Abstract
Two critical interactions within the poliovirus RNA replication complex are those of the RNA-dependent RNA polymerase 3D with the viral proteins 3AB and VPg. 3AB is a membrane-binding protein responsible for the localization of the polymerase to the membranous vesicles at which replication occurs. VPg (a peptide comprising the 3B region of 3AB) is the 22-residue soluble product of 3AB cleavage and serves as the protein primer for RNA replication. The detailed interactions of these proteins with the RNA-dependent RNA polymerase 3D were analyzed to elucidate the precise roles of 3AB and VPg in the viral RNA replication complex. Using a membrane-based pull-down assay, we have identified a binding “hot-spot” spanning residues 100 to 104 in the 3B (VPg) region of 3AB which plays a critical role in mediating the interaction of 3AB with the polymerase. Isothermal titration calorimetry shows that the interaction of VPg with 3D is enthalpically driven, with a dissociation constant of 11 μM. Mutational analyses of VPg indicate that a subset of the residues important for 3AB-3D binding are also important for VPg-3D binding. Two residues in particular, P14 and R17, were shown to be absolutely critical for the binding interaction. This work provides the direct characterization of two binding interactions critical for the replication of this important class of viruses and identifies a conserved polymerase binding sequence responsible for targeting the polymerase.
Since the major polio outbreaks of the early 1900s, poliovirus has been the focus of extensive genetic, molecular, and biochemical investigation. The robust growth of the virus in cultured cells and the ability to produce wild-type infection from RNA alone have made poliovirus one of the best-characterized viruses and the prototypical member of the Picornaviridae family. As a model for other viruses and as a significant pathogen in its own right, a detailed understanding of the mechanisms of action of this important virus may yet provide therapies for a number of human and livestock diseases.
Like other members of the Picornaviridae family, the poliovirus virion consists of a small, icosahedral capsid containing a single positive strand of RNA with a small covalently linked peptide (reviewed in references 30 and 35). Upon insertion of the RNA genome into a host cell, a single open reading frame is translated. The resulting polypeptide is then proteolytically processed to ultimately form four structural and seven nonstructural proteins, as well as at least four functionally significant precursor proteins. These viral proteins cause disruption of a number of cellular functions, including inhibition of host-cell transcription and translation and the rearrangement of the host-cell endoplasmic reticulum to form membranous vesicles throughout the cytoplasm (4, 47). The viral proteins, along with the viral RNA, then assemble into a replication complex at the surface of the membranous vesicles (2). This replication machinery is critical for the progression of infection as it is responsible for transcribing the positive, viral RNA genome to form negative RNA strands and then transcribing these to form the positive RNA strands used for further translation, replication, and packaging into progeny virions.
A number of critically important components of the replication complex are encoded in the C-terminal 752 residues of the poliovirus polyprotein, also known as the P3 region. The functional precursor protein 3CD is a required component of the viral replication complex and also contains a protease activity required for proper processing of the viral polypeptide. The cleavage of 3CD leads to the formation of 3C, a functionally distinct protease, and 3D, the viral RNA-dependent RNA polymerase. The other precursor encoded by the P3 region is 3AB. This well-conserved 12-kDa protein binds to the membranous vesicles in infected cells through a 22-residue hydrophobic region which inserts into the membrane, leaving both the N and C termini of the protein on the cytoplasmic side of the vesicle (Fig. 1) (6, 38, 41). 3AB also binds to the RNA polymerase 3D (17, 24, 29, 45), localizing the replication complex to the membranous vesicles and enhancing initiation (31, 32).
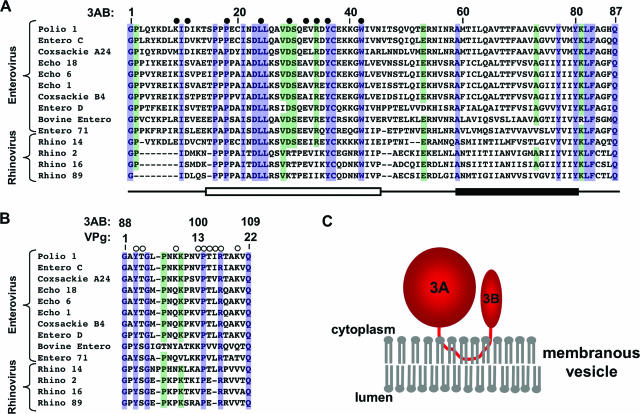
FIG. 1.
Sequence alignment of 3A (A) and VPg (B) from members of the enterovirus and rhinovirus genera of the Picornaviridae family. Completely conserved (100% identity) residues are highlighted in blue, and highly conserved (>75% identity) residues are highlighted in green. Filled circles indicate residues of the 3A region which were mutated and tested by the polymerase recruitment assay. Open circles indicate residues of VPg which were mutated and tested by ITC analysis. Below, an open box indicates the folded region of 3A, and the closed box indicates the 22-residue hydrophobic region. (C) Cartoon illustrating the proposed topology model for 3AB binding to membranous vesicles in infected cells.
Further proteolytic processing of 3AB yields 3A and the VPg peptide (associated with the 3B region of 3AB). 3A consists of the first 87 residues of 3AB, and since it contains the hydrophobic region, it also binds to membranes. The solution structure of the N-terminal soluble domain of 3A indicates that the protein forms a dimer, with each monomer adopting a helical hairpin structure with significant unstructured regions at its N terminus and preceding the hydrophobic region (36). The functional role of 3A in RNA replication is not clear. The amino acid sequence of the 3A coding region is well-conserved (Fig. 1), and genetic studies have linked the 3A coding region with viral RNA replication (12, 14, 15, 44, 46); however, it remains unclear whether the 3A protein alone plays a role in RNA replication after cleavage from 3AB. Two functions which have been characterized for the 3A protein include the ability to alter the structures of host-cell membranes (8, 37) and the ability to disrupt cellular endoplasmic reticulum-to-Golgi complex trafficking, an activity potentially associated with viral evasion of the host's immune response (5, 8, 9, 43). The other product of 3AB cleavage is VPg, a 22-residue peptide which acts as the protein primer for RNA replication (28) and is therefore required for initiation of replication by the viral polymerase 3D. Initiation of both negative- and positive-strand syntheses requires that uridylate residues first be added to the side chain hydroxyl group of the tyrosine at position 3 (13, 25, 26, 42). This uridylylation reaction is catalyzed by the RNA-dependent RNA polymerase 3D and can be mimicked in vitro using the enzyme 3D, the substrates VPg and UTP, and a poly(A) RNA template strand or the cis-acting replication enhancing (2CCRE) RNA hairpin (28).
Investigations into the detailed mechanisms of the P3 proteins have been complicated by a variety of factors. In particular, genetic studies of poliovirus can be difficult to interpret since the polio proteins display multiple activities which often differ from those of their proteolytic precursors and products. For example, a 3AB K96A K97A mutation yields a nonviable virus (46). However, these same residues have been implicated in 3AB-3D binding (45), 3AB-RNA binding (46), VPg-3D binding (27), and VPg-uridylylation (27), and it is difficult to determine which of these functions is responsible for the observed phenotype. Another significant obstacle in investigations of these proteins is the lack of structural information. Although structural studies of the viral polymerase have yielded important insights into its functions, no empirical structural information has been reported for the critical interactions of the polymerase with either 3AB or VPg, despite a number of efforts. In an attempt to overcome this obstacle, two recent studies have used computational methods to dock VPg to the 3D polymerase structure (34, 39). In each case, a structural model of the VPg peptide was determined either using nuclear magnetic resonance data or by sequence homology, and the corresponding peptide was docked to a previously proposed binding site on the polymerase surface. Although each method provides a plausible structural model, additional biochemical data are essential to validate these proposed models.
