Abstract
The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen (LANA) protein interacts with glycogen synthase kinase 3 (GSK-3) and relocalizes GSK-3 in a manner that leads to stabilization of β-catenin and upregulation of β-catenin-responsive cell genes. The LANA-GSK-3 interaction was further examined to determine whether there were additional downstream consequences. In the present study, the nuclear GSK-3 bound to LANA in transfected cells and in BCBL1 primary effusion lymphoma cells was found to be enriched for the inactive serine 9-phosphorylated form of GSK-3. The mechanism of inactivation of nuclear GSK-3 involved LANA recruitment of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) and the ribosomal S6 kinase 1 (RSK1). ERK1/2 and RSK1 coprecipitated with LANA, and LANA was a substrate for ERK1 in vitro. A model is proposed for the overall inactivation of nuclear GSK-3 that incorporates the previously described GSK-3 phosphorylation of LANA itself. Functional inactivation of nuclear GSK-3 was demonstrated by the ability of LANA to limit phosphorylation of the known GSK-3 substrates C/EBPβ and C/EBPα. The effect of LANA-mediated ablation of C/EBP phosphorylation on differentiation was modeled in the well-characterized 3T3L1 adipogenesis system. LANA-expressing 3T3L1 cells were impaired in their ability to undergo differentiation and adipogenesis. C/EBPβ induction followed the same time course as that seen in vector-transduced cells, but there was delayed and reduced induction of C/EBPβ transcriptional targets in LANA-expressing cells. We conclude that LANA inactivates nuclear GSK-3 and modifies the function of proteins that are GSK-3 substrates. In the case of C/EBPs, this translates into LANA-mediated inhibition of differentiation.
The latency-associated nuclear antigen (LANA) protein is one of a very limited number of Kaposi's sarcoma-associated herpesvirus (KSHV) proteins synthesized during KSHV latency and in the KSHV-associated tumors of Kaposi's sarcoma, primary effusion lymphoma (PEL), and multicentric Castleman's disease (25, 41, 52). LANA is encoded by KSHV open reading frame 73 and is expressed in latently infected cells from alternatively spliced transcripts that also encode the FLICE inhibitory protein v-FLIP and the D-type cyclin v-cyclin (12, 51, 57). A second downstream promoter that is responsive to the KSHV lytic transactivator known as the replication and transcription activator (RTA) also drives LANA expression (35, 56), and this promoter may be important for the synthesis of LANA in cells immediately following KSHV infection (27). LANA is a multifunctional protein with DNA replication, chromosome tethering, antiapoptotic, cell cycle regulatory, and gene regulatory functions. LANA binds to sequences in the KSHV terminal repeats (8, 19, 22, 23) that are essential for replication of the episomal KSHV genomes during latency (13, 21). LANA also supports long-term episomal maintenance through the tethering of KSHV genomes to chromosomes (2, 4, 43). Interactions between the LANA N terminus and C terminus with the core histones H2A and H2B (5), the methyl CpG binding protein MeCP2 (28), and the chromatin-associated proteins DEK, HP1, RING3, and Brd4 (28, 32, 38, 62, 73) facilitate long-term genome maintenance. An antiapoptotic function for LANA was linked to an interaction with p53 (14), and LANA stimulation of cell cycle S-phase entry is associated with the upregulation of cyclin D1 through stabilization of β-catenin (18), interaction with pRb (44), and overcoming G1 cell cycle arrest mediated by p16 (1) and by BRD4 and BRD2/RING3 (38).
Gene array studies have found that KSHV infection reprograms cell gene expression (63) and that expression of LANA alone is sufficient to modulate cell gene expression both positively and negatively (1, 45, 54, 65). The direct binding of LANA to the KSHV terminal repeat sequences or the targeting of LANA to DNA as a Gal4 fusion construct results in transcriptional repression (19, 29, 53). One component of this repression is the association of LANA with corepressors such as mSin3 and histone deacetylases (29). The mechanisms of promoter-specific LANA targeting in KSHV-infected cells are incompletely understood; however, LANA can be detected in association with repressed cell promoters by chromatin immunoprecipitation assays, and long-term cell gene repression is in some cases linked to LANA-induced CpG methylation of the targeted promoter (54). The ability of LANA to epigenetically modify promoter DNA and repress transcription in this setting derives from a coordination of recruitment of de novo methyltransferases, the methyl CpG binding protein MeCP2, and the histone methyl transferase SUV39H1 (28, 50, 54). Given the association of LANA with multiple proteins that participate in transcriptional repression, it seems likely that upregulation of cell genes by LANA is mediated indirectly. Genes regulated by a variety of transcription factors (E2F, Sp1, Ap1, RBP-Jκ, ATF4, CBP, and Id-1) have been reported to be modulated by LANA (31, 33, 44, 58, 61). There is also an indirect regulation of Ets-1-responsive promoters through a LANA-Daxx interaction (37).
LANA-mediated stabilization of β-catenin contributes significantly to the positive changes in cell gene regulation seen in LANA-expressing cells (1, 18). Accumulation of β-catenin in KSHV-infected cells occurs as a consequence of LANA interaction with glycogen synthase kinase 3 (GSK-3). LANA mediates a cell cycle-regulated nuclear relocalization of GSK-3 which depletes GSK-3 from the cytoplasmic β-catenin destruction complex. β-Catenin then becomes available for the transcriptional activation of β-catenin-Tcf/Lef-regulated genes in the nucleus. LANA deletion variants that are unable to bind GSK-3 are defective for GSK-3 relocalization, β-catenin accumulation, and increased β-catenin gene regulatory activity (16, 18). There are two GSK-3 isoforms, GSK-3α and GSK-3β (42), and LANA binds to both forms (16). Interaction with GSK-3 requires two separate regions of LANA, an N-terminal region between amino acids 241 and 275 that contains multiple consensus sites for GSK-3 phosphorylation and a C-terminal domain that was initially mapped using C-terminal deletions and was confirmed by mutagenesis to be functionally homologous to the GSK-3 interaction domain of axin (16, 17). Efficient binding of LANA to GSK-3 is dependent on GSK-3 phosphorylation of the N-terminal domain, and mutagenesis of the phosphorylation sites abolishes the ability to coprecipitate GSK-3 with LANA (17).
Limited shuttling of GSK-3 into the nucleus occurs in cells during S phase (10) in response to apoptotic stimuli (67) and in senescent cells (74). GSK-3 phosphorylation of several known nuclear protein targets results in negative regulation of their function or a functionally negative outcome for the cell. For example, GSK-3 stimulates the transcriptional activity of p53, increasing p53 activation of proapoptotic and cell cycle arrest genes (66), and GSK-3 phosphorylation of c-Myc and c-Jun generates a high-affinity binding site for the E3 ligase Fbw7, which regulates polyubiquitination and proteasomal degradation of these proteins (68, 69). LANA-mediated relocalization of GSK-3 and increased levels of nuclear GSK-3 would consequently be expected to be problematic for cell survival in the absence of regulation of GSK-3 enzymatic activity. The GSK-3 associated with LANA was found to be capable of phosphorylating LANA but was unable to phosphorylate a primed peptide substrate in an in vitro kinase assay (17), suggesting that some form of negative regulation of nuclear GSK-3 activity might occur.
We have further addressed the status of GSK-3 in LANA-expressing cells and now show that LANA recruits extracellular signal-regulated kinase 1 (ERK1) and ribosomal S6 kinase 1 (RSK1), which together mediate the serine 9 inactivation of nuclear GSK-3β. Thus, LANA increases nuclear GSK-3 protein accumulation but decreases both cytoplasmic and nuclear GSK-3 activity. A number of transcription factors are nuclear substrates for GSK-3 (24). The consequences of a reduction of nuclear GSK-3 activity were evaluated by examining the phosphorylation and activity of C/EBP proteins which are known GSK-3 substrates and key participants in cellular differentiation (39, 48). We provide evidence that LANA expression blocks C/EBP phosphorylation, which translates, in a model differentiation system, into LANA-mediated interference with the terminal differentiation response.
