sufficient to serve as an NLS, DNA encoding this motif was fused in frame to the N terminus of the gene encoding EGFP. Plasmids encoding EGFP and the putative pUL15 NLS fused to EGFP were transfected into Hep2 cells, and the intracellular distributions of EGFP and the chimeric protein were determined by fluorescence microscopy. As shown in Fig. 4, in cells expressing EGFP alone, the fluorescence was diffuse and localized within both the cytoplasm and nucleus. In contrast, the NLS-EGFP localized predominantly within the nucleus. These observations indicate that pUL15 contains a functional NLS between amino acids 183 and 189.
FIG. 4.
Nuclear import of EGFP by the UL15 NLS. The sequence encoding the UL15 NLS (amino acids 182 to 189) was cloned upstream of the EGFP gene in vector pEGFP-N1 as shown on the right. Plasmid DNA encoding EGFP or the EGFP fusion protein was transfected into Hep2 cells, and the cellular distribution of NLS-EGFP was analyzed by fluorescence microscopy 24 h posttransfection. Nuclei are identified with Hoechst counterstain. The data are representative of three independent experiments.
pUL28 targeting to the nucleus in infected cells requires the NLS of pUL15.
Thus far, previously reported studies were conducted using transiently expressed proteins in uninfected cells. Because it is possible that in infected cells, other viral proteins might affect the nuclear import of terminase components, we constructed recombinant viruses as detailed in Materials and Methods. Genotypes of viruses used in these studies are listed in Table 2 and are as follows: vJB12 lacks the pUL15 NLS as a consequence of a deletion of UL15 codons 182 to 189, vJB13 contains DNA encoding the FLAG epitopic tag fused to the C terminus of pUL15, and vJB16 was derived from vJB13 and encodes both the UL15-FLAG fusion and a deletion of the putative pUL15 NLS.
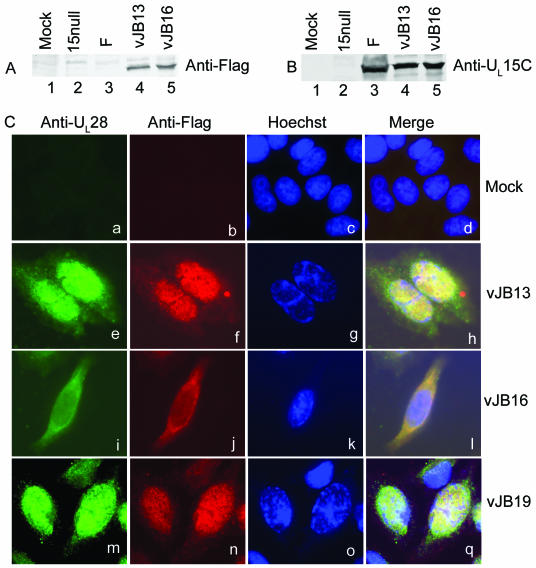
That the recombinant viruses expressed FLAG-tagged pUL15 was determined by infection of cells followed by immunoblotting with anti-FLAG antibody. As shown in Fig. 5, the FLAG-tagged pUL15 was readily detected in lysates of cells infected with either vJB13 or vJB16 (Fig. 5A, lanes 4 and 5). In contrast, no FLAG-specific immunoreactivity was detected in lysates of cells that were mock infected (Fig. 5A, lane 1) or that were infected with wild-type HSV-1(F) (Fig. 5A, lane 3) or the UL15 deletion mutant (Fig. 5A, lane 2). The finding that the FLAG epitope was fused to pUL15 was further confirmed upon probing the same blot with anti-pUL15C antibody. Specifically, the bands containing FLAG-specific immunoreactivity also reacted with the pUL15-specific antiserum. (Fig. 5B).
FIG. 5.
pUL28 and pUL15 localization in virus-infected cells. (A) Immunoblot probed with antibodies directed against the FLAG epitope or the C terminus of pUL15. CV1 cells were mock infected or infected at an MOI of 5 PFU/cell with a UL15 deletion virus (15 null), wild-type HSV-1(F) (designated F), vJB13 encoding a FLAG-tagged pUL15, or vJB16 encoding a FLAG-tagged pUL15 lacking amino acids 182 to 189. Whole-cell lysates were prepared 18 hpi and separated on a denaturing 12% SDS-polyacrylamide gel. After transferring onto a nitrocellulose membrane, immunoblotting was performed using anti-FLAG antibodies. (B) The nitrocellulose sheet was stripped of immunoreactivity and reprobed with antibody directed against the C terminus of pUL15. (C) Indirect immunofluorescence of pUL15 and pUL28 in infected cells. Hep2 cells were mock infected or infected with 0.1 PFU/cell vJB13, vJB16, or vJB19 (derived from vJB16 but containing restored UL15 codons 182 to 189). Infected cells were fixed in 3% paraformaldehyde and permeabilized with 0.2% Triton X-100 18 hpi and then coimmunostained with anti-FLAG monoclonal antibody and preadsorbed anti-pUL28 rabbit polyclonal antibody. Bound primary antibody was visualized by reaction with Alexa Fluor 488-labeled goat anti-rabbit IgG (green) and Alexa Fluor 568-labeled goat anti-mouse IgG (red). Nuclei were identified by Hoechst staining. The images are representative of three independent experiments.
To test the effects of the deletion of the pUL15 NLS and insertion of the FLAG epitope at the pUL15 C terminus on viral replication, Vero cells were infected with 0.01 PFU per cell wild-type HSV-1(F) or the recombinant virus vJB13 or vJB16. At 24 hpi, the yields of infectious virus were determined by plaque assay on Vero cells (in the case of vJB13) or a UL15 complementing cell line (for vJB12 and vJB16). As shown in Table 2, vJB13 replicated in Vero cells to titers that were similar to those of the wild-type virus, indicating that the insertion of the FLAG epitope into the C terminus of pUL15 did not greatly affect virus replication. In contrast, viruses lacking the NLS, whether they encoded a FLAG-tagged or untagged pUL15 (vJB12 and vJB16, respectively) (Table 2), replicated to titers in Vero cells that were reduced more than 100,000-fold relative to those of wild-type HSV-1(F) or vJB13 bearing a FLAG-tagged pUL15 with an intact NLS.