In vitro studies of 3AB and VPg have given some insights into the functions of these proteins in replication, yet these have also presented challenges. For instance, since 3AB is a membrane protein and is not normally soluble in aqueous solution, many in vitro studies have utilized a detergent-solubilized from of 3AB. However, detergent-solubilized 3AB fails to act as a substrate for 3CD, the viral protease responsible for cleaving 3AB in vivo, suggesting that it may not retain its native structure in that environment (20). In work where the protein is in an environment closer to the native one, 3AB bound to Escherichia coli membranes has been used to identify residues present in 3D that mediate the 3AB-3D interaction, and a binding site has been proposed based on 3D mutations which abolish binding (17, 22). To date, the only study to investigate the contributions of 3AB residues to 3AB-3D binding used a yeast two-hybrid assay to analyze clusters of mutations spanning the 3AB sequence (45). These researchers observed a strong effect on binding from two mutations in the 3B region of 3AB. However, significant effects were also seen from mutations in the 3A region, making it difficult to pinpoint the exact binding site. Additionally, since the structure of 3AB may be affected by solubilization, it is difficult to predict whether 3AB in the yeast two-hybrid assay is indicative of native 3AB bound to membranes. Fortunately, analysis of VPg is more straightforward since this peptide is readily soluble, and two studies have investigated binding of this peptide to the polymerase 3D. One of these utilized a yeast two-hybrid analysis, and although this analysis provides only qualitative results, it did identify three alleles that had significant effects on VPg-3D binding, Y3F, the double mutant K9A K10A, and R17E, suggesting that these residues may play roles at the binding interface (27). However, less than half (only 9 of the 22) of VPg residues have been analyzed for their contributions to this critical interface, allowing for the significant possibility that other important residues are present.
In this study, we use direct binding assays to determine the key residues involved in the interactions of 3AB and VPg with the viral polymerase 3D. We utilize membrane-bound 3AB to show that the 3A region of 3AB plays no part in the interaction with 3D, while a “hot spot” of binding spanning five residues within the 3B region of 3AB is critical for this interaction. We also use isothermal titration calorimetry (ITC) to directly and quantitatively analyze the binding reaction of VPg with 3D. Thermodynamic characterization of this interaction shows that it is enthalpically driven with a dissociation constant of 11 μM. Analysis of VPg mutants identifies a similar pattern of sequence requirements to that of 3AB; notably, two residues, P14 and R17, play crucial roles at both the VPg-3D and 3AB-3D interfaces. This straightforward in vitro binding analysis allows for the comparison of the interactions of 3AB and VPg with the polymerase and the critical evaluation of previous in vitro and modeling studies.
MATERIALS AND METHODS
Expression and purification of poliovirus 3D and membrane-bound 3AB.
Full-length poliovirus 3D and the L446D R455D 3D mutant (3D-DD) were expressed from pKKT7E-3D and pKKT7E-DD plasmids, respectively (generous gifts from Olve Peersen, Colorado State University). The proteins were expressed and purified as described previously (16). For ITC experiments, 3D-DD was also recycled from previous ITC reactions by performing the final Q-Sepharose chromatography step of the purification with extensive washing to remove bound VPg before elution. 3D-DD recovered in this manner was indistinguishable from newly expressed 3D-DD by ITC analysis.
Membrane-bound poliovirus 3AB was expressed and purified based on a previously described method (22). Briefly, E. coli BL21(DE3) cells (Novagen) transformed with the pT7lac3AB plasmid (a generous gift from Aniko Paul and Eckard Wimmer, State University of New York at Stony Brook) were grown at 37°C to an optical density at 600 nm of 0.8 in minimal medium [25 mM Na2HPO4, 22 mM KH2PO4, 25 mM NaCl, 11.4 mM (NH4)2SO4, and 2 g/liter glucose] enriched with vitamins (1% Gibco Eagle Basal Medium Vitamin Solution) and trace metals (46 μM H3BO4, 102 μM CaCl2, 0.19 μM CoCl2, 0.8 μM CuSO4, 1 μM FeSO4, 1 mM MgCl2, 1 μM MnCl2, 3 nM MoO3, 1.7 μM ZnCl2) and containing 50 mg/liter ampicillin. The cultures were then cooled on ice, protein expression was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and the cells were shaken at 22°C overnight. The cells were harvested by centrifugation, washed once in 50 mM Tris (pH 7.5)-100 mM NaCl, pelleted again, and stored at −20°C. Frozen pellets were thawed and resuspended in 3AB lysis buffer (5% glycerol, 50 mM Tris, pH 7.5, 100 mM NaCl, 1 mM Na2-EDTA, 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml pepstatin A). Cells were lysed in a French pressure cell and centrifuged at 12,000 × g at 4°C for 30 min to remove cellular debris. The supernatant was then saved and centrifuged at 100,000 × g at 4°C for 60 min to collect cellular membranes. This pellet was washed once by resuspension in 3AB lysis buffer, collected again by centrifugation at 60,000 × g for 30 min, and resuspended in 3AB lysis buffer by nutation at 4°C overnight. The total protein concentration was adjusted to approximately 1.5 mg/ml, and samples were stored at −80°C. Control membranes were purified in the same fashion from E. coli containing no plasmid and were grown without ampicillin.
Mutations were introduced into the 3AB sequence by site-directed mutagenesis using the QuikChange mutagenesis protocol (Stratagene). Full-length 3A protein was created by mutagenesis of the codons for the first two residues of 3B to stop codons (GGAGCA to TGATAA). All mutations were confirmed by DNA sequencing, and mutant proteins were purified using exactly the same protocol as used for wild-type 3AB.
Polymerase recruitment assay.
The polymerase recruitment assay was performed based on a method described previously (22). Briefly, reaction mixtures consisting of 10 μl of 3AB-containing or control membrane (in 3AB lysis buffer), 20 μl of 5 μM 3D, 8 μl of glycerol, and 4 μl of 5 M NaCl were placed on ice for 60 min, incubated at 30°C for 20 min, and then spun at 14,000 rpm for 10 min in a microcentrifuge at 4°C. The pellets were then resuspended in wash buffer (25 mM Tris, pH 7.5, 500 mM NaCl, 10 mM dithiothreitol) and centrifuged again as before. The pellets were finally resuspended in sodium dodecyl sulfate protein gel buffer and separated by electrophoresis on a 4 to 20% gradient gel (Bio-Rad). Gels were stained with Sypro-red dye (Molecular Probes), visualized on a Typhoon scanner (GE Healthcare), and quantified using ImageQuant software (Molecular Dynamics). To calculate the fraction of 3AB binding, the volume of the 3D band from a control membrane was subtracted from each of the other 3D bands, and these were then normalized to the 3D band pulled down by wild-type 3AB. Assays were performed in triplicate, and the mean and the standard error of the mean are reported.