MATERIALS AND METHODS
Plasmids and antibodies.
Flag-LANA, hemagglutinin (HA)-GSK-3β, and the glutathione S-transferase fusion constructs containing the N-terminal amino acids 1 to 340 of LANA (GST-LANA-N), the C-terminal amino acids 936 to 1162 of LANA (GST-LANA-C), and both the N-terminal and C-terminal regions of LANA [GST-LANA(C+N)] have been described previously (16, 29).
The antibodies used include anti-GSK-3β and anti-phospho-GSK-3β (Ser 9) (Transduction Laboratories); anti-LANA rat monoclonal (Advanced Biotechnologies, Inc.); anti-phospho-ERK1/2(Thr 202/Tyr 204), anti-ERK1/2, anti-RSK1, and anti-peroxisome proliferator-activated receptor gamma (anti-PPARγ) (Cell Signaling); anti-adiponectin, anti-C/EBPα, and anti-CEBPβ (Santa Cruz); and anti-Myc and anti-Flag (Sigma).
Cell culture and differentiation assays.
3T3L1 preadipocytes (obtained from Daniel Lane, Johns Hopkins School of Medicine) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). 3T3L1 cells were transduced with recombinant retroviruses either lacking an insert (3T3L1-vector) or containing an insert that encodes LANA(C+N) (3T3L1-LANA), and transduced cells were selected with 2 μg/ml puromycin. To induce differentiation, 3T3L1-derived cultures at 2 days postconfluence were switched to DMEM containing 10% FBS, 1 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine (designated day 0). Two days later, the cells were cultured in DMEM supplemented with 10% FBS and 1 μg/ml insulin for two additional days, after which culture medium (DMEM plus 10% FBS) was replaced every other day. Adipogenesis was visualized using oil red O staining. Cells were washed three times with prechilled 1× phosphate-buffered saline (PBS) (pH 7.4) and fixed for 5 min with 2% paraformaldehyde. Oil red O (0.5% in isopropanol) was diluted with water (3:2), filtered through a 0.45-μm filter, and incubated with the fixed cells for 1 h at room temperature. Cells were rinsed with water twice and the stained fatty droplets photographed by inverted light microscopy.
Immunoprecipitations.
HeLa cells seeded at 106 per 10-cm dish were transfected by calcium phosphate precipitation with Flag-LANA (7 μg) and HA-GSK-3β (7 μg) or Flag-LANA (7 μg) and Myc-ERK2-MEK1 (7 μg) (46). Cells were harvested 48 h posttransfection, resuspended in 2 ml of ice-cold lysis buffer (50 mM Tris-HCl [pH 7.8], 0.2% Nonidet P-40, 5% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM EDTA, 50 mM NaCl, 0.5 mM phenylmethylsulfonyl fluoride, 1 μg of pepstatin per ml, 5 μg of aprotinin per ml, 0.5 mM NaF, and 1 mM sodium pyrophosphate) and lysed with rotation for 30 min. Lysates were precleared by mixing them with Sepharose beads (50 μl) followed by centrifugation at 5,000 rpm for 5 min. The supernatant was subjected to a second centrifugation at 15,000 rpm for 15 min. The supernatant was then incubated with anti-HA monoclonal antibody (1 μg), anti-Myc monoclonal antibody (1 μg), or an anti-Flag M2 affinity gel for 2 h at 4°C, followed by incubation with protein G-Sepharose beads (50 μl, swollen volume) for 2 h at 4°C. The beads were washed four times with ice-cold lysis buffer and aliquoted for in vitro kinase assays, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting.
For immunoprecipitation, 108 BCBL1 PEL cells were lysed with 2 ml of ice-cold lysis buffer, and the lysates were centrifuged at 15,000 rpm for 15 min to remove the cell debris. Precleared lysates were then incubated with 1 μg of anti-ERK1/2 antibodies, anti-LANA antibody, or normal mouse immunoglobulin G (IgG) at 4°C for 2 h and further incubated with protein G-Sepharose beads at 4°C for 2 h. The resulting immunoprecipitates were washed and subjected to in vitro kinase assays and SDS-PAGE analyses.
Cell viability assay.
The CellTiter-Glo luminescence assay kit (Promega) was employed to assess the relative levels of cellular ATP as a measure of cell viability. 3T3L1, 3T3L1-LANA(C+N), and 3T3L1-vector preadipocytes cultured in 6-well plates were lysed with prechilled RIPA buffer (50 mM Tris-HCl [pH 7.4], 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA) supplemented with protease inhibitor cocktail and treated with CellTiter-Glo reagent according to the manufacturer's instructions. Fluorescence was measured using clear tissue culture plates (Promega). Cell viability was quantified by measurement of the sample fluorescence intensity at 560 nm/590 nm.
In vitro kinase assay.
To assay GSK-3β kinase activity, immunoprecipitates were washed twice with GSK-3 kinase buffer (50 mM Tris-HCl [pH 7.9], 10 mM MgCl2, 2 mM DTT, 100 mM NaCl, 20 μM cold ATP). The resulting beads were resuspended in 20 μl of kinase buffer containing 10 μCi of [γ-32P]ATP with 20 μM of primed glycogen synthase substrate peptide [RRRPASVPPSPSLSRHS(pS)HQRR; Upstate]. Reaction mixtures were incubated for 30 min at 30°C and then washed twice with ice-cold washing buffer (50 mM Tris-HCl [pH 7.9], 10 mM MgCl2, 2 mM DTT, 100 mM NaCl) and separated by Tris-Tricine gel electrophoresis. Radiolabeled polypeptides were detected by autoradiography. Immunoprecipitates were assayed for ERK1/2 kinase activity after being washed twice with ERK kinase buffer (25 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 1 mM DTT, 1 mM EGTA, 20 μM cold ATP). The resulting beads were resuspended in 20 μl of kinase buffer containing 10 μCi of [γ-32P]ATP with 20 μM of synthetic polypeptide corresponding to LANA amino acids 256 to 277 and containing a consensus mitogen-activated protein kinase (MAPK) site and a consensus GSK-3 site (PRQPPTSPISIGSSSPSEGSWG) or with a control peptide containing only a consensus GSK-3 site (VGSSSDGETPPRQ). Peptides were synthesized by the Johns Hopkins Core Facility. Reaction mixtures were incubated for 30 min at 30°C and then separated by Tris-Tricine gel electrophoresis. Radiolabeled polypeptides were detected by autoradiography. To assay RSK1 activity, immunoprecipitates were prepared as described above, incubated with GST-GSK-3β, and then separated and analyzed as described above.
To examine phosphorylation of LANA expressed in prokaryotic cells, GST-LANA-N, GST-LANA-C, and GST-LANA(C+N) were prepared as previously described (29). Bound proteins were washed twice with ERK kinase buffer and then incubated in ERK kinase buffer with 10 μCi of [γ-32P]ATP and 25 mU of activated ERK1 (Upstate) for 30 min at 30°C. The polypeptides were separated and autoradiographed as described above.
To examine ERK1 priming phosphorylation for GSK-3, GST and GST-LANA-N were washed twice with kinase buffer and then (i) incubated in kinase buffer with 10 μCi of [γ-32P]ATP and 0.1 U of GSK-3β (Cell Signaling) for 30 min at 30°C, (ii) incubated in 20 μM cold ATP and 25 mU of activated ERK1 (Upstate) for 30 min at 30°C and washed six times with lysis buffer followed by incubation in reaction buffer containing 10 uCi of [γ-32P]ATP, or (iii) treated as described above [see (ii)] but with the addition of 0.1 U of GSK-3β (Cell Signaling). Samples were separated by SDS-PAGE and analyzed by autoradiography.