To ensure that the reduction in titer was a consequence of a deletion of the pUL15 NLS, the NLS deletions in the recombinant BACs bJB12 and bJB16 were restored by using en passant mutagenesis, as described in Materials and Methods and as described previously (35), to yield the BACs bJB15 and bJB19, respectively. Viruses derived from the transfection of these BACs into rabbit skin cells were designated vJB15 and vJB19, respectively. Infection of Vero cells with these viruses followed by titration of infectivity by plaque assay 24 h later indicated that they replicated as well as wild-type virus HSV-1(F) (Table 2). Together, these data indicate that while an insertion of the FLAG epitope into pUL15 did not affect virus yields, pUL15 amino acids 182 to 189 were essential for virus replication.
To determine the role of the pUL15 NLS in the nuclear import of pUL28, Hep2 cells were mock infected or infected with vJB13, vJB16, or vJB19 at an MOI of 0.1 PFU/cell. At 18 hpi, cells were fixed and permeabilized and reacted with preadsorbed anti-pUL28 rabbit polyclonal antiserum and anti-FLAG mouse monoclonal antibody, followed by reaction with the appropriate conjugated secondary antibodies and Hoechst stain to identify host chromosomal DNA. As shown in Fig. 5C, little immunofluorescence was detected in mock-infected cells, indicating that the antibodies did not recognize host proteins. In cells infected with vJB13, encoding a FLAG-tagged pUL15, or vJB19, bearing a restored NLS within FLAG-tagged pUL15, pUL28- and pUL15-specific immunofluorescence colocalized predominantly in cell nuclei. In contrast to these results, pUL15-specific and pUL28-specific immunofluorescence localized mostly in the cytoplasm of cells infected with vJB16 lacking the UL15 NLS. This pattern of immunofluorescent staining was present in approximately 80% of over 300 cells examined (data not shown). These results indicate that the NLS of pUL15 is required for the normal intranuclear localization of pUL28.
We next wanted to determine whether the interaction between pUL15 and pUL28 was necessary for the nuclear import of the latter, as might be expected if the proteins interact in the cytoplasm, followed by nuclear import of the complex via recognition of the pUL15 NLS. To test this possibility, we used two recombinant viruses bearing two different mutations in UL28. In cells infected with the 741i recombinant virus, bearing an insertion of the amino acids ARSR at position 741 of UL28, viral replication occurs, and pUL15 and pUL28 coimmunoprecipitate normally (18). In cells infected with the 741s virus, bearing an insertion encoding ARS stop at position 741, viral replication does not occur, and pUL28 coimmunoprecipitates with pUL15 poorly (18). The hypothesis that pUL15 interacts with pUL28 to transport it to the nucleus predicts that pUL28 should be located in the cytoplasm of cells infected with 741S but in the nuclei of cells infected with 741i.
Hep2 cells were infected with gCB (a UL28 null mutant virus), HSV-1(F), 741s, or 741i at an MOI of 0.1 PFU/cell, and the intracellular localization of pUL28 was determined 18 hpi by immunofluorescence using preadsorbed anti-pUL28 antiserum. As a marker of HSV-infected nuclei, cells were also stained with anti-ICP35 monoclonal antibody (MCA406; Serotec). As shown in Fig. 6, no pUL28-specific immunofluorescence was visualized upon immunostaining of gCB-infected cells, despite prominent ICP35-specific immunostaining indicating that the cells were infected (Fig. 6A, panels a through d). This indicated that the preadsorbed anti-pUL28 antiserum did not cross-react with cellular or viral proteins other than pUL28. As expected, pUL28 localized in the nuclei of cells infected with HSV-1(F) (Fig. 6A, panels e through h). In cells infected with UL28 mutant 741s, pUL28 localized primarily in the cytoplasm (Fig. 6A, panels i through l), whereas pUL28 localized in the nuclei of cells infected with 741i (panels m through p). To ensure that pUL15 was expressed in infected nuclei, nuclear pellets of cells infected with HSV-1(F), 741s, and the UL15 and UL28 null mutants were subjected to immunoblotting with the pUL15 antiserum. As shown in Fig. 6B, infected nuclei contained two proteins with apparent molecular weights of approximately 83,000 and 72,000 that reacted with the pUL15 antiserum. That these were products of pUL15 was indicated by their absence from cells infected with the UL15 null mutant. Collectively, these results suggest that (i) the interaction between pUL15 and pUL28 is required for the translocation of pUL28 into the nucleus, (ii) pUL15 can enter the nucleus independently of UL28, and (iii) the pUL28/pUL15 complex forms primarily in the cytoplasm of virus-infected cells.
FIG. 6.
Intracellular localization of pUL28 and pUL15 in virus-infected cells. Hep2 cells were infected with 0.1 PFU/cell gCB (a UL28 null mutant), wild-type HSV-1(F), 741s, or 741i, containing stop or insertion mutations at codon 741 of UL28, respectively. At 18 hpi, cells were fixed in 3% paraformaldehyde, permeabilized with 0.2% Triton X-100, and coimmunostained with preadsorbed anti-pUL28 rabbit polyclonal antibody and a monoclonal antibody to ICP35, the major HSV scaffold protein. The secondary antibodies were Alexa Fluor 488-labeled goat anti-rabbit IgG (green) and Alexa Fluor 568-labeled goat anti-mouse IgG (red). Nuclei were identified by Hoechst staining. The images are representative of three independent assays. (B) Nuclear pellets of cells infected with the indicated viruses were solubilized in SDS and immunoblotted with pUL15-specific antiserum.