VPg expression and purification.
Wild-type VPg and VPg mutants were expressed in E. coli as fusion proteins with the B1 immunoglobulin binding domain of streptococcal protein G (GB1) (18, 21). The GB1-VPg fusion construct was created using the following oligonucleotides: oligo 1, 5′-CTAGCATGGGAGCATACACTGGTTTACCAAACAAAAAACCCAACGTG CCCACCATTCGGACAGCAAAGGTACAAATGCCCCGCGGGCCCGGGC-3′; oligo 2, 5′-TCGAGCCCGGGCCCGCGGGGCATTTGTACCTTTGCTGTCCGAATGGT GGGCACGTTGGGTTTTTTGTTTGGTAAACCAGTGTATGCTCCCATG-3′.
Upon annealing, these oligonucleotides form a double-stranded insert containing the coding sequence for VPg flanked by methionine residues. The insert also contains 5′ and 3′ overhangs for ligation into an XhoI- and NheI-cleaved plasmid and an SmaI site for identification of positive ligation clones. The oligonucleotides were dissolved to 50 nM each in 100 mM NaCl, annealed by incubation at 95°C for 1 h and slow cooling to 25°C, and ethanol precipitated. A T7 expression plasmid containing the GB1 domain and a six-His tag (GEV-1 vector, a generous gift from Marius Clore, National Institutes of Health) was simultaneously digested with XhoI and NheI endonucleases and agarose gel purified. The annealed insert was then ligated into the vector overnight at 14°C; the resulting plasmid (GB1-VPg) was transformed into XL-1 Blue E. coli competent cells (Novagen), and positive colonies were identified by SmaI digestion. Mutations were introduced into the VPg sequence by site-directed mutagenesis using the QuikChange mutagenesis protocol (Stratagene) and verified by DNA sequencing.
The GB1-VPg plasmid was transformed into E. coli BL21(DE3)pLysS cells (Novagen), which were then grown at 37°C to an optical density at 600 nm of 0.8 in 2× yeast extract-tryptone broth (16 g tryptone, 10 g yeast extract, 5 g NaCl in 1 l) containing 50 μg/μl ampicillin and 34 μg/μl chloramphenicol. Protein expression was induced with 1 mM IPTG, and the cultures were shaken for 4 h at 37°C. The cells were collected by centrifugation and stored at −20°C. Frozen cell pellets were thawed, resuspended in VPg lysis buffer (50 mM Tris, pH 8.0, 0.01% NaN3), lysed by sonication, and centrifuged to remove cellular debris. The fusion protein was loaded onto a 5-ml HiTrap chelating column (GE Healthcare) preloaded with 5 ml of 0.1 NiCl2, washed extensively with double-distilled H2O and His buffer A (20 mM Tris, pH 8.0, 500 mM NaCl, 5 mM imidazole), and eluted with a linear gradient of imidazole. Eluted protein was concentrated using YM-3 Centriprep filters (Amicon), desalted into 10 mM NH4HCO3 solution using PD-10 columns (GE Healthcare) and lyophilized to dryness. Dried samples were dissolved in 0.1N HCl to 5 mg/ml in a sealed pear-shaped flask in a fume hood, and 3 M cyanogen bromide (CNBr) in acetonitrile was added to a final molar ratio of 300:1 for CNBr:protein. This reaction chemically cleaves at methionine residues, which removes the GB1 domain and the six-His tag, leaving the peptide with a native N terminus and a homoserine lactone at the C terminus. The cleavage reaction was allowed to proceed with stirring overnight at ambient temperature in the fume hood and was quenched by the addition of 5 to 10 volumes of cold 10 mM NH4HCO3. The solution was stirred uncovered in the fume hood for 1 h and then lyophilized to dryness. The VPg peptide was purified by reversed-phase high-pressure liquid chromatography, followed by two rounds of lyophilization to remove residual organic molecules.
All mutations were introduced into the VPg sequence by site-directed mutagenesis using the QuikChange mutagenesis protocol (Stratagene) and confirmed by DNA sequencing. The composition of the wild-type construct was verified by amino acid analysis (Molecular Structure Facility, University of California, Oavis, CA), and the molecular weights of the wild-type and mutant peptides were confirmed by matrix-assisted laser desorption ionization—time of flight mass spectrometry. The ability of the peptide to act as a substrate for uridylylation by wild-type 3D was determined using a uridylylation assay described previously (3).
ITC.
Lyophilized VPg samples were dissolved in ITC buffer (15% glycerol, 25 mM HEPES, pH 8.5, 50 mM NaCl, 2 mM β-mercaptoethanol) to a concentration of approximately 1.2 mM. VPg concentrations were determined by absorbance at 275 nm using an extinction coefficient of 1,136 M−1 cm−1 as determined by amino acid analysis. The concentration of the VPg Y3A mutant, which did not absorb at 275 nm, was determined by bicinchoninic acid assay (Pierce) using a standard curve constructed with VPg peptide. Purified 3D-DD was concentrated to approximately 120 μM with a YM-10 Centriprep filter (Amicon). VPg and 3D-DD solutions were each dialyzed twice against ITC buffer, and the pH of each sample was checked.
Isothermal titrations were performed on a VP-ITC isothermal titration calorimeter (MicroCal). Data were recorded at 4°C with a syringe stirring speed of 300 rpm and a baseline of 12 μcal/sec. Samples were degassed by stirring under vacuum for 5 min before each titration. For each data point, 10 μl of VPg solution was added to polymerase solution over a 20-s interval with 180 s of equilibration time between each injection. Data were analyzed using the ITC module in the Origin software (version 5.0; MicroCal). The average heat of dilution of the peptide, determined by adding peptide solution to the ITC buffer alone, was first subtracted from the titration data. The average heat of dilution of 3D, determined by adding ITC buffer to 3D in the sample cell, was found to be negligible. Thermograms were then analyzed with a standard 1:1 binding model included in the software to obtain the binding enthalpy (ΔH), association constant (Ka), and binding stoichiometry (N). The dissociation constant was then calculated as 1/Ka, and the binding free energy (ΔG) was calculated as −RT[ln(Ka)], where R is the gas constant and T is the temperature in Kelvins. The binding entropy term (TΔS) was then calculated as ΔH − ΔG after rearrangement of the Gibbs free energy equation. Each titration was performed in triplicate, and the mean and standard error of the mean are reported for each of the thermodynamic parameters determined.
VPg competition for 3AB-3D binding.
The ability of VPg to compete with 3AB for binding of 3D was determined using a variation of the polymerase recruitment assay. Briefly, reaction mixtures lacking 3AB-containing membranes were prepared as described above. To these, either 5 μl of 1.5 mM VPg in ITC buffer or ITC buffer alone was added, and the reaction was allowed to sit on ice for 30 min to allow prebinding of the peptide to the polymerase. 3AB-containing membranes were then added to the reaction and the assay was carried out as described above.
RESULTS
3AB binding to 3D occurs through interactions with the 3B region with negligible contributions from the soluble 3A domain.