In vivo labeling with [32P]orthophosphate.
Two days after transfection, HeLa cells were rinsed once with 1× PBS, pH 7.4, washed once with phosphate-free DMEM, starved in phosphate-free DMEM plus 10% dialyzed FBS medium for 1 h, and incubated for 4 h with the same medium containing [32P]orthophosphate (carrier free; Amersham) (4 mCi/10-cm dish, 250 μCi/ml), with or without 25 mM LiCl. Labeling was stopped by washing the cells twice with ice-cold PBS, and labeled cells were lysed in 1 ml of ice-cold lysis buffer. C/EBPα or C/EBPβ was purified by immunoprecipitation and separated by SDS-PAGE, followed by autoradiography and Western blotting.
RESULTS
Association of serine 9-phosphorylated, inactivated GSK-3 with LANA.
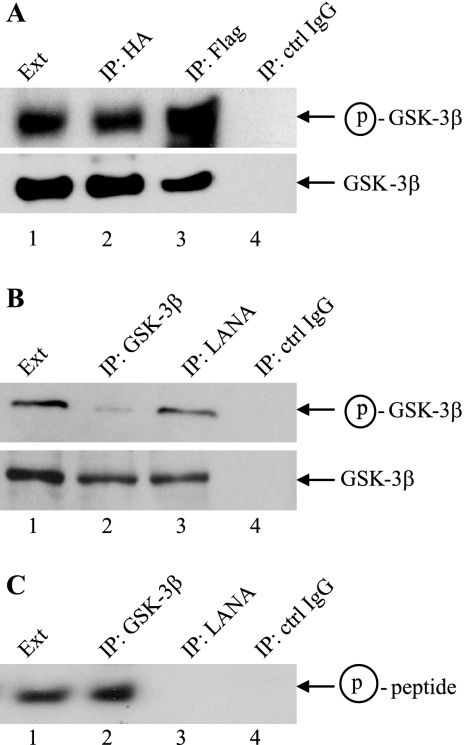
We have previously shown that LANA-bound GSK-3 was able to phosphorylate LANA but was ineffective at phosphorylating a primed peptide substrate in vitro (17). The activity of nuclear GSK-3 was further examined using anti-GSK-3β antibody that is specific for the serine 9-phosphorylated, inactive form of GSK-3β. LANA-mediated relocalization of GSK-3 is cell cycle dependent, and in unsynchronized cultures the majority of GSK-3 remains cytoplasmic. In cotransfected HeLa cells, the ratio of serine 9-phosphorylated HA-GSK-3β to total HA-GSK-3β was greater for HA-GSK-3β that coprecipitated with Flag-LANA and was therefore nuclear than for HA-GSK-3β that was directly precipitated from the cells with anti-HA antibody and that represented total cellular GSK-3 (Fig. 1A, compare lanes 3 and 2). The same increased ratio of serine 9-phosphorylated GSK-3β to total GSK-3β was observed when GSK-3β from BCBL1 PEL cells was either coprecipitated with LANA or directly precipitated (Fig. 1B, compare lanes 3 and 2). Examination of GSK-3 enzymatic activity in these same BCBL1 fractions showed that the increased serine 9 phosphorylation of LANA-associated GSK-3β isolated from PEL cells correlated with an inability of this GSK-3β to effectively phosphorylate a primed peptide substrate (Fig. 1C). In an in vitro kinase assay, the peptide was phosphorylated by total PEL extract (Fig. 1C, lane 1) and by directly precipitated GSK-3β (Fig. 1C, lane 2) but not by the control IgG immunoprecipitate (Fig. 1C, lane 4) or by GSK-3β that coprecipitated with LANA (Fig. 1C, lane 3).
FIG. 1.
LANA bound GSK-3β is phosphorylated at Ser 9 and is inactive in an in vitro kinase assay. (A) Western blots probed with anti-Ser 9-GSK-3β (upper) or anti-GSK-3β (lower) antibodies to determine the phosphorylation status of HA-GSK-3β in Flag-LANA-cotransfected HeLa cells. Lane 1, transfected cell extract (Ext; 5% of that used for immunoprecipitation [IP]); lane 2, anti-HA precipitate; lane 3, anti-Flag precipitate; lane 4, control IgG precipitate. (B) Western blots probed with anti-Ser 9-GSK-3β (upper) or anti-GSK-3β (lower) antibodies to determine the phosphorylation status of GSK-3β in BCBL1 PEL cells. Lane 1, BCBL1 cell extract (5% of that used for immunoprecipitation); lane 2, anti-GSK-3β precipitate; lane 3, anti-LANA precipitate; lane 4, control IgG precipitate. (C) In vitro kinase assay using the BCBL1 samples from (B) and a primed GSK-3 peptide substrate. The peptide was subjected to gel electrophoresis and autoradiography to detect incorporation of [γ-32P]ATP. Lane 1, BCBL1 cell extract; lane 2, anti-GSK-3β precipitate; lane 3, anti-LANA precipitate; lane 4, control IgG precipitate. Circled p, phospho.
LANA interacts with enzymatically active ERK1/2.
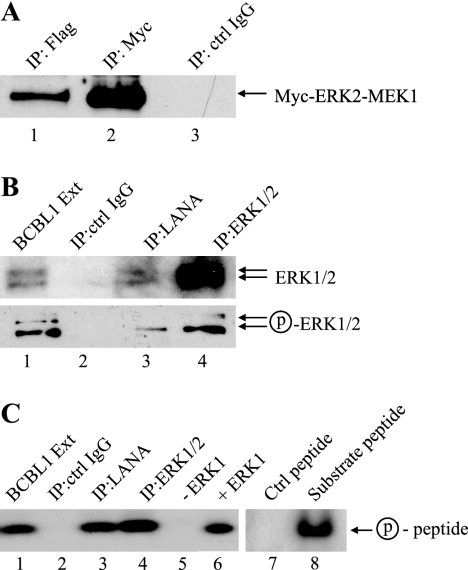
It has recently been found that one mechanism of GSK-3 inactivation is via ERK phosphorylation of GSK-3β at Thr 43, which primes GSK-3β for subsequent phosphorylation at Ser 9 by RSK1 (ribosomal S6 kinase 1; p90RSK) (11). We therefore tested whether there was any interaction between LANA and ERK1/2. Immunoprecipitation assays performed on cotransfected HeLa cells revealed that Myc-tagged ERK2-MEK1 (a constitutively activated form of ERK2) coprecipitated with Flag-LANA (Fig. 2A, lane 1). Figure 2A shows direct immunoprecipitation of Myc-ERK2-MEK1 (lane 2) and lack of precipitation by control IgG (lane 3). Endogenous LANA immunoprecipitated from BCBL1 PEL cells was also found to coprecipitate endogenous ERK1/2 (Fig. 2B, lane 3). Probing of the Western blot with an antibody that recognizes phosphorylated, activated ERK1/2 gave a positive signal, suggesting that at least some of the ERK1/2 associated with LANA was in the enzymatically active form (Fig. 2A, lower panel, lane 3). Phosphorylated ERK1/2 was also detected in the BCBL1 cell extract and in the direct ERK1/2 immunoprecipitate (Fig. 2A, lower panel, lanes 1 and 4). Phosphorylation of ERK is associated with ERK activation, and the detection of phospho-ERK1/2 in the anti-LANA immunoprecipitates suggested that LANA was associated with enzymatically active ERK. To address this point, an in vitro kinase assay was performed using the same BCBL1 samples that are shown in Fig. 2B and a MAPK peptide substrate (Fig. 2C). An extract prepared from BCBL1 PEL cells phosphorylated the peptide, indicating that PEL cells contain activated MAPKs (Fig. 2C, lane 1). The peptide was also phosphorylated by ERK1/2 directly immunoprecipitated from BCBL1 PEL cells with anti-ERK1/2 antibodies (Fig. 2C, lane 4) and by the MAPK that was coprecipitated with LANA in an anti-LANA immunoprecipitate (Fig. 2C, lane 3). No kinase activity was present in a control IgG immunoprecipitate (Fig. 2C, lane 2). In the controls containing kinase and peptide (Fig. 2C, lanes 6 and 8), the peptide was incubated in kinase buffer containing purified ERK1, and in the control without kinase (Fig. 2C, lane 5), the peptide was incubated with kinase buffer only. A control peptide that lacked a MAPK consensus sequence was not phosphorylated by purified ERK1, indicating the specificity of the kinase assay (Fig. 2C, lane 7). Thus, LANA associates with enzymatically active MAPK(s), and ERK1/2 is a likely candidate.