The pUL15/pUL28/pUL33 complex forms exclusively in the cytoplasm of cells infected with a mutant virus lacking the pUL15 NLS.
A reasonable objection to experiments using viral mutants bearing deletions in the NLS within pUL15 was the possibility that this deletion might interfere with the formation of the pUL28/pUL15/pUL33 complex. Thus, the lack of nuclear import of pUL28 observed in cells infected with vJB16 might be the consequence of a failure of pUL15 to interact with pUL28 rather than a specific effect on nuclear import. To exclude this possibility, we examined whether the pUL15 lacking an NLS could interact with pUL28 and pUL33 and, if so, whether the complex was located in the cytoplasm or nucleus.
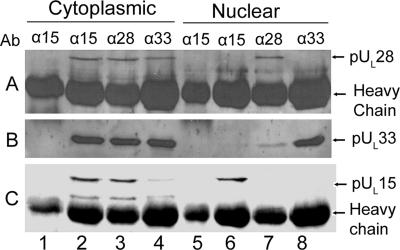
CV1 cells were mock infected or infected with vJB12 at an MOI of 5.0 PFU/cell. Cytoplasmic and nuclear fractions were prepared at 18 hpi and reacted with antiserum against pUL15, pUL28, or pUL33. Whether pUL15, pUL28, or pUL33 was present in the immunoprecipitated material was then determined by immunoblotting. As shown in Fig. 7, pUL15, pUL28, or pUL33 was immunoprecipitated from the cytoplasmic fraction by its cognate antibody (Fig 7C, lane 2, A, lane 3, and B, lane 4, respectively). Importantly, pUL28 was readily coimmunoprecipitated from the cytoplasmic fraction by both anti-pUL15 antibodies (Fig. 7A, lane 2) and anti-pUL33 antibodies (Fig. 7A, lane 4).
FIG. 7.
Immunoprecipitation of the pUL15/pUL28/pUL33 complex from cytoplasmic and nuclear lysates of cells infected with a virus lacking the NLS of pUL15. CV1 cells were mock infected (lanes 1 and 5) or infected with 5.0 PFU/cell vJB12, a virus lacking the NLS of pUL15. Eighteen hours after infection, cells were lysed and separated into cytoplasmic and nuclear fractions. Immunoprecipitation from the respective lysates was performed with antibodies to the N terminus of pUL15 or full-length pUL28 or pUL33. The immunoprecipitates were separated on a 12% SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane, followed by immunoblotting with antibodies against pUL28 (A), pUL33 (B), or the C terminus of pUL15 (C). Ab indicates the row of immunoprecipitating antibodies indicated to the right and above each lane.
Similar to the results with pUL28, pUL33 coimmunoprecipitated from the cytoplasmic fraction reacted with either anti-pUL15 (Fig. 7B, lane 2) or anti-pUL28 antibodies (Fig. 7B, lane 3), and pUL15 was coimmunoprecipitated from the cytoplasmic fraction by anti-pUL28 (Fig. 7C, lane 3) and anti-pUL33 (Fig. 7C, lane 4) antisera.
These observations ruled out the possibility that the deletion of codons 183 to 189 of UL15 precluded an interaction with pUL28 and pUL33 and were consistent with above-described data (Fig. 1, 5, and 6) indicating that the complex of pUL28, pUL15, and pUL33 forms in the cytoplasm of virus-infected cells.
To determine the effects of the pUL15 NLS on nuclear forms of the putative terminase, a similar experiment was performed using nuclear extracts. The results shown in Fig. 7 and repeated in at least three separate experiments indicated that pUL15, pUL28, and pUL33 were immunoprecipitated from the nuclear fractions of cells infected with vJB12 with their cognate antibodies (Fig. 7A, lane 7, C, lane 6, and B, lane 8, respectively). This was not surprising, given the observation that immunofluorescence specific for pUL15 and pUL28 was detected within the nuclei of vJB16-infected cells by indirect immunofluorescence (Fig. 5), and pUL33 is normally present in the cytoplasm and nucleus late in infection (28).
In stark contrast to the results with cytoplasmic extracts of cells infected with vJB16, however, coimmunoprecipitation of pUL15, pUL28, and pUL33 from nuclear extracts was, at best, inefficient. Specifically, pUL15 did not coimmunoprecipitate with detectable pUL28 or pUL33 (Fig. 7, lane 6), and pUL33 did not coimmunoprecipitate with detectable pUL15 or pUL28 (Fig. 7, lane 8). Moreover, the anti-pUL28 antiserum coimmunoprecipitated no detectable pUL15 and only a small amount of pUL33 (Fig. 7, lane 7). These observations were also in contrast to the results obtained upon immunoprecipitation of pUL15, pUL28, and pUL33 from nuclear extracts of cells infected with wild-type HSV-1(F) (Fig. 1). These data suggest that efficient formation of the pUL15/pUL28/pUL33 complex requires that interactions between these proteins occur in the cytoplasm of cells infected with wild-type viruses. We speculate that in the absence of a cytoplasmic interaction, interactions in the nucleus either cannot occur or occur only very inefficiently. Alternatively, the data do not exclude the possibility that the interactions between pUL15, pUL33, and pUL28 within the nucleus require amino acids 182 to 189, whereas interactions in the cytoplasm do not.
The portal encoded by pUL6 is translocated into the nucleus independently of pUL15.
We reasoned that if pUL6 and pUL15 were to interact in the cytoplasm, retention of some pUL15 in the cytoplasm by the deletion of its NLS should also retain pUL6 in the cytoplasm in both infected and uninfected cells.