The interaction between poliovirus 3AB and the polymerase 3D is key to the assembly of the viral replication complex at membranous vesicles. Little is known, however, about the specific sequence requirements for poliovirus 3AB binding to 3D. Yeast two-hybrid analyses of 3AB mutants have indicated that residues in the 3B region of 3AB are critical for the 3AB-3D interaction (45). In those studies, moderate effects were also observed from mutations throughout the 3A region, suggesting that this region of the protein may be involved in the interaction. To identify the specific sites which are required for the interaction of membrane-bound 3AB with the viral polymerase 3D, the effects of mutations within the 3AB sequence were analyzed by the polymerase recruitment assay.
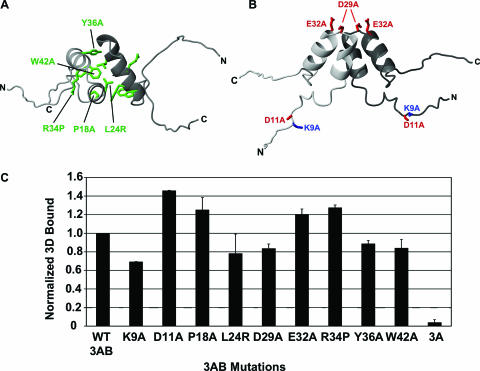
The N-terminal region of 3A (3A-N) is known to form a structured dimer in solution (36). Although dimerization of membrane-bound 3AB has not been definitively observed, the presence of dimerization in yeast two-hybrid analysis of 3AB and glutaraldehyde cross-linking of detergent-solubilized 3AB (45) suggest that the 3A-N region forms a dimeric structure in the membrane-bound form of this protein as well. In order to determine the role of this folded region in 3AB-3D binding, mutations were designed which were intended to disrupt the dimer structure (Fig. 2A). Specifically, alanine mutations were created at three highly conserved positions that make important contacts in the 3A-N structure (P18A, Y36A, and W42A), and two additional mutations were created that were specifically intended to disrupt the hydrophobic, dimer interface (L24R) or the helical secondary structure (R34P). Analysis of 3D-binding by each of these 3AB single mutants (P18A, L24R, R34P, Y36A, and W42A) indicates that they each bind to 3D similarly to wild-type protein (Fig. 2C). In order to characterize the roles of charged residues in the 3A region on 3AB-3D binding, four charged residues were individually mutated to alanine (Fig. 2B), and these mutants (K9A, D11A, D29A, and E32A) were analyzed for their ability to bind to 3D. In each case, no substantial changes in binding were observed compared to wild-type 3AB (Fig. 2C). These results suggest that neither the structured region of 3A nor the charged residues play significant roles in 3AB binding to 3D.
FIG. 2.
Effects of point mutations in the 3A region of membrane-bound 3AB on binding to the 3D polymerase. (A) Ribbon diagram of 3A-N viewed from the “top.” Hydrophobic residues in the dimer interface and other structurally critical residues mutated for this study are labeled, and side chain carbons are displayed. (B) Ribbon diagram of the structure of the soluble, N-terminal region of 3AB (3A-N) viewed from the “side.” Charged surface residues mutated for this study are labeled, and side chain carbons are displayed. (C) Results of the polymerase recruitment assay using membranes containing a 3AB construct containing the indicated mutation. Bars indicate the amount of 3D bound by the membranes corrected for the amount bound to control membranes and normalized to the amount bound to wild-type 3AB. The assays were performed in triplicate, and error bars indicate the standard error of the mean.
The inability of mutations in the 3A region of 3AB to disrupt binding to 3D suggests that the 3B region of 3AB is responsible for the interaction of 3AB with 3D. In order to determine if the 3B region of 3AB is required for binding, the ability of membrane-bound 3A alone to bind to 3D was determined. Polymerase recruitment analysis indicates that membranes containing 3A do not pull down 3D more efficiently than the control blank membranes (Fig. 2C). This complete loss of binding indicates that it is the 3B region of 3AB that is critical for 3AB-3D binding.
Identification of five residues in the 3B region critical for 3AB-3D binding.
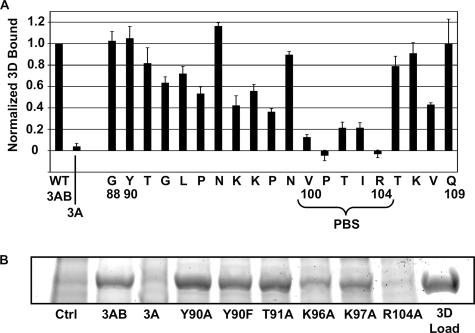
To pinpoint the residues of the 3B region that participate in 3D-binding, a complete alanine scan of the 3B region was performed, and changes in binding were assessed with the polymerase recruitment assay (Fig. 3). Of the 20 positions tested in the 3B region of 3AB, alanine mutations at positions 100, 101, 102, 103, and 104 display the most dramatic effects on binding, reducing 3AB-3D binding levels to less than 30% of the wild-type level. These residues form a contiguous region which we have termed the polymerase binding sequence (PBS). Additionally, five other 3AB mutants exhibit a moderate reduction in binding to 30 to 60% of the levels observed for wild-type binding. These residues are primarily located in the regions flanking the PBS, including positions 94, 96, 97, 98, and 108. Although these residues may have a significant role in the interaction with 3D, the qualitative nature of the polymerase recruitment assay makes it difficult to interpret what role they might play. Overall, these results suggest that a novel binding surface spanning a contiguous region from position 100 to 104 is responsible for the majority of the interaction of 3AB with 3D, while a number of other residues play minor parts in 3D binding.
FIG. 3.
Effects of point mutations in the 3B region of membrane-bound 3AB on binding to the 3D polymerase. (A) Results of the polymerase recruitment assay using membranes containing wild-type 3A or 3AB with the indicated 3B residue mutated to alanine. Note that the alanine residues at positions 89 and 106 were not tested. The residues making up the PBS are labeled. Bars and error bars are as described in the legend of Fig. 2. (B) Sample of the 3D region of an sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel used for quantifying the polymerase recruitment assay.
Thermodynamic analysis of VPg binding to 3D by ITC.
Analysis of the binding of membrane-bound 3AB to 3D indicates that the 3B region of 3AB is primarily responsible for that interaction. As a cleavage product of 3AB, the VPg peptide contains the entire sequence found to be necessary for 3AB-3D binding. VPg is also known to bind to 3D and act as a substrate for the uridylylation reaction catalyzed by the polymerase (28, 45). In order to quantitate and gain a better understanding of the binding interaction of VPg with the polymerase 3D, we performed a thermodynamic analysis of the 3D-binding reactions of both wild-type and mutant VPg peptides by ITC.