FIG. 2.
LANA coprecipitates with phospho-ERK1/2. (A) Western blot probed with anti-Myc antibody showing coprecipitation of Myc-ERK2-MEK1 with Flag-LANA in cotransfected HeLa cells. Lane 1, anti-Flag coprecipitate; lane 2, anti-Myc direct precipitate; lane 3, control (ctrl) IgG precipitate. (B) Western blots probed with anti-ERK1/2 antibody (upper) and anti-phospho-ERK1/2 antibody (lower) to determine the phosphorylation status of endogenous LANA bound ERK1/2 in BCBL1 PEL cells. Lane 1, BCBL1 cell extract (Ext; 5% of that used for immunoprecipitation [IP]); lane 2, control IgG precipitate; lane 3, anti-LANA precipitate; lane 4, anti-ERK1/2 precipitate. (C) In vitro ERK1/2 kinase assay using the BCBL1 samples shown in panel B and a MAPK peptide substrate. The autoradiograph examines the incorporation of [γ-32P]ATP in the presence of BCBL1 extract (lane 1), control IgG precipitate (lane 2), anti-LANA precipitate (lane 3), anti-ERK1/2 precipitate (lane 4), no added kinase (lane 5), purified ERK1 (lane 6), control peptide plus ERK1 kinase (lane 7), and substrate peptide plus ERK1 kinase (lane 8). Circled p, phospho. Lanes 7 and 8 were processed separately.
ERK1/2 phosphorylates LANA and can prime LANA for GSK-3 phosphorylation.
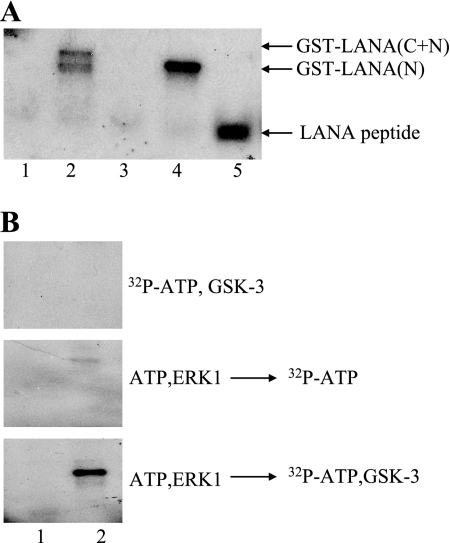
Coprecipitation of ERK1/2 with LANA could be a consequence of the fact that LANA is a substrate for ERK1/2 phosphorylation. To test this possibility, purified, commercially available ERK1 was incubated with bacterially expressed, purified GST and GST-LANA fusion proteins in an in vitro kinase assay (Fig. 3A). GST was not phosphorylated by ERK1 in this assay, and neither was the GST-LANA-C fusion protein. (Fig. 3A, lanes 1 and 3). The GST fusion proteins containing the LANA N terminus and the LANA N terminus plus C terminus were phosphorylated by ERK1 (Fig. 3A, lanes 2 and 4). Amino acid Thr 254 in the LANA N terminus is a predicted substrate for MAPKs, and we have previously shown that p38 MAPK could phosphorylate Thr 254 in vitro (17). A synthetic peptide representing LANA amino acids 246 to 258 was included in the assay and was readily phosphorylated by ERK1 (Fig. 3A, lane 5). The phosphorylation of the peptide by ERK1 was more robust than that previously seen with p38 MAPK, suggesting that ERK1 may be the more biologically relevant MAPK for phosphorylation of LANA Thr 254.
FIG. 3.
ERK1 phosphorylates LANA and can prime for GSK-3 phosphorylation in vitro. (A) In vitro ERK1 kinase assay. The autoradiograph examines the incorporation of [γ-32P]ATP into control GST (lane 1), GST-LANA(C+N) (lane 2), GST-LANA-C (lane 3), GST-LANA-N (lane 4), and synthetic peptide LANA(246-258) (lane 5). (B) In vitro ERK1 priming and GSK-3 kinase assays. The autoradiograph examines the incorporation of [γ-32P]ATP in a GSK-3 kinase assay performed without priming (top), a control ERK1 priming kinase assay to detect ERK background activity (middle), and an assay in which ERK1 primes for GSK-3 phosphorylation (bottom). Lane 1, control GST; lane 2, GST-LANA-N.
The ability of ERK1 to phosphorylate LANA and the LANA(246-258) peptide raised the possibility that ERK might also be one of the kinases capable of priming LANA for GSK-3 phosphorylation. An in vitro kinase assay was performed using control GST protein and GST-LANA-N (Fig. 3B). As previously shown, GSK-3 was unable to phosphorylate LANA-N in the absence of preincubation with a priming kinase (Fig. 3B, upper panel). The GST proteins were then primed with ERK1 using cold ATP in the kinase buffer, washed extensively, and incubated with kinase buffer containing [32P]ATP plus purified GSK-3β (Fig. 3B, lower panel). GSK-3 phosphorylation of GST-LANA-N was observed (Fig. 3B, lower panel, lane 2), indicating that ERK1 can prime LANA for GSK-3 phosphorylation. A control assay was also performed in which the ERK-primed GST proteins were subsequently incubated with [32P]ATP containing kinase buffer to ensure that the signal observed in the GSK-3 assay did not result from residual ERK1 activity that was not removed by washing. The washing step was effective at removing most of the added ERK1, and only a weak background signal was observed for the GST-LANA fusion protein in the control assay (Fig. 3B, center panel, lane 2).
RSK1 coprecipitates with LANA.