To test these possibilities, pUL15-FLAG with or without its NLS was transiently coexpressed with pUL6 in Hep2 cells, and the subcellular localizations of pUL6 and pUL15 were determined by immunofluorescence using an anti-pUL6 polyclonal antiserum and anti-FLAG monoclonal antibody to detect pUL15. Nuclei were identified with Hoechst stain. As expected, when full-length pUL15-FLAG and pUL6 were coexpressed, both localized exclusively in cell nuclei (Fig. 8a to d). In contrast, when pUL15-FLAG-NLS(−) was coexpressed with pUL6, the pUL15-specific signal localized predominantly in the cytoplasm, whereas pUL6-specific immunoreactivity localized in the nucleus in a smooth, even pattern (Fig. 8e to h). This observation agrees with previous results indicating that both pUL6 and pUL15 can translocate to nuclei in the absence of other viral proteins but also indicates that these proteins can localize into nuclei independently of one another.
FIG. 8.
Indirect immunofluorescence of pUL6 and pUL15. Hep2 cells were either transiently transfected with expression plasmids (a through h) or infected with recombinant viruses (i through u) as indicated. Cells were fixed at 24 h posttransfection or 18 h after infection, and the intracellular localizations of pUL6 and pUL15 were determined by indirect immunofluorescence microscopy using mouse anti-FLAG antibody (to detect pUL15) or rabbit antiserum directed against pUL6. The cells were reacted with the secondary antibodies Alexa Fluor 488-labeled goat anti-rabbit IgG (green) and Alexa Fluor 568-labeled goat anti-mouse IgG (red). Nuclei were identified by Hoechst staining. The results are representative of three independent assays.
To investigate the effects of the pUL15 NLS on the localization of pUL6 in infected cells, Hep2 cells were infected with vJB13, vJB16, or vJB19 at an MOI of 0.1 PFU/cell, and the intracellular distributions of pUL6 and pUL15 were examined 18 hpi by indirect immunofluorescence as described above. As shown in Fig. 8m to p, pUL15 localized predominantly in the cytoplasm of cells infected with vJB16 lacking the UL15 NLS, whereas pUL6 localized in small foci or larger globular regions within the nucleus. Parenthetically, this pattern of immunostaining was different from the smooth pattern obtained upon the transient expression of pUL6 in uninfected cells (Fig. 8, compare b and f with j, n, and s). In contrast, FLAG-tagged pUL15 containing an NLS as in vJB13-infected cells or a restored NLS as in vJB19-infected cells localized in a diffuse pattern throughout the DNA replication compartment (27), whereas pUL6 localized in small foci or larger globular regions within or adjacent to the DNA replication compartment (Fig. 8i to l and r to u, respectively). These data suggest that pUL15 and pUL6 are imported separately into the nucleus of the infected cell.
pUL15 lacking the NLS is a dominant-negative inhibitor of HSV-1 replication.
As shown in Table 2 and as discussed above, deletion of the NLS of pUL15 as in recombinant viruses vJB12 and vJB16 precluded viral replication in Vero cells. Of additional interest, however, was the observation that the pUL15 NLS deletion mutant vJB12 also replicated to titers about 17-fold lower than that of the UL15 null mutant in the UL15 complementing cell line clone 17 (data not shown) and CV15 (Table 2). Moreover, in UL15 complementing cells, vJB12 replicated to titers about 33-fold lower than those of the corresponding genetically repaired virus vJB15 or wild-type HSV-1(F) (Table 2). Inasmuch as the NLS-repaired virus vJB15 derived from vJB12 replicated in Vero cells normally, the limited replication of vJB12 in complementing cells was not attributable to a mutation other than a deletion of the NLS in UL15. These data suggest that pUL15 lacking an NLS interferes with the function of wild-type pUL15 in the complementing cell lines.
To clarify whether the deletion of the NLS in pUL15 created a dominant-negative protein, we constructed a new cell line, CV15M, containing pUL15-NLS(−) as detailed in Materials and Methods. CV15M cells were infected with HSV-1(F), and the amount of infectious virus was determined by plaque assay. As shown in Table 2, HSV-1(F) titers in CV15M cell lines were reduced about 80-fold compared to those obtained from Vero cells or CV15 cells. As expected, a UL15 null virus lacking most of the second exon of UL15 did not replicate to an appreciable extent in CV15M cells, indicating that the NLS was necessary for complementing the replication of viral mutants bearing lethal mutations in UL15. These results also indicate that mutant pUL15 lacking an NLS acts in a dominant-negative fashion to inhibit the replication of wild-type HSV.
Interaction between terminase components and the portal protein pUL6.
Previous attempts to coimmunoprecipitate putative terminase components and the portal protein were unsuccessful (9, 43). Those results might be explained by the insolubility of the interacting proteins, the interference of pUL6 interactions by antibody binding, or the possibility that the proteins do not interact in infected cells. A systematic study was therefore undertaken to determine conditions under which the pUL6 protein could be solubilized and, if it could be solubilized, whether pUL6 could be coimmunoprecipitated with terminase components.
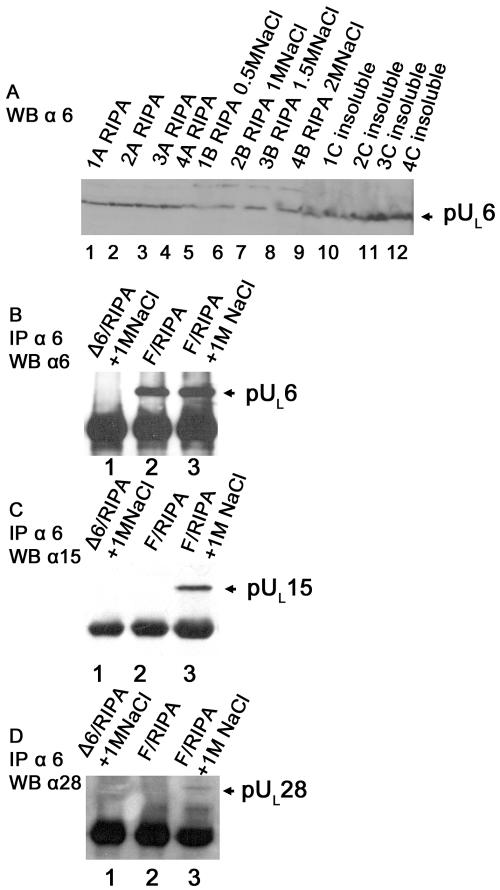
Cells were infected with 5 PFU per cell of wild-type virus HSV-1(F) and were extracted in RIPA buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, and 1 mM EDTA. Alternatively, the cells were extracted in RIPA buffer supplemented with 500 mM NaCl, 1.0 M NaCl, 1.5 M NaCl, or 2 M NaCl (Fig. 9A). In all cases, insoluble material was separated from soluble material by centrifugation, and proteins in the pellets and supernatants were treated with SDS sample buffer, followed by denaturing gel electrophoresis and immunoblotting with a pUL6-specific antibody. As shown in Fig. 9A, some pUL6 was soluble in the presence of RIPA buffer alone, although a substantial amount of the protein was not solubilized in RIPA buffer. Increasing the salt concentration did not greatly increase the amount of pUL6 that was solubilized, at least as assessed by immunoblotting. Even infected cells extracted in 2 M NaCl retained some pUL6 in the insoluble fraction (Fig. 9A, compare lanes 4, 8, and 12).