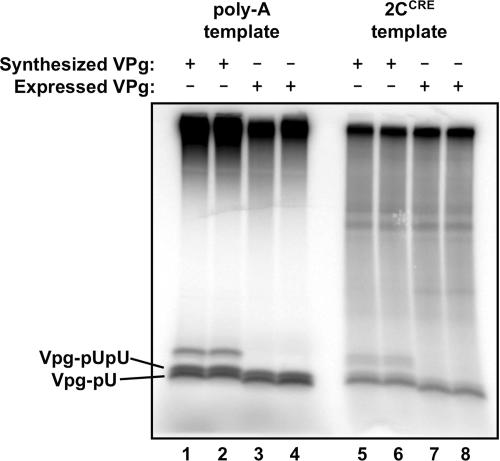
To obtain the large quantity and high concentration of 3D polymerase necessary for ITC analysis, the highly soluble mutant 3D-DD was used (40). Although this mutant exhibits about twofold decreases in elongation activity and 3AB-binding compared to wild-type 3D (40; D. M. Strauss and D. S. Wuttke, unpublished data), the enzyme's 10-fold increase in solubility makes it critical for these experiments. To obtain sufficient quantities of VPg, the peptide was expressed in E. coli as a fusion to a soluble globular protein (GB1) and chemically cleaved by a CNBr reaction. Large quantities of VPg peptide could be produced by this method which were observed to act like synthesized VPg as a substrate for uridylylation using either poly(A) RNA or the 2CCRE RNA hairpin as a template (Fig. 4).
FIG. 4.
Uridylylation of E. coli-derived VPg. Electrophoretic gel indicating uridylylation of VPg which was either obtained by peptide synthesis (lanes 1 and 2 and lanes 5 and 6) or expressed as a fusion protein in E. coli (lanes 3 and 4 and lanes 7 and 8). The reactions are performed using either a poly(A) RNA template (lanes 1 to 4) or the 2CCRE RNA hairpin template (lanes 5 to 8). Uridylylated VPg peptides are labeled. Larger-molecular-weight bands in the synthesized peptide lanes (lanes 1 and 2 and lanes 5 and 6) represent peptide heterogeneity not present in the E. coli-expressed peptides.
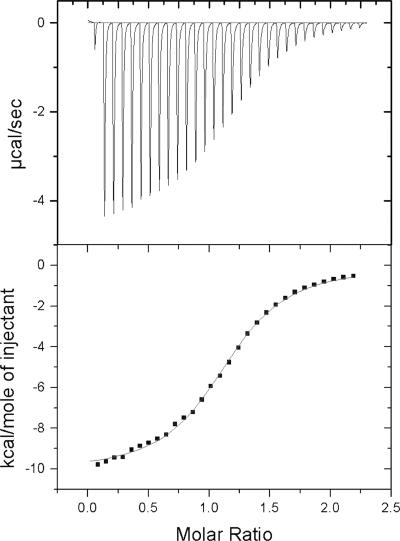
Titration of VPg into 3D-DD yields a highly reproducible thermogram, which fits well to a two-state binding curve (Fig. 5 and Table 1). The dissociation constant (Kd) for the binding reaction under these conditions is 11.3 ± 0.9 μM. The floating parameter N, which reports on the stoichiometry of the reaction, is 1.21 ± 0.06, indicating that VPg and 3D-DD bind at approximately a 1:1 ratio (likely varying from unity due to uncertainty in the extinction coefficient of the polymerase). Furthermore, the binding reaction is exothermically driven, as indicated by a ΔH value of −10.2 ± 0.2 Kcal/mol and is entropically disfavored, as indicated by a TΔS value of −4.0 ± 0.3 Kcal/mol.
FIG. 5.
ITC analysis of wild-type VPg binding to 3D. (Top) Thermogram displaying the change in heat required by the instrument to maintain an isothermal condition between the sample cell and reference cell. Each peak occurs upon the addition of an aliquot of VPg to the 3D solution in the sample cell. (Bottom) Points displaying the integration of the thermogram peaks indicate the heat released per mole of VPg during each injection. The line connecting the points is the best fit to the data of a two-state binding model using a nonlinear least squares algorithm to solve for the stoichiometry (N), the dissociation constant (Kd), and the change in enthalpy (ΔH) for the reaction. The change in entropy, ΔS, is calculated from these values.
TABLE 1.
Thermodynamic data from ITC analysis of the VPg-3D binding reaction
| VPga | Kd (μM)b | ΔΗ (Kcal/mol) | TΔS (Kcal/mol) | N |
|---|---|---|---|---|
| WT | 11.3 ± 0.9 | −10.2 ± 0.2 | −4.0 ± 0.3 | 1.21 ± 0.06 |
| Y3A | 4.7 ± 0.3 | −12.60 ± 0.06 | −5.84 ± 0.01 | 0.84 ± 0.01 |
| T4A | 6.5 ± 0.2 | −10.77 ± 0.01 | −4.19 ± 0.02 | 1.09 ± 0.01 |
| K9A | 16 ± 2 | −10.7 ± 0.4 | −4.6 ± 0.5 | 1.19 ± 0.02 |
| V13A | 7.2 ± 1.1 | −9.6 ± 0.5 | −3.1 ± 0.6 | 1.28 ± 0.02 |
| P14A | NB | |||
| T15A | 22 ± 5 | −9.2 ± 0.7 | −3.3 ± 0.8 | 1.09 ± 0.06 |
| I16A | 30 ± 2 | −9.5 ± 0.3 | −3.8 ± 0.4 | 1.07 ± 0.08 |
| R17A | NB | |||
| R17K | NB | |||
| K20A | 17.0 ± 1.2 | −10.16 ± 0.18 | −4.1 ± 0.2 | 1.58 ± 0.13 |
Isothermal titration analysis of the L446D R455D mutant of poliovirus 3D with either wild-type VPg (WT) or VPg containing the indicated mutation.
ΔH, change in enthalpy; ΔS, change in entropy; N, stoichiometry of the binding reaction; NB, no appreciable binding was detected; T, 277 K.
Identification of two VPg residues critical for VPg-3D binding.
Even though 3AB contains all of the residues that make up the VPg peptide, 3AB is not uridylylated to a significant extent by the polymerase in vitro. One possible explanation for this phenomenon is that 3AB and VPg may bind to different sites on 3D, or they may bind to the same site but in a different manner. In order to compare the interactions of VPg and 3AB with 3D, the sequence requirements for VPg-3D binding were determined. Based on our 3AB-3D binding data and previously published data, nine sites were selected for mutation to alanines. Specifically, mutations at each of the five positions in the PBS (V13A, P14A, T15A, I16A, and R17A) and at one position which has a moderate effect on 3AB-3D binding (K9A) were analyzed. Additionally, an alanine mutation at position 3 (Y3A) was analyzed since this residue is the substrate for the uridylylation reaction and was indicated previously to be important for binding to 3D (27). Finally, two control mutations were made of partially conserved residues that showed no effect on 3AB binding and have not previously been indicated to affect VPg binding (T4A and K20A).