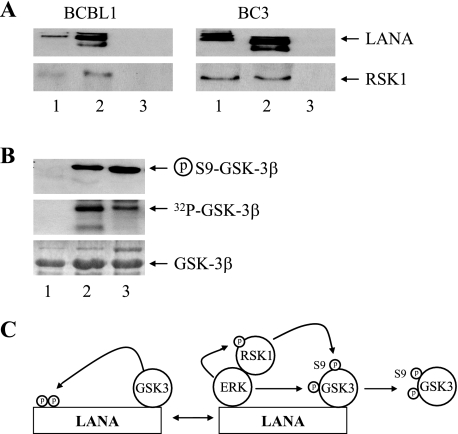
ERK1/2 can act as an adaptor and priming kinase by binding to GSK-3β and facilitating the phosphorylation of GSK-3β at Ser 9 by the ERK1/2-associated kinase RSK1 (p90RSK) (11). The discovery of an ERK1/2 association with LANA led us to investigate whether RSK1 was also complexed with LANA. RSK1 is a serine-threonine kinase that is a member of the ribosomal S6 kinase family (15). The kinase activity of RSK1 is activated upon ERK phosphorylation of the C-terminal kinase domain, and RSK1 contains an ERK docking site at the C terminus (20, 55). Western blot analysis of immunoprecipitates from BCBL1 and BC3 PEL cells revealed that endogenous RSK1 present in the PEL cell extracts (Fig. 4A, lanes 1) coprecipitated with LANA in an anti-LANA immunoprecipitate (Fig. 4A, lanes 2) and was not detected in control immunoglobulin precipitate (Fig. 4A, lanes 3). To demonstrate that the LANA-associated RSK/ERK complex was capable of phosphorylating GSK-3β at Ser 9, bacterially expressed and purified GST-GSK-3β was incubated in vitro with the anti-LANA immunoprecipitate from BCBL1 cells (Fig. 4B, lane 2), with a control IgG BCBL1 immunoprecipitate (Fig. 4B, lane 1), or with commercial RSK1 and ERK1 (Fig. 4B, lane 3). A Western blot probed with anti-Ser 9-GSK-3 antibody revealed that GST-GSK-3β became phosphorylated at Ser 9 after incubation with either the anti-LANA immunoprecipitate or purified RSK1 plus ERK1 (Fig. 4B, upper panel). Phosphorylation of the GSK-3β could also be observed using autoradiography (Fig. 4B, middle panel). The input GST-GSK-3β protein used in each kinase assay is shown by Coomassie brilliant blue staining (Fig. 4B, lower panel). Thus, RSK1 is also associated with LANA, and the data support a model of LANA-induced GSK-3β Ser 9 inactivation mediated by the tethered ERK1/2 and RSK1 kinases (Fig. 4C).
FIG. 4.
LANA-associated RSK1 phosphorylates GSK-3β. (A) Western blots probed with anti-LANA (upper) or anti-RSK1 (lower) antibodies showing coprecipitation of RSK1 and LANA from BCBL1 and BC3 PEL cell extracts. Lane 1, cell extract; lane 2, anti-LANA immunoprecipitate; lane 3, control IgG immunoprecipitate. The extract lane contained 5% of the protein used in immunoprecipitation. (B) In vitro phosphorylation assay showing Ser 9 inactivation of GSK-3β by ERK1 plus RSK1. GST-GSK-3β was incubated with kinase buffer containing [γ-32P]ATP and control IgG immunoprecipitate from BCBL1 cells (lane 1), anti-LANA immunoprecipitate from BCBL1 cells (lane 2), or purified RSK1 and ERK1 (lane 3). Samples were separated by SDS-PAGE. A Western blot was probed with anti-Ser 9-GSK-3β antibody (upper). Samples were also subjected to autoradiography (middle) and Coomassie brilliant blue staining (lower). (C) Summary of LANA-associated ERK1/RSK-mediated serine 9 phosphorylation of nuclear GSK-3. Circled p, phospho.
LANA blocks GSK-3 phosphorylation of the transcription factors C/EBPα and C/EBPβ.
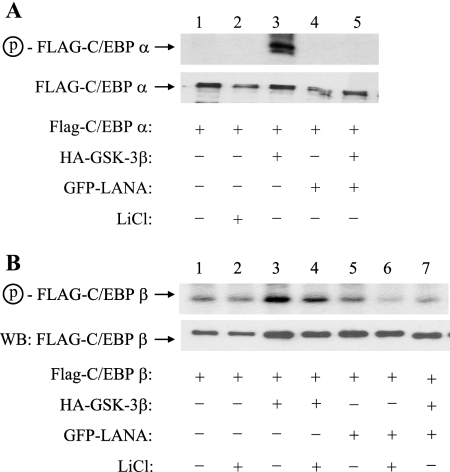
LANA-associated GSK-3 is inactivated to the extent that it is ineffective at phosphorylating a peptide substrate in vitro. If the majority of nuclear GSK-3 is inactive in the presence of LANA, then this would be expected to affect the phosphorylation of nuclear GSK-3 substrates. To examine this point, GSK-3 phosphorylation of two known GSK-3 substrates, C/EBPα and C/EBPβ, was evaluated in transfected HeLa cells. Cells were transfected with Flag-C/EBPα (Fig. 5A) or Flag-C/EBPβ (Fig. 5B) alone or in the presence of HA-GSK-3β or green fluorescent protein (GFP)-LANA or cotransfected with HA-GSK-3β plus GFP-LANA. Expression of the Flag-C/EBP proteins in the transfected cell extracts was demonstrated by Western blotting (Fig. 5A and B, lower panels). Phosphorylation of the Flag-C/EBP proteins was detected by pulsing the transfected cells with [32P]orthophosphate for 4 h prior to harvesting and immunoprecipitation with anti-Flag antibody. No phosphorylation of C/EBPα was detectable in the absence of cotransfected GSK-3β (Fig. 5A, lanes 1 and 2). Cotransfection of LANA alone had no apparent effect, but LANA plus GSK-3β eliminated detectable C/EBPα phosphorylation (Fig. 5A, lane 4).
FIG. 5.
GSK-3 phosphorylation of C/EBPα and C/EBPβ is inhibited by LANA. (A) HeLa cells transfected with Flag-C/EBPα, HA-GSK-3β, and GFP-LANA, as indicated, were metabolically labeled for 4 h with [32P]orthophosphate 2 days posttransfection. Flag-C/EBPα was immunoprecipitated with anti-Flag M2 affinity beads and subjected to Western blotting to detect Flag-C/EBPα (lower) and to autoradiography to detect 32P-labeled C/EBPα (upper). Detection of phosphorylated Flag-C/EBPα required cotransfection with HA-GSK-3β (lane 3), and this phosphorylation was blocked by cotransfected LANA (lane 5). (B) HeLa cells transfected with Flag-C/EBPβ, HA-GSK-3β, and GFP-LANA, as indicated, were metabolically labeled with [32P]orthophosphate as described above. Flag-C/EBPβ was immunoprecipitated with anti-Flag M2 affinity beads and subjected to Western blotting to detect Flag-C/EBPβ (lower) and to autoradiography to detect 32P-labeled C/EBPβ (upper). 32P labeling of Flag-C/EBPβ increased in the presence of HA-GSK-3β (lane 3), and this increase was abolished by cotransfection of LANA (lane 7). LiCl is a GSK-3 inhibitor. Circled p, phospho.
The basal level of phosphorylation of transfected Flag-C/EBPβ was minimally affected by the addition of the GSK-3 inhibitor LiCl, indicating that this basal C/EBPβ phosphorylation was not predominantly GSK-3 mediated (Fig. 5B, lanes 1 and 2). The increased phosphorylation of Flag-C/EBPβ seen with cotransfection of GSK-3β is indicative of GSK-3 phosphorylation (Fig. 5B, lanes 1 and 3). The addition of LANA alone had little effect on the basal level of endogenous C/EBPβ phosphorylation (Fig. 5B, lanes 1 and 5) and significantly decreased the phosphorylation that occurred in the presence of cotransfected GSK-3β (Fig. 5B, lanes 3 and 7).
LANA blocks differentiation-associated adipogenesis.
To obtain evidence that LANA-mediated changes in C/EBP phosphorylation could have functional consequences, we sought a biological assay system in which C/EBP proteins are known to be critical. One such well-characterized system is induction of adipogenesis in 3T3L1 preadipocytes. Key effectors of this cascade are C/EBPβ, C/EBPα, and PPARγ, since cells lacking these proteins do not undergo adipogenesis (3, 47). In adipogenesis, C/EBPβ induction precedes that of C/EBPα and PPARγ, and C/EBPβ upregulation of C/EBPα and PPARγ is dependent upon prior C/EBPβ modification. Transactivation by C/EBPβ requires GSK-3 phosphorylation that is primed by MAPK phosphorylation (59), and the MAPK that has been implicated in C/EBPβ phosphorylation is ERK1/2 (40). Active C/EBPβ is phosphorylated by MAP/ERK kinases at Thr 188 and subsequently GSK-3 phosphorylated at Ser 184 and Thr 179 (40, 59).