FIG. 9.
Interaction between pUL6 and putative component of terminase in virus-infected cells detected by coimmunoprecipitation. CV1 cells were infected with 5 PFU per cell of HSV-1(F) or the UL6 null mutant. (A) At 18 hpi, cells were extracted with RIPA buffer (containing detergent and 150 mM NaCl) (lanes designated A) or RIPA buffer supplemented with NaCl at various concentrations for 30 min at room temperature. Each number above the lanes corresponds to the same sample or partitions thereof. Insoluble material was pelleted by centrifugation, and the materials in the supernatant (soluble) (B's) and pellet (insoluble) (C's) were denatured, electrophoretically separated, transferred onto nitrocellulose, and probed with antibody against pUL6. (B, C, and D) Infected cell lysates harvested at the same time point as those in A were subjected to immunoprecipitation (IP) with the pUL6-specific antibody. Immunoprecipitated proteins were separated in an SDS-polyacrylamide gel, transferred onto nitrocellulose, and identified by immunoblotting with antibodies to pUL6 (B), pUL15 (C), or pUL28 (D). WB, Western blot.
pUL6 solubilized in RIPA buffer and at various concentrations of NaCl was then subjected to immunoprecipitation with a pUL6-specific polyclonal antibody, and the presence of pUL6, pUL15, pUL28, and pUL33 in the immunoprecipitates was assessed by immunoblotting. The results using RIPA buffer alone or containing 1 M NaCl are shown in Fig. 9B to D. As a control, cells infected with 5 PFU per cell of a UL6 null virus were treated similarly.
pUL6 was readily immunoprecipitated from cells infected with HSV-1(F) that were lysed in the presence of unsupplemented RIPA buffer (Fig. 9B, lane 2), but the immunoprecipitated material did not contain pUL15, pUL28 (Fig. 9C and D, lanes 2), or pUL33 (data not shown). The finding that the immunoprecipitated protein was the product of UL6 was confirmed by its absence from immunoprecipitation reactions in which lysates of cells infected with the UL6 null virus were reacted with the pUL6-specific antibody (Fig. 9B, lane 1). The lack of coimmunoprecipitation of pUL15, pUL28, and pUL33 with pUL6 in RIPA lysates of HSV-1(F)-infected cells is consistent with previous observations suggesting that highly soluble pUL6 does not interact with the terminase components pUL15, pUL28, and pUL33. This result was also consistent with conclusions presented above indicating that pUL6 does not interact with pUL15 in the cytoplasm.
Like pUL6 solubilized in RIPA buffer, pUL6 extracted from infected cells in RIPA buffer containing 1.0 M NaCl was readily immunoprecipitated with the pUL6-specific antibody (Fig. 9B, lane 3), indicating that the high salt concentration did not affect the immunoreactivity of pUL6 with its cognate antibody. In the negative control reaction, neither pUL15 nor pUL28 was coimmunoprecipitated with pUL6 in 1 M NaCl extracts of cells infected with the pUL6 deletion mutant (Fig. 9C and D, lanes 1). Importantly, pUL15 was readily coimmunoprecipitated with pUL6-specific antibody in extracts produced in the presence of 1.0 M NaCl (Fig. 9C, lane 3). Whereas pUL28 immunoreactivity was also detectable in the pUL6 immunoprecipitates under these conditions, the pUL28-specific signal was considerably weaker than that of pUL15 (Fig. 9D, lane 3). pUL33 did not coimmunoprecipitate with pUL6 in 1.0 M NaCl above background levels (data not shown). These results suggest that at least the pUL15 and pUL28 components interact with the portal encoded by UL6 in infected cells, thus supporting previous results using heterologously expressed proteins (40). The data also suggest that the pUL6/pUL15/pUL28 complex is considerably less soluble than the pUL15/pUL28/pUL33 complex in infected cells. Less solubility might be expected of proteins associated with the nucleus, cytoskeletal components, or large complexes (e.g., procapsids or portal rings) that must be disrupted.
DISCUSSION
Nuclear localization of the putative terminase.
This study identifies a functional NLS within pUL15 and shows that this is necessary to import normal levels of the complex of pUL15 and pUL28 into the nucleus. In addition, when the interaction of pUL28 and pUL15 is reduced by the truncation of UL28 at codon 741 (18), pUL28 remains in the cytoplasm. Thus, both the interaction between pUL28 and pUL15 and the NLS of pUL15 are required for the nuclear localization of pUL28 in infected cells. The disruption of the NLS in pUL15 did not preclude an interaction between pUL28 and pUL33 inasmuch as all three proteins were successfully coimmunoprecipitated from the cytoplasm of the UL15 NLS(−) mutant. Together, these data suggest that the terminase forms in the cytoplasm and is incorporated into the nucleus as a complex of all three proteins by virtue of the NLS in pUL15.