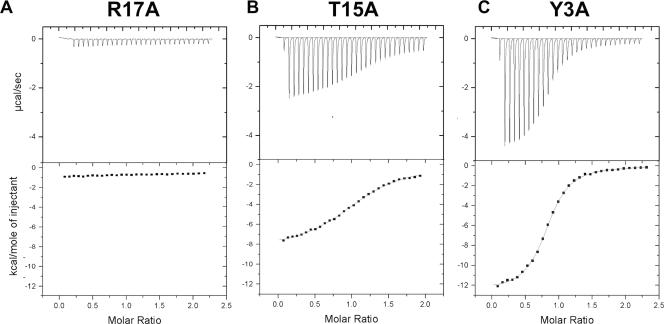
ITC analysis was used to accurately measure the effects of these mutations on VPg binding affinity to 3D (Table 1). A wide range of effects was observed, and for the purposes of discussion, the mutations were clustered into three categories based on their effects on binding affinity. Alanine mutations at positions P14 and R17, which correspond to critical positions P101 and R104 in 3AB, drastically disrupt binding of VPg to 3D (Fig. 6A). The binding affinity of these mutants is too weak to be detected by ITC due to the negligible heat released upon injection. To determine if the effect of the R17A mutation was due to the loss of a positive charge at that position, we analyzed binding of an R17K mutant by ITC. Although this mutant retains the positive charge at position 17, no binding to 3D is observed. Two other VPg mutants, T15A and I16A, exhibit two- to threefold reductions in binding, with Kd values of 22 ± 5 and 30 ± 2 μM, respectively (Fig. 6B). Finally, five of the VPg mutants have little effect on binding compared with wild-type VPg. These include Y3A, T4A, K9A, V13A, and K20A with Kd values of 4.7 ± 0.3, 6.5 ± 0.2, 16 ± 2, 7.2 ± 1.1, and 17.0 ± 1.2 μM, respectively (Fig. 6C).
FIG. 6.
ITC analysis of VPg mutants. Representative ITC data illustrate the effects on 3D-binding of the three classes of VPg mutations. (A) VPg mutant R17A does not bind to 3D, displaying little or no heat released upon addition to 3D. (B) VPg mutant T15A binds to 3D with a somewhat reduced affinity, displaying smaller peaks and a shallower curve than wild-type VPg but still allowing for accurate determination of thermodynamic binding parameters. (C) VPg mutant Y3A binds to 3D much like wild-type VPg, displaying similar thermograms and allowing for accurate determination of thermodynamic binding parameters.
DISCUSSION
Localization of the poliovirus replication complex to membranous vesicles is essential for virus replication and is rigorously dependent on the interaction between the poliovirus RNA-polymerase 3D and membrane-bound 3AB. We have identified a novel five-residue region within the 3B domain of 3AB which is required for binding to 3D (Fig. 6). Mutation of any one of the residues within the VPTIR sequence spanning positions 100 to 104 reduces 3AB-3D binding to levels observed when the entire 3B region is removed. Of these five residues, only R104 has been previously identified as playing a role in 3AB-3D binding (45), while the other four residues have never been tested for their roles in this interaction. Strikingly, both P101 and R104 are completely conserved in 3AB sequences among all enteroviruses and rhinoviruses, indicating that the interaction may be conserved among these virus families (Fig. 1B). In contrast, we found that mutations of charged residues in the 3A region, as well as mutations targeted to disrupt the folded structure of the 3A region, had no effect on 3AB-3D binding. Interestingly, one of the latter mutations, L24R, designed to insert a charged arginine residue into the hydrophobic dimer interface, disrupts the ability of 3A to inhibit endoplasmic reticulum (ER)-to-Golgi trafficking (S.S. Choe and K. Kirkegaard, personal communication), suggesting that the 3A structure is necessary for inhibition of ER-to-Golgi trafficking. Thus, although the folded 3A-N dimer structure is dispensable for 3AB binding to 3D, it is important for other viral functions.
ITC has provided quantitative information on the molecular determinants of the polymerase 3D binding site within the VPg peptide. The Kd value for the binding of wild-type VPg to 3D is 11.3 ± 0.9 μM. This value is consistent with the inhibition constant (Ki) of 20 μM measured for VPg-derived peptide (39). The VPg-3D binding affinity is relatively low compared to other known primer-polymerase interactions. For instance, the bacteriophage T4 DNA polymerase gp43 binds to a DNA primer-template duplex with a Kd as low as 8 nM (7), and the hepatitis C virus RNA polymerase NS5B catalyzes elongation of a DNA primer with a Km of 25 nM (23). The observation that poliovirus 3D also binds to an RNA primer-template duplex with weak affinity (Kd = 1 μM) (1) may suggest that the low affinity for VPg is, in fact, a general characteristic of the 3D polymerase. The tight binding affinities observed in other viral systems may not be needed for functional poliovirus complexes, as the concentrations of viral proteins are high in poliovirus-infected cells, and the formation of membranous vesicles makes the effective local concentrations of these proteins even higher, particularly if they are localized at the active site through their precursor forms.
Two positions within the VPg peptide, P14 and R17, were found in which alanine mutations completely disrupt VPg-3D binding (Fig. 7). Since circular dichroism and nuclear magnetic resonance studies of the VPg peptide have shown that it is completely unstructured in solution (33; D. M. Strauss, C. A. Fowler, and D. S. Wuttke, unpublished data), these residues must play important roles at the binding interface rather than stabilizing the free VPg structure. Both sites are completely conserved among enteroviruses and rhinoviruses (Fig. 1B), and mutations at both of these positions have been shown to yield nonviable or quasi-infectious virus (27, 46). Since these positions are also required for the interaction between 3AB and 3D, the defective phenotypes may be due to due to disruption of 3AB-3D binding, VPg-3D binding, or both. In vitro experiments with R17 mutants have shown this residue to be critical for VPg uridylylation (27), and the loss of binding by the R17 mutants observed is sufficient to explain the loss of function of VPg as a substrate. Additionally, the strict conservation of arginine rather than lysine at this position, combined with the observation that VPg containing an R17K mutation does not recover 3D binding activity, suggests that the guanidinium group of arginine mediates specific interactions with 3D. Furthermore, our studies suggest that it is unlikely that this residue plays a role in binding UTP, as has been suggested (28, 34).
FIG. 7.
Comparison of 3AB and VPg binding to the 3D polymerase. The sequences of 3AB and VPg are shown with their corresponding residue numbers, and the polymerase binding sequence of 3AB is indicated. A lowercase h indicates the homoserine residue remaining at the C terminus of VPg due to CNBr cleavage. Residues not tested for their effects on 3D-binding are shown in black, residues tested but not displaying a significant effect on binding are shown in blue, residues tested and displaying a moderate effect on 3D-binding are shown in orange, and residues tested and displaying a large effect on 3D-binding are shown in red and underlined.
Mutation of tyrosine 3, the site of uridylylation, to alanine does not affect binding to 3D by ITC analysis. In contrast, a previous study found that a phenylalanine mutation at that position disrupted a yeast two-hybrid interaction between VPg and 3D (27). This discrepancy may be due to a disruption introduced by the phenylalanine side chain not observed when tyrosine or alanine is present or could be a false negative result of the yeast two-hybrid assay. Our observation that the substrate tyrosine does not play a role in the binding interaction of VPg to 3D is somewhat surprising since the substrate hydroxyl must ultimately bind to the active site of the polymerase in order for 3D to catalyze the uridylylation reaction. Since the 3D polymerase active site normally utilizes a ribose 3′ hydroxyl as a substrate, it may not be optimized to bind a tyrosine hydroxyl at that site. The role of the binding site at the C terminus of VPg may therefore be to bind to a region on the polymerase near the active site and effectively increase the local concentration of the N-terminal tyrosine at the active site, allowing a relatively inefficient reaction to take place.