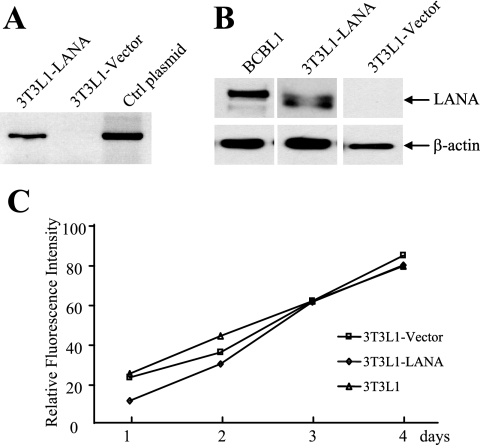
Passaging 3T3L1 cells leads to a loss of differentiation potential, and hence the establishment of clonal cell lines of LANA-expressing cells was not practical. Cultures enriched for LANA expression were obtained by retroviral transduction of a LANA construct with the central repeat region deleted, followed by selection for 3 days in puromycin. 3T3L1 cells transduced with the empty retroviral vector were selected in parallel and served as controls for subsequent experiments. Expression of LANA in the transduced cells was confirmed by reverse transcriptase-PCR for LANA transcripts (Fig. 6A) and by Western blotting for LANA protein (Fig. 6B). The amount of LANA protein expressed in the converted 3T3L1 cells was similar to that seen in BCBL1 PEL cells (Fig. 6B). The growth rate of the 3T3L1-LANA and 3T3L1-vector cells was not grossly altered from that of the parental 3T3L1 cells (Fig. 6C).
FIG. 6.
Expression of LANA in transduced 3T3L1 preadipocytes. (A) Verification of LANA expression in retrovirus-transduced, puromycin-selected 3T3L1 cultures. Reverse transcriptase PCR detected LANA expression in 3T3L1-LANA but not in 3T3L1-vector transduced cultures. The LANA plasmid formed a positive PCR control. Ctrl, control. (B) Western blot probed with anti-LANA antibody comparing LANA expression with that in BCBL1 PEL cells. (C) Comparison of the growth rates of the parental 3T3L1 and transduced 3T3L1-vector and 3T3L1-LANA cultures. Cell growth was measured using CellTiter-Glo.
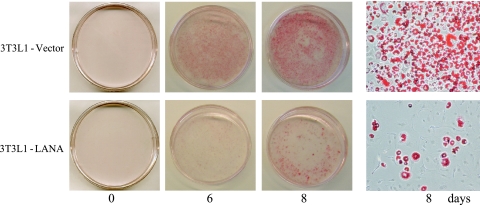
The ability of the 3T3L1-vector and 3T3L1-LANA cells to undergo differentiation and adipogenesis after stimulation with a cocktail of insulin, dexamethasone, and isobutyl-1-methylxanthine was examined by incubating the cells with oil red O, which stains accumulated fat droplets (60) (Fig. 7). In the control 3T3L1-vector cells, no red staining was visible on day 0, but there was significant staining on day 6 after treatment and an increased intensity of oil red O staining on day 8. The 3T3L1-LANA culture showed both a delay in the development of staining which was not readily apparent until day 8 and a severe reduction in the numbers of the cells exhibiting oil red O staining (Fig. 7, lower panel).
FIG. 7.
LANA inhibits differentiation of 3T3L1 preadipocytes. 3T3L1-vector and 3T3L1-LANA cultures were subjected to a standard 3T3L1 differentiation protocol. Cells were fixed and incubated with oil red O to stain accumulated fat droplets on days 0, 6, and 8 postinduction. Right panel, high-magnification photomicrograph of day 8 cultures (×200).
LANA inhibits induction of C/EBPα and PPARγ.
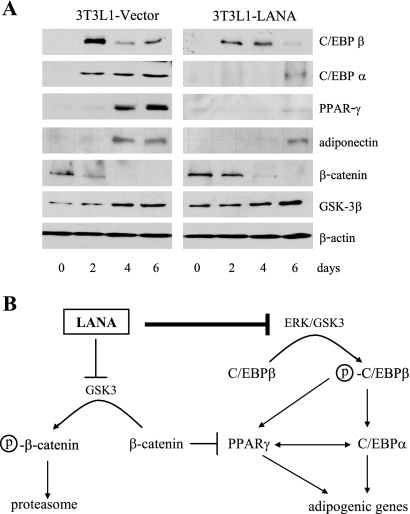
Expression of the key proteins required for adipogenesis was examined in extracts of the induced 3T3L1-LANA and 3T3L1-vector cells (Fig. 8A). In the vector-transduced cells, C/EBPβ expression was maximal at day 2, and expression decreased over the following days. C/EBPα was initially detected at day 2 after induction, and C/EBPα expression continued through day 6. PPARγ was first detected on day 4 postinduction and showed increased expression on day 6. The adipocyte-specific protein adiponectin, which is a marker for adipogenesis, was also detected on days 4 and 6 after induction. This profile of sequential gene expression is characteristic of 3T3L1 cells undergoing adipogenesis (39, 40). In the 3T3L1-LANA culture, C/EBPβ expression was similar to that in the vector control cells in that expression peaked on day 2 and then declined (Fig. 8A). However, induction of C/EBPα and PPARγ was delayed in the 3T3L1-LANA cells relative to that in vector control cells and was significantly reduced. The delay in adiponectin expression in the 3T3L1-LANA cells is consistent with the delayed and impaired induction of C/EBPα and PPARγ, which together regulate adiponectin. Western blotting for LANA found no change in expression over the time course of the experiment (data not shown).
FIG. 8.
Expression of adipogenic mediators and markers in 3T3L1-vector and 3T3L1-LANA cultures. (A) Western blots examining expression of the differentiation regulators C/EBPβ, C/EBPα, PPARγ, β-catenin, and GSK-3β and the differentiation marker adiponectin in 3T3L1-vector and 3T3L1-LANA cultures subjected to a standard differentiation protocol. (B) Summary of the differentiation process showing the regulatory events blocked by LANA. Circled p, phospho.
Wnt signaling in preadipocytes stabilizes β-catenin and blocks adipogenesis by inhibiting induction of C/EBPα and PPARγ (6). LANA also stabilizes β-catenin by blocking GSK-3-mediated β-catenin degradation. To evaluate the potential contribution of this LANA function, β-catenin expression was also examined. β-Catenin expression was initially slightly higher in the 3T3L1-LANA cells than in the 3T3L1-vector control cells. However, β-catenin expression was downregulated by day 4 in both 3T3L1-LANA and 3T3L1-vector cultures (Fig. 8A), and so β-catenin expression seems insufficient to account for the observed reduction in PPARγ and C/EBPα expression. GSK-3β itself was expressed in both 3T3L1-vector and 3T3L1-LANA cells (Fig. 8A). Since C/EBPβ was induced with the same kinetics in both 3T3L1-LANA and 3T3L1-vector cells, the impaired induction of its downstream targets C/EBPα and PPARγ is most likely the result of impaired GSK-3 phosphorylation and activation of C/EBPβ. A summary of the proposed mechanism for interference with C/EBP-dependent differentiation is presented in Fig. 8B.