We did not perform indirect immunofluorescence to determine the localization of pUL33 in these studies for the following reasons. First, pUL33 is arguably smaller than the diffusion limit of the nuclear pore, and a portion would be expected to be imported into the nucleus independently of the pUL15/pUL28/pUL33 complex. Thus, any effects of the UL15 NLS mutation on pUL33 localization would be difficult to discern. This expectation is consistent with the observation that pUL33 is present in both the cytoplasm and nucleus by immunofluorescence assay, even late in infection (28). Second, pUL33 interacts directly with pUL28 but only indirectly with pUL15 (43). Thus, any influence of pUL15 mutations on pUL33 localization would likely be mediated through effects on pUL28. These studies therefore focused primarily on the effects of UL15 mutations on the localization of pUL28.
Although the nuclear import of pUL15 and pUL28 is greatly affected by the ablation of the pUL15 NLS, some pUL15 and pUL28 can be imported into the nucleus in the absence of the pUL15 NLS. This is supported by both indirect immunofluorescence and immunoblots of nuclear lysates probed with pUL15- and pUL28-specific antibodies (Fig. 5 and 7). (As a caveat, it should be noted that the signals on the immunoblots may not reflect the relative quantities of the probed proteins inasmuch as such quantification requires that the proteins be in the linear range of detection.) It is currently unclear how some pUL15 lacking an NLS and pUL28 are imported into the nuclei of cells infected with the mutant virus, although it seems likely that NLSs other than amino acids 183 to 189 of pUL15 are used. In any event, the data indicate that the nuclear import of the pUL15/pUL28 complex is negatively affected in the absence of the classical NLS within pUL15.
In nuclear lysates of cells infected with wild-type virus, pUL15, pUL28, and pUL33 could be coimmunoprecipitated, suggesting that the interactions between these proteins are maintained in the nucleus after nuclear import. In contrast, these proteins either did not coimmunoprecipitate (pUL15 and pUL28) or coimmunoprecipitated poorly (pUL28 and pUL33) from nuclear lysates of cells infected with the viral mutant lacking the NLS within pUL15. These data argue that in the event that the putative terminase does not form in the cytoplasm, components that become incorporated into the nucleoplasm through independent means cannot interact efficiently. We cannot exclude the possibilities that (i) the NLS of UL15 is necessary for the interaction of these proteins in the nucleus but dispensable for interactions in the cytoplasm or (ii) the smaller amounts of proteins imported into nuclei in the absence of the pUL15 NLS preclude the detection of coimmunoprecipitated proteins.
Interaction between the portal protein and terminase components.
The current data suggest that pUL6 is imported into the nucleus independently of the putative terminase components pUL15, pUL33, and pUL28. This hypothesis is supported by the observations that (i) UL6 is incorporated into the nucleus whether or not the NLS within pUL15 is ablated and (ii) the pUL6 purified from lysates produced under mild conditions did not contain either pUL15 (Fig. 9C, lane 2), pUL28 (Fig. 9D, lane 2), or pUL33 (data not shown). These conclusions are in contrast to those from previous studies in which pUL6 was mutated such that it remained cytoplasmic and, when transiently expressed, precluded coexpressed pUL15 from translocation into the nucleus (40). These disparate results may be a consequence of transient expression in the previous study versus the use of infected cells in the current study. Given that in infected cells, the proteins of interest are expressed at physiological levels and in the presence of other viral proteins that likely influence the nuclear import of portal, capsid, and terminase components, we give more credence to the current results.
Although they use different routes to the intranuclear DNA packaging reaction, the current data also suggest that pUL6 and the putative terminase interact (Fig. 9). The possibility that such interactions can occur is also supported by (i) the coimmunoprecipitation of pUL6 with pUL28 and pUL15 with pUL6 when overexpressed in a recombinant baculovirus system (40), (ii) the observation that treatment with an antiviral drug directed against UL6 decreases the incorporation of both pUL6 and pUL15 into capsids (22, 38), and (iii) the finding that decreased amounts of pUL15 associate with UL6(−) capsids (46). On the other hand, one to two copies of pUL15 and pUL28 and an unknown number of copies of pUL33 can associate with capsids isolated from cells infected with a UL6 deletion mutant, suggesting that the interactions of terminase proteins and the capsid also involve capsid components other than pUL6 (7, 8). It is also worth noting that like pUL6, the UL15, UL28, and UL33 proteins have been shown to associate with the external surface of the capsid, as might be expected when the terminase docks with the capsid (23, 41).
It is of interest that pUL15 coimmunoprecipitates with pUL6 more readily than does pUL28 or pUL33. UL15 and UL28 coimmunoprecipitate with one another in 1 M NaCl (not shown); therefore, the relatively high amount of salt in the extraction buffer cannot fully explain why less pUL28 than pUL15 coimmunoprecipitates with pUL6. Although this observation might simply reflect pUL6-specific antibody interference with the pUL28 interaction, it is also consistent with an emerging model in which pUL15, as the ATPase subunit, is more intimately associated with the portal than its interaction partners encoded by pUL28 and pUL33. The latter two proteins interact directly with one another and might represent the DNA binding portion of the terminase (1, 43). Supporting this model are the observations that pUL15 is highly enriched in capsids believed to result from aborted DNA packaging events (type A capsids), whereas pUL28 was not detected in these capsids (8). In that work, it was speculated that in binding to the DNA, pUL28 was lost from the capsid along with the DNA.