The similar sequence requirements for the 3AB and VPg binding interactions (Fig. 7) with 3D strongly suggest that these two peptides bind to a similar site on 3D. This hypothesis is supported by the ability of 3AB to inhibit VPg uridylylation in vitro (3). Competition experiments show that VPg competes for 3AB-3D binding, exhibiting a 35% decrease in 3AB binding in 160 μM VPg (data not shown). This degree of inhibition suggests that 3AB binds significantly tighter than VPg, a conclusion supported by the reported Ki of 100 nM for uridylylation of detergent-solubilized 3AB (3).
While the 3D binding surface is not known, conflicting data from the structure of a homologue and biochemical studies suggest two very different surfaces: one near the active site on the “front” and one directly behind the active site on the “back” of the polymerase. The front site is based on the crystal structure of the closely related picornavirus foot-and-mouth disease virus (FMDV) 3D polymerase bound to its VPg substrate (10), in which VPg binds near the active site. The high degree of sequence similarity between the poliovirus and FMDV polymerases suggests that these proteins utilize a similar mechanism and binding site for VPg uridylylation. However, due to the poor alignment between their sequences, a direct correlation between the FMDV and poliovirus VPg structures cannot be made. Proline and arginine residues at positions 6 and 9 play critical roles at the FMDV binding interface. These residues may correspond to poliovirus VPg P14 and R17 found here to be biochemically key for 3D binding. We suggest that a register shift may structurally align the two VPg sequences, such that proline 6 of FMDV VPg corresponds to proline 14 of poliovirus VPg, which would place the thermodynamically important VPg residues in contact positions near the active site. In contrast, the proposed site on the back of the polymerase lies at the base of the “thumb” domain and is based on the location of mutations which decrease both 3AB-binding and VPg uridylylation (17, 22). However, this location affords only limited access of the peptide substrate to the active site of the polymerase, and it differs substantially from other structures of RNA-dependent polymerases with bound primers (10, 11, 19). Although two recent studies have used computational docking methods to develop plausible structural models of VPg bound to this site, little experimental data are available to support these models (33, 34, 39). Based on preliminary data in our lab (D. M. Strauss and D. S. Wuttke, unpublished data), we suspect that the 3D mutations at this site may not disrupt binding directly but may instead cause a conformational shift in the polymerase structure near a key hinge region which then affects a binding site distantly located on the protein.
In summary, we have identified regions of the poliovirus 3AB protein and VPg peptide that contribute to the binding interaction with the polymerase 3D. Using membrane-bound 3AB, we have identified a short, contiguous stretch of residues in the 3B region that are critical for the 3AB-3D interaction, and we have shown that a subset of these are required for VPg binding to the polymerase as well. We have also determined the thermodynamic contributions of VPg residues for 3D-binding and identified a proline at position 14 and an arginine at position 17 as playing key roles at the binding interface. This work provides a detailed understanding of the direct contributions of 3AB and VPg sequence elements that govern their binding interactions with the viral polymerase.
Acknowledgments
We thank Oliver Richards for performing the uridylation assay, for assistance with the 3AB expression protocol, and for helpful advice; Leslie Glustrom for enthusiastic discussions; Olve Peersen for valuable conversations and for providing the 3D-DD construct; Karla Kirkegaard and Sunny Choe for characterizing the ER-to-Golgi inhibition activities of 3A mutants; Eckard Wimmer for providing the 3AB construct; and Marius Clore for providing the GB1 construct.
This work was supported by a National Science Foundation Career Award (MCB9875663), and a National Institutes of Health Genetics Training Grant (GM07135).
Footnotes
Published ahead of print on 4 April 2007.
REFERENCES
- 1.Arnold, J. J., and C. E. Cameron. 2000. Poliovirus RNA-dependent RNA polymerase (3Dpol). Assembly of stable, elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub). J. Biol. Chem. 275:5329-5336. [DOI] [PubMed] [Google Scholar]
- 2.Bienz, K., D. Egger, M. Troxler, and L. Pasamontes. 1990. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J. Virol. 64:1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boerner, J. E., J. M. Lyle, S. Daijogo, B. L. Semler, S. C. Schultz, K. Kirkegaard, and O. C. Richards. 2005. Allosteric effects of ligands and mutations on poliovirus RNA-dependent RNA polymerase. J. Virol. 79:7803-7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bushell, M., and P. Sarnow. 2002. Hijacking the translation apparatus by RNA viruses. J. Cell Biol. 158:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe, S. S., D. A. Dodd, and K. Kirkegaard. 2005. Inhibition of cellular protein secretion by picornaviral 3A proteins. Virology 337:18-29. [DOI] [PubMed] [Google Scholar]
- 6.Choe, S. S., and K. Kirkegaard. 2004. Intracellular topology and epitope shielding of poliovirus 3A protein. J. Virol. 78:5973-5982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delagoutte, E., and P. H. Von Hippel. 2003. Function and assembly of the bacteriophage T4 DNA replication complex: interactions of the T4 polymerase with various model DNA constructs. J. Biol. Chem. 278:25435-25447. [DOI] [PubMed] [Google Scholar]
- 8.Doedens, J. R., T. H. Giddings, Jr., and K. Kirkegaard. 1997. Inhibition of endoplasmic reticulum-to-Golgi traffic by poliovirus protein 3A: genetic and ultrastructural analysis. J. Virol. 71:9054-9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doedens, J. R., and K. Kirkegaard. 1995. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 14:894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrer-Orta, C., A. Arias, R. Agudo, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2006. The structure of a protein primer-polymerase complex in the initiation of genome replication. EMBO J. 25:880-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Orta, C., A. Arias, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2004. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J. Biol. Chem. 279:47212-47221. [DOI] [PubMed] [Google Scholar]