DISCUSSION
The discovery that LANA stabilizes β-catenin, the nuclear effector of the Wnt signaling pathway, through an interaction with GSK-3 led to the following model: GSK-3 entering the nucleus in early S phase is bound by LANA, which interacts through an axin/Frat-like C-terminal GSK-3 interaction domain. This interaction prevents the nuclear export of GSK-3 leading to a cell cycle-regulated cytoplasmic depletion of GSK-3. GSK3 is then unavailable for participation in the cytoplasmic β-catenin destruction complex, in which GSK-3 phosphorylation of β-catenin targets the protein for ubiquitination and proteasomal degradation (18). One question immediately raised by this model was the status of the nuclear GSK-3 associated with LANA. GSK-3 phosphorylation has been documented to be a signal that leads to protein degradation and growth inhibitory or proapoptotic outcomes (26). GSK-3 has a large number of potential substrates in the nucleus, including transcription factors and the cell cycle regulatory proteins Myc, cyclin D1, and cyclin E (10, 69, 70). Increasing the amount of nuclear GSK-3 would therefore seem to be problematic for cell survival.
A previous examination of GSK-3 activity found that LANA-bound GSK-3 phosphorylated multiple consensus GSK-3 sites in the N terminus of LANA. Moreover, phosphorylation of the LANA N terminus was necessary for high-affinity interaction between LANA and GSK-3 (17). GSK-3 is a constitutively active kinase that is regulated by inactivation mediated by phosphorylation at Ser 9 of GSK-3β or Ser 21 of GSK-3α (9). In the cytoplasm, Akt is one of the kinases that mediates Ser 9/Ser 21 phosphorylation of GSK-3 (71), and this inactivation of GSK-3 contributes to the antiapoptotic effects of Akt in part by preventing GSK-3 phosphorylation of the BCL-2 family member MCL-1, an event that targets MCL-1 for ubiquitinylation and degradation (36). Probing with anti-Ser 9 GSK-3β-specific antibody revealed that the Ser 9/total GSK-3β ratio was greater for LANA-associated GSK-3β than for the total cell extract. Stabilization of β-catenin in hepatitis B virus-infected cells has been ascribed to a Ser 9 inactivation of GSK-3β that is brought about by phosphorylation at Ser 9 by RSK1/p90RSK, an event that is dependent upon an interaction between RSK1 and ERK kinase and ERK priming phosphorylation of GSK-3β (11). When we tested to determine whether a similar mechanism might account for the Ser 9 inactivation of LANA, we found that LANA coprecipitated with ERK1/2 and RSK1. The LANA-associated ERK1/2 reacted with phospho-ERK antibody, indicating the presence of an activated form. We had previously found that p38 MAPK was able to prime for GSK-3 phosphorylation of the LANA N terminus. We now show that the ERK1 MAPK family member is also capable of priming for GSK-3, and ERK1/2 may be the biologically more relevant priming kinase. The kinases present in anti-LANA immunoprecipitates isolated from PEL cells were capable of mediating Ser 9 phosphorylation of GSK-3 in vitro. The evidence suggests that Ser 9 inactivation of GSK-3 is mediated by the concerted activity of LANA-tethered ERK1/2 and RSK1 kinases.
The observation that the GSK-3 bound to LANA is enriched for Ser 9-inactivated GSK-3 initially seems at odds with the fact that LANA is a GSK-3 substrate. However, integrating these two findings leads to a model (Fig. 9) in which the activity of nuclear GSK-3 in LANA-expressing cells can be regulated. Enzymatically active GSK-3 entering the nucleus in early S phase or upon apoptotic stimulation is bound by LANA in a manner that is regulated by GSK-3 phosphorylation of the LANA N terminus. LANA can also associate with ERK1/2 and RSK1. The combined activity of these kinases inactivates GSK-3β by Ser 9 phosphorylation. The inactivated GSK-3 has a reduced affinity for LANA and is released into the nucleoplasm. In this way, the LANA-mediated increase in nuclear GSK-3 would not lead to an increased phosphorylation of nuclear protein substrates. On the contrary, the sequestering of LANA-associated enzymatically active GSK-3 would produce an overall reduction in nuclear GSK-3 activity in LANA-expressing cells. This last possibility was tested for two known GSK-3 nuclear substrates, C/EBPβ and C/EBPα, and evidence was obtained that LANA can block GSK-3 phosphorylation of both C/EBPα and C/EBPβ in transfected cells.
FIG. 9.
Proposed mechanisms of LANA-mediated inhibition of nuclear GSK-3 activity and downstream modification of cell gene expression. LANA binds to GSK-3 entering the nucleus in S phase and prevents nuclear export, resulting in nuclear accumulation of GSK-3. GSK-3 phosphorylation of LANA is required for interaction between the two proteins. LANA also binds ERK1/2 and RSK1, which participate in Ser 9 inactivation of GSK-3. Thus, active nuclear GSK-3 is LANA bound, whereas inactivated GSK-3 is released from LANA, with the paradoxical outcome that LANA-mediated nuclear accumulation of GSK-3 is associated with a reduction in nuclear GSK-3 enzymatic activity and phosphorylation of downstream targets. Changes in transcription factor phosphorylation are likely to be reflected in a reprogramming of cell gene expression. Circled p, phospho.
C/EBPs (CCAAT enhancer binding proteins) are a family of bZIP transcription factors that contain N-terminal domains that regulate transcriptional activity and C-terminal DNA binding and dimerization domains. C/EBP proteins regulate the transition from proliferation to cell cycle arrest and differentiation through both transcriptional and non-DNA-binding mechanisms. C/EBPα is phosphorylated by GSK-3 on Thr 222 and Thr 226 (Thr 226 and Thr 230 in human C/EBPα) (34, 49). The functional consequence of C/EBPα phosphorylation at these residues is not clear, but phosphorylation is believed to change the structure of the C/EBPα protein and may affect binding site preferences. In the preadipocyte differentiation system, phosphorylation of C/EBPβ at Thr 188 by MAPK is required for the mitotic expansion and terminal differentiation activities of C/EBPβ, while subsequent hyperphosphorylation by GSK-3 at Ser 184 and Thr 179 is necessary for the efficient upregulation of C/EBPα- and C/EBPα-regulated genes (59). Transduction of 3T3L1 preadipocytes with LANA did not affect the growth rate of the cells but did impair their ability to differentiate into adipocytes. The block in LANA-expressing cells could be accounted for by the inefficient induction of C/EBPα (Fig. 8Α).
In most cases, GSK-3 phosphorylation is a negative regulator of substrate protein stability or activity. GSK-3β phosphorylation of C/EBPβ is an exception in its impact on C/EBPα expression and cellular differentiation. Differentiation and proliferation are antagonistic processes, and hence the ability of LANA to block differentiation would enhance the KSHV-associated proliferative response. In addition, KSHV lytic gene expression is dependent upon a C/EBPα-driven exit from the cell cycle (64, 72) and, in the case of Epstein-Barr virus, lytic gene expression has been shown to be associated with a more differentiated cell phenotype (30). LANA-mediated antagonism of the differentiation process would therefore also serve to reinforce KSHV latency at the expense of lytic induction. A variety of other transcription factors are substrates for GSK-3 phosphorylation (7). LANA-mediated depletion of nuclear GSK-3 activity could consequently have a significant impact on cellular gene reprogramming.
Acknowledgments
We thank Daniel Lane (Johns Hopkins School of Medicine) for 3T3L1 cells and advice on the adipogenesis assay and Melanie Cobb (University of Texas Southwestern Medical Center) for the Myc-ERK2-MEK1 expression plasmid.
This work was supported by PHS grant PO1 CA113239 to S.D.H.
Footnotes
Published ahead of print on 21 February 2007.