The relative insolubility of the pUL6/pUL15 complex suggests localization in the nucleus (14), association with cytoskeletal elements, or interaction with larger complexes that must be disrupted by extraction in high salt. Such larger complexes might include the procapsid/terminase complex, which may or may not be associated with nuclear matrix loci that augment procapsid formation or function (10, 39, 42). The resistance of the pUL15/pUL6 complex to extraction under mild conditions helps to explain previous and current results that show interactions between putative terminase components but not between the terminase and the portal protein (7, 43). pUL33 interacts directly with pUL28 but only indirectly with pUL15 (43). We speculate that the inability to coimmunoprecipitate pUL33 with pUL6 reflects antibody interference or the rather stringent extraction conditions required to solubilize the portal/terminase complex. It seems unlikely that the result can be explained by the absence of pUL33 from capsids inasmuch as a relatively stable association of pUL33 with the capsid surface has been documented (7).
Assuming that a single terminase complex mediates all terminase functions, the simplest model to explain the current results would propose that pUL15, pUL28, and pUL33 form a complex in the cytoplasm. The complex is then imported into the nucleus via an NLS in pUL15. The terminase then presumably identifies terminal ends of concatameric genomes. The terminase/DNA complex then interacts with the UL6-encoded portal vertex primarily through the pUL15 moiety, followed by the cleavage/packaging of the DNA. Further experimentation is clearly warranted to test aspects and implications of this model and to elucidate the reactions in more molecular detail.
Acknowledgments
We thank David Binder for his help in the construction of recombinant viruses; Klaus Osterrieder, Karsten Tischer, Jens von Einem, and Sascha Trapp for their helpful advice; Arvind Patel for the UL6 deletion virus; and Gary Whittaker for use of the Nikon fluorescence microscope.
These studies were supported by R01 grants GM50740 (J.D.B.) and AI060836 (F.H.) from the National Institutes of Health.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Adelman, K., B. Salmon, and J. D. Baines. 2001. Herpes simplex DNA packaging sequences adopt novel structures that are specifically recognized by a component of the cleavage and packaging machinery. Proc. Natl. Acad. Sci. USA 98:3086-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kobaisi, M. F., F. J. Rixon, I. McDougall, and V. G. Preston. 1991. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology 180:380-388. [DOI] [PubMed] [Google Scholar]
- 3.Baines, J. D., C. Cunningham, D. Nalwanga, and A. J. Davison. 1997. The UL15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the UL15 gene product. J. Virol. 71:2666-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines, J. D., and C. Duffy. 2006. Nucleocapsid assembly and envelopment of herpes simplex virus, p. 175-204. In R. M. Sandri-Goldin (ed.), Alpha herpesviruses: pathogenesis, molecular biology and infection control. Caister Scientific Press, Norfolk, United Kingdom.
- 5.Baines, J. D., A. P. W. Poon, J. Rovnak, and B. Roizman. 1994. The UL15 gene of herpes simplex virus encodes two proteins and is required for cleavage of viral DNA. J. Virol. 68:8118-8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines, J. D., and S. K. Weller. 2004. Cleavage and packaging of herpes simplex virus 1 DNA, p. 1-16. In C. Catalano (ed.), Viral genome packaging. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 7.Beard, P. M., and J. D. Baines. 2004. The DNA cleavage and packaging protein encoded by the UL33 gene of herpes simplex virus 1 associates with capsids. Virology 324:475-482. [DOI] [PubMed] [Google Scholar]
- 8.Beard, P. M., C. Duffy, and J. D. Baines. 2004. Quantification of the DNA cleavage and packaging proteins UL15 and UL28 in A and B capsids of herpes simplex virus type 1. J. Virol. 78:1367-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beard, P. M., N. S. Taus, and J. D. Baines. 2002. The DNA cleavage and packaging proteins encoded by genes UL28, UL15, and UL33 of herpes simplex virus 1 form a complex in infected cells. J. Virol. 76:4785-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bibor-Hardy, V., M. Pouchelet, E. St.-Pierre, M. Herzberg, and R. Simard. 1982. The nuclear matrix is involved in herpes simplex virogenesis. Virology 121:296-306. [DOI] [PubMed] [Google Scholar]
- 11.Black, L. W. 1989. DNA packaging in dsDNA bacteriophages. Annu. Rev. Microbiol. 43:267-292. [DOI] [PubMed] [Google Scholar]
- 12.Cokol, M., R. Nair, and B. Rost. 2000. Finding nuclear localization signals. EMBO Rep. 1:411-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davison, A. J. 1992. Channel catfish virus: a new type of herpesvirus. Virology 186:9-14. [DOI] [PubMed] [Google Scholar]
- 14.Dignam, J. D., R. M. Lebovitz, and R. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejercito, P. M., E. D. Kieff, and B. Roizman. 1968. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J. Gen. Virol. 2:357-364. [DOI] [PubMed] [Google Scholar]
- 16.Heymann, J. B., N. Cheng, W. W. Newcomb, B. L. Trus, J. C. Brown, and A. C. Steven. 2003. Dynamics of herpes simplex virus capsid maturation visualized by time-lapse cryo-electron microscopy. Nat. Struct. Biol. 10:334-341. [DOI] [PubMed] [Google Scholar]
- 17.Hodge, P. D., and N. D. Stow. 2001. Effects of mutations within the herpes simplex virus type 1 DNA encapsidation signal on packaging efficiency. J. Virol. 75:8977-8986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobson, J. G., K. Yang, J. D. Baines, and F. L. Homa. 2006. Linker insertion mutations in the herpes simplex virus type 1 UL28 gene: effects on UL28 interaction with UL15 and UL33 and identification of a second-site mutation in the UL15 gene that suppresses a lethal UL28 mutation. J. Virol. 80:12312-12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koslowski, K. M., P. R. Shaver, J. T. I. Casey, T. Wilson, G. Yamanaka, A. K. Sheaffer, D. J. Tenny, and N. E. Pedersen. 1999. Physical and functional interactions between the herpes simplex virus UL15 and UL28 DNA cleavage and packaging proteins. J. Virol. 73:1704-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koslowski, K. M., P. R. Shaver, X.-Y. Wang, D. J. Tenny, and N. Pedersen. 1997. The pseudorabies virus UL28 protein enters the nucleus after coexpression with the herpes simplex virus UL15 protein. J. Virol. 71:9118-9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang, L., M. Tanaka, Y. Kawaguchi, and J. D. Baines. 2004. Cell lines that support replication of a novel herpes simplex 1 UL31 deletion mutant can properly target UL34 protein to the nuclear rim in the absence of UL31. Virology 329:68-76. [DOI] [PubMed] [Google Scholar]