- 12.Giachetti, C., S. S. Hwang, and B. L. Semler. 1992. cis-acting lesions targeted to the hydrophobic domain of a poliovirus membrane protein involved in RNA replication. J. Virol. 66:6045-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodfellow, I., Y. Chaudhry, A. Richardson, J. Meredith, J. W. Almond, W. Barclay, and D. J. Evans. 2000. Identification of a cis-acting replication element within the poliovirus coding region. J. Virol. 74:4590-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinz, B. A., and L. M. Vance. 1996. Sequence determinants of 3A-mediated resistance to enviroxime in rhinoviruses and enteroviruses. J. Virol. 70:4854-4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinz, B. A., and L. M. Vance. 1995. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 69:4189-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobson, S. D., E. S. Rosenblum, O. C. Richards, K. Richmond, K. Kirkegaard, and S. C. Schultz. 2001. Oligomeric structures of poliovirus polymerase are important for function. EMBO J. 20:1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hope, D. A., S. E. Diamond, and K. Kirkegaard. 1997. Genetic dissection of interaction between poliovirus 3D polymerase and viral protein 3AB. J. Virol. 71:9490-9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huth, J. R., C. A. Bewley, B. M. Jackson, A. G. Hinnebusch, G. M. Clore, and A. M. Gronenborn. 1997. Design of an expression system for detecting folded protein domains and mapping macromolecular interactions by NMR. Protein Sci. 6:2359-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobo-Molina, A., J. Ding, R. G. Nanni, A. D. Clark, Jr., X. Lu, C. Tantillo, R. L. Williams, G. Kamer, A. L. Ferris, P. Clark, et al. 1993. Crystal structure of human immunodeficiency virus type 1 reverse transcriptase complexed with double-stranded DNA at 3.0 Å resolution shows bent DNA. Proc. Natl. Acad. Sci. USA 90:6320-6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lama, J., A. V. Paul, K. S. Harris, and E. Wimmer. 1994. Properties of purified recombinant poliovirus protein 3AB as substrate for viral proteinases and as co-factor for RNA polymerase 3Dpol. J. Biol. Chem. 269:66-70. [PubMed] [Google Scholar]
- 21.Lindhout, D. A., M. X. Li, D. Schieve, and B. D. Sykes. 2002. Effects of T142 phosphorylation and mutation R145G on the interaction of the inhibitory region of human cardiac troponin I with the C-domain of human cardiac troponin C. Biochemistry 41:7267-7274. [DOI] [PubMed] [Google Scholar]
- 22.Lyle, J. M., A. Clewell, K. Richmond, O. C. Richards, D. A. Hope, S. C. Schultz, and K. Kirkegaard. 2002. Similar structural basis for membrane localization and protein priming by an RNA-dependent RNA polymerase. J. Biol. Chem. 277:16324-16331. [DOI] [PubMed] [Google Scholar]
- 23.McKercher, G., P. L. Beaulieu, D. Lamarre, S. LaPlante, S. Lefebvre, C. Pellerin, L. Thauvette, and G. Kukolj. 2004. Specific inhibitors of HCV polymerase identified using an NS5B with lower affinity for template/primer substrate. Nucleic Acids Res. 32:422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molla, A., K. S. Harris, A. V. Paul, S. H. Shin, J. Mugavero, and E. Wimmer. 1994. Stimulation of poliovirus proteinase 3Cpro-related proteolysis by the genome-linked protein VPg and its precursor 3AB. J. Biol. Chem. 269:27015-27020. [PubMed] [Google Scholar]
- 25.Morasco, B. J., N. Sharma, J. Parilla, and J. B. Flanegan. 2003. Poliovirus cre(2C)-dependent synthesis of VPgpUpU is required for positive- but not negative-strand RNA synthesis. J. Virol. 77:5136-5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murray, K. E., and D. J. Barton. 2003. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J. Virol. 77:4739-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul, A. V., J. Peters, J. Mugavero, J. Yin, J. H. van Boom, and E. Wimmer. 2003. Biochemical and genetic studies of the VPg uridylylation reaction catalyzed by the RNA polymerase of poliovirus. J. Virol. 77:891-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paul, A. V., J. H. van Boom, D. Filippov, and E. Wimmer. 1998. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393:280-284. [DOI] [PubMed] [Google Scholar]
- 29.Plotch, S. J., and O. Palant. 1995. Poliovirus protein 3AB forms a complex with and stimulates the activity of the viral RNA polymerase, 3Dpol. J. Virol. 69:7169-7179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Racaniello, V. 2001. Picornaviridae: the viruses and their replication, p. 685-722. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 31.Richards, O. C., and E. Ehrenfeld. 1998. Effects of poliovirus 3AB protein on 3D polymerase-catalyzed reaction. J. Biol. Chem. 273:12832-12840. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Wells, V., S. J. Plotch, and J. J. DeStefano. 2001. Primer-dependent synthesis by poliovirus RNA-dependent RNA polymerase (3Dpol). Nucleic Acids Res. 29:2715-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schein, C. H., N. Oezguen, D. E. Volk, R. Garimella, A. Paul, and W. Braun. 2006. NMR structure of the viral peptide linked to the genome (VPg) of poliovirus. Peptides 27:1676-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schein, C. H., D. E. Volk, N. Oezguen, and A. Paul. 2006. Novel, structure-based mechanism for uridylylation of the genome-linked peptide (VPg) of picornaviruses. Proteins 63:719-726. [DOI] [PubMed] [Google Scholar]
- 35.Semler, B. L., and E. Wimmer. 2002. Molecular biology of picornaviruses. ASM Press, Washington, DC.
- 36.Strauss, D. M., L. W. Glustrom, and D. S. Wuttke. 2003. Towards an understanding of the poliovirus replication complex: the solution structure of the soluble domain of the poliovirus 3A protein. J. Mol. Biol. 330:225-234. [DOI] [PubMed] [Google Scholar]
- 37.Suhy, D. A., T. H. Giddings, Jr., and K. Kirkegaard. 2000. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 74:8953-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takegami, T., B. L. Semler, C. W. Anderson, and E. Wimmer. 1983. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology 128:33-47. [DOI] [PubMed] [Google Scholar]
- 39.Tellez, A. B., S. Crowder, J. F. Spagnolo, A. A. Thompson, O. B. Peersen, D. L. Brutlag, and K. Kirkegaard. 2006. Nucleotide channel of RNA-dependent RNA polymerase used for intermolecular uridylylation of protein primer. J. Mol. Biol. 357:665-675. [DOI] [PubMed] [Google Scholar]
- 40.Thompson, A. A., and O. B. Peersen. 2004. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 23:3462-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Towner, J. S., T. V. Ho, and B. L. Semler. 1996. Determinants of membrane association for poliovirus protein 3AB. J. Biol. Chem. 271:26810-26818. [DOI] [PubMed] [Google Scholar]
- 42.van Ooij, M. J., D. A. Vogt, A. Paul, C. Castro, J. Kuijpers, F. J. van Kuppeveld, C. E. Cameron, E. Wimmer, R. Andino, and W. J. Melchers. 2006. Structural and functional characterization of the coxsackievirus B3 CRE(2C): role of CRE(2C) in negative- and positive-strand RNA synthesis. J. Gen. Virol. 87:103-113. [DOI] [PubMed] [Google Scholar]
- 43.Wessels, E., D. Duijsings, R. A. Notebaart, W. J. Melchers, and F. J. van Kuppeveld. 2005. A proline-rich region in the coxsackievirus 3A protein is required for the protein to inhibit endoplasmic reticulum-to-Golgi transport. J. Virol. 79:5163-5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wessels, E., R. A. Notebaart, D. Duijsings, K. Lanke, B. Vergeer, W. J. Melchers, and F. J. van Kuppeveld. 2006. Structure-function analysis of the coxsackievirus protein 3A: identification of residues important for dimerization, viral RNA replication, and transport inhibition. J. Biol. Chem. 281:28232-28243. [DOI] [PubMed] [Google Scholar]
- 45.Xiang, W., A. Cuconati, D. Hope, K. Kirkegaard, and E. Wimmer. 1998. Complete protein linkage map of poliovirus P3 proteins: interaction of polymerase 3Dpol with VPg and with genetic variants of 3AB. J. Virol. 72:6732-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiang, W., A. Cuconati, A. V. Paul, X. Cao, and E. Wimmer. 1995. Molecular dissection of the multifunctional poliovirus RNA-binding protein 3AB. RNA 1:892-904. [PMC free article] [PubMed] [Google Scholar]
- 47.Yalamanchili, P., U. Datta, and A. Dasgupta. 1997. Inhibition of host cell transcription by poliovirus: cleavage of transcription factor CREB by poliovirus-encoded protease 3Cpro. J. Virol. 71:1220-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]