- 22.Newcomb, W. W., and J. C. Brown. 2002. Inhibition of herpes simplex virus replication by WAY-150138: assembly of capsids depleted of the portal and terminase proteins involved in DNA encapsidation. J. Virol. 76:10084-10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcomb, W. W., R. M. Juhas, D. R. Thomsen, F. L. Homa, A. D. Burch, S. K. Weller, and J. C. Brown. 2001. The UL6 gene product forms the portal for entry of DNA into the herpes simplex virus capsid. J. Virol. 75:10923-10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcomb, W. W., B. L. Trus, N. Cheng, A. C. Steven, A. K. Sheaffer, D. J. Tenney, S. K. Weller, and J. C. Brown. 2000. Isolation of herpes simplex virus procapsids from cells infected with a protease-deficient mutant virus. J. Virol. 74:1663-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel, A. H., F. J. Rixon, C. Cunningham, and A. J. Davison. 1996. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology 217:111-123. [DOI] [PubMed] [Google Scholar]
- 26.Poon, A. P. W., and B. Roizman. 1993. Characterization of a temperature-sensitive mutant of the UL15 open reading frame of herpes simplex virus 1. J. Virol. 67:4497-4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds, A. E., Y. Fan, and J. D. Baines. 2000. Characterization of the UL33 gene product of herpes simplex virus 1. Virology 266:310-318. [DOI] [PubMed] [Google Scholar]
- 29.Salmon, B., D. Nalwanga, Y. Fan, and J. D. Baines. 1999. Proteolytic cleavage of the amino terminus of the UL15 gene product of herpes simplex virus 1 UL15 protein is coupled with maturation of viral DNA into unit length genomes. J. Virol. 73:8338-8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed., p. 15. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 31.Sheaffer, A. K., W. W. Newcomb, M. Gao, D. Yu, S. K. Weller, J. C. Brown, and D. J. Tenney. 2001. Herpes simplex virus DNA cleavage and packaging proteins associate with the procapsid prior to its maturation. J. Virol. 75:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanaka, M., H. Kagawa, Y. Yamanashi, T. Sata, and Y. Kawaguchi. 2003. Construction of an excisable bacterial artificial chromosome containing a full-length infectious clone of herpes simplex virus type 1: viruses reconstituted from the clone exhibit wild-type properties in vitro and in vivo. J. Virol. 77:1382-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taus, N. S., B. Salmon, and J. D. Baines. 1998. The herpes simplex virus 1 UL17 gene is required for localization of capsids and major and minor capsid proteins to intranuclear sites where viral DNA is cleaved and packaged. Virology 252:115-125. [DOI] [PubMed] [Google Scholar]
- 34.Tengelsen, L. A., N. E. Pedersen, P. R. Shaver, M. W. Wathen, and F. L. Homa. 1993. Herpes simplex virus type 1 DNA cleavage and capsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J. Virol. 67:3470-3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tischer, B. K., J. von Einem, B. Kaufer, and N. Osterrieder. 2006. Two-step RED-mediated recombination for versatile high-efficiency markerless DNA manipulation in Escherichia coli. BioTechniques 40:191-197. [DOI] [PubMed] [Google Scholar]
- 36.Trus, B. L., F. P. Booy, W. W. Newcomb, J. C. Brown, F. L. Homa, D. R. Thomsen, and A. C. Steven. 1996. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19C and VP23 in assembly. J. Mol. Biol. 263:447-462. [DOI] [PubMed] [Google Scholar]
- 37.Trus, B. L., N. Cheng, W. W. Newcomb, F. L. Homa, J. C. Brown, and A. C. Steven. 2004. Structure and polymorphism of the UL6 portal protein of herpes simplex virus type 1. J. Virol. 78:12668-12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Zijl, M., J. Fairhurst, T. R. Jones, S. K. Vernon, J. Morin, J. LaRocque, B. Feld, B. O'Hara, J. D. Bloom, and S. V. Johann. 2000. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene J. Virol. 74:9054-9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, P. L., W. O. Ogle, and B. Roizman. 1996. Assemblons: nuclear structures defined by aggregation of immature capsids and some tegument proteins of herpes simplex virus 1. J. Virol. 70:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White, C. A., N. D. Stow, A. H. Patel, M. Hughes, and V. G. Preston. 2003. Herpes simplex virus type 1 portal protein UL6 interacts with the putative terminase subunits UL15 and UL28. J. Virol. 77:6351-6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wills, E., L. Scholtes, and J. D. Baines. 2006. Herpes simplex virus 1 DNA packaging proteins encoded by UL6, UL15, UL17, UL28, and UL33 are located on the external surface of the viral capsid. J. Virol. 80:10894-10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilson, D. W., and G. A. Church. 1997. Study of herpes simplex virus maturation during a synchronous wave of assembly. J. Virol. 71:3603-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, K., and J. D. Baines. 2006. The putative terminase subunit of herpes simplex virus 1 encoded by UL28 is necessary and sufficient to mediate interaction between pUL15 and pUL33. J. Virol. 80:5733-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu, D., A. K. Shaeffer, D. J. Tenny, and S. K. Weller. 1997. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J. Virol. 71:2656-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu, D., and S. K. Weller. 1998. Genetic analysis of the UL15 gene locus for the putative terminase of herpes simplex virus type 1. Virology 243:32-44. [DOI] [PubMed] [Google Scholar]
- 46.Yu, D., and S. K. Weller. 1998. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J. Virol. 72:7428-7439. [DOI] [PMC free article] [PubMed] [Google Scholar]