REFERENCES
- 1.An, F. Q., N. Compitello, E. Horwitz, M. Sramkoski, E. S. Knudsen, and R. Renne. 2005. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus modulates cellular gene expression and protects lymphoid cells from P16INK4A-induced cell cycle arrest. J. Biol. Chem. 280:3862-3874. [DOI] [PubMed] [Google Scholar]
- 2.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 3.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 4.Barbera, A. J., M. E. Ballestas, and K. M. Kaye. 2004. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 N terminus is essential for chromosome association, DNA replication, and episome persistence. J. Virol. 78:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbera, A. J., J. V. Chodaparambil, B. Kelley-Clarke, V. Joukov, J. C. Walter, K. Luger, and K. M. Kaye. 2006. The nucleosomal surface as a docking station for Kaposi's sarcoma herpesvirus LANA. Science 311:856-861. [DOI] [PubMed] [Google Scholar]
- 6.Bennett, C. N., S. E. Ross, K. A. Longo, L. Bajnok, N. Hemati, K. W. Johnson, S. D. Harrison, and O. A. MacDougald. 2002. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 277:30998-31004. [DOI] [PubMed] [Google Scholar]
- 7.Beurel, E., and R. S. Jope. 2006. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog. Neurobiol. 79:173-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotter, M. A. II, C. Subramanian, and E. S. Robertson. 2001. The Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen binds to specific sequences at the left end of the viral genome through its carboxy-terminus. Virology 291:241-259. [DOI] [PubMed] [Google Scholar]
- 9.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 10.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding, Q., W. Xia, J. C. Liu, J. Y. Yang, D. F. Lee, J. Xia, G. Bartholomeusz, Y. Li, Y. Pan, Z. Li, R. C. Bargou, J. Qin, C. C. Lai, F. J. Tsai, C. H. Tsai, and M. C. Hung. 2005. Erk associates with and primes GSK-3beta for its inactivation resulting in upregulation of beta-catenin. Mol. Cell 19:159-170. [DOI] [PubMed] [Google Scholar]
- 12.Dittmer, D., M. Lagunoff, R. Renne, K. Staskus, A. Haase, and D. Ganem. 1998. A cluster of latently expressed genes in Kaposi's sarcoma-associated herpesvirus. J. Virol. 72:8309-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fejer, G., M. M. Medveczky, E. Horvath, B. Lane, Y. Chang, and P. G. Medveczky. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus interacts preferentially with the terminal repeats of the genome in vivo and this complex is sufficient for episomal DNA replication. J. Gen. Virol. 84:1451-1462. [DOI] [PubMed] [Google Scholar]
- 14.Friborg, J. J., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 15.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol. 151:65-77. [DOI] [PubMed] [Google Scholar]
- 16.Fujimuro, M., and S. D. Hayward. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus manipulates the activity of glycogen synthase kinase-3β. J. Virol. 77:8019-8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimuro, M., J. Liu, J. Zhu, H. Yokosawa, and S. D. Hayward. 2005. Regulation of the interaction between glycogen synthase kinase 3 and the Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen. J. Virol. 79:10429-10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimuro, M., F. Y. Wu, C. ApRhys, H. Kajumbula, D. B. Young, G. S. Hayward, and S. D. Hayward. 2003. A novel viral mechanism for dysregulation of beta-catenin in Kaposi's sarcoma-associated herpesvirus latency. Nat. Med. 9:300-306. [DOI] [PubMed] [Google Scholar]
- 19.Garber, A. C., J. Hu, and R. Renne. 2002. Latency-associated nuclear antigen (LANA) cooperatively binds to two sites within the terminal repeat, and both sites contribute to the ability of LANA to suppress transcription and to facilitate DNA replication. J. Biol. Chem. 277:27401-27411. [DOI] [PubMed] [Google Scholar]
- 20.Gavin, A. C., and A. R. Nebreda. 1999. A MAP kinase docking site is required for phosphorylation and activation of p90(rsk)/MAPKAP kinase-1. Curr. Biol. 9:281-284. [DOI] [PubMed] [Google Scholar]
- 21.Grundhoff, A., and D. Ganem. 2003. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus permits replication of terminal repeat-containing plasmids. J. Virol. 77:2779-2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu, J., A. C. Garber, and R. Renne. 2002. The latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus supports latent DNA replication in dividing cells. J. Virol. 76:11677-11687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu, J., and R. Renne. 2005. Characterization of the minimal replicator of Kaposi's sarcoma-associated herpesvirus latent origin. J. Virol. 79:2637-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jope, R. S., and G. V. Johnson. 2004. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem. Sci. 29:95-102. [DOI] [PubMed] [Google Scholar]
- 25.Katano, H., Y. Sato, T. Kurata, S. Mori, and T. Sata. 1999. High expression of HHV-8-encoded ORF73 protein in spindle-shaped cells of Kaposi's sarcoma. Am. J. Pathol. 155:47-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoepfler, P. S., and A. M. Kenney. 2006. Neural precursor cycling at sonic speed: N-Myc pedals, GSK-3 brakes. Cell Cycle 5:47-52. [DOI] [PubMed] [Google Scholar]
- 27.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krithivas, A., M. Fujimuro, M. Weidner, D. B. Young, and S. D. Hayward. 2002. Protein interactions targeting the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus to cell chromosomes. J. Virol. 76:11596-11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krithivas, A., D. B. Young, G. Liao, D. Greene, and S. D. Hayward. 2000. Human herpesvirus 8 LANA interacts with proteins of the mSin3 corepressor complex and negatively regulates Epstein-Barr virus gene expression in dually infected PEL cells. J. Virol. 74:9637-9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan, K., D. A. Kuppers, and E. S. Robertson. 2005. Kaposi's sarcoma-associated herpesvirus reactivation is regulated by interaction of latency-associated nuclear antigen with recombination signal sequence-binding protein Jκ, the major downstream effector of the Notch signaling pathway. J. Virol. 79:3468-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim, C., D. Lee, T. Seo, C. Choi, and J. Choe. 2003. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus functionally interacts with heterochromatin protein 1. J. Biol. Chem. 278:7397-7405. [DOI] [PubMed] [Google Scholar]
- 33.Lim, C., H. Sohn, Y. Gwack, and J. Choe. 2000. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) binds ATF4/CREB2 and inhibits its transcriptional activation activity. J. Gen. Virol. 81:2645-2652. [DOI] [PubMed] [Google Scholar]
- 34.Liu, H. K., S. Perrier, C. Lipina, D. Finlay, H. McLauchlan, C. J. Hastie, H. S. Hundal, and C. Sutherland. 2006. Functional characterisation of the regulation of CAAT enhancer binding protein alpha by GSK-3 phosphorylation of Threonines 222/226. BMC Mol. Biol. 7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsumura, S., Y. Fujita, E. Gomez, N. Tanese, and A. C. Wilson. 2005. Activation of the Kaposi's sarcoma-associated herpesvirus major latency locus by the lytic switch protein RTA (ORF50). J. Virol. 79:8493-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurer, U., C. Charvet, A. S. Wagman, E. Dejardin, and D. R. Green. 2006. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol. Cell 21:749-760. [DOI] [PubMed] [Google Scholar]
- 37.Murakami, Y., S. Yamagoe, K. Noguchi, Y. Takebe, N. Takahashi, Y. Uehara, and H. Fukazawa. 2006. Ets-1-dependent expression of vascular endothelial growth factor receptors is activated by latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus through interaction with Daxx. J. Biol. Chem. 281:28113-28121. [DOI] [PubMed] [Google Scholar]
- 38.Ottinger, M., T. Christalla, K. Nathan, M. M. Brinkmann, A. Viejo-Borbolla, and T. F. Schulz. 2006. Kaposi's sarcoma-associated herpesvirus LANA-1 interacts with the short variant of BRD4 and releases cells from a BRD4- and BRD2/RING3- induced G1 cell cycle arrest. J. Virol. 80:10772-10786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otto, T. C., and M. D. Lane. 2005. Adipose development: from stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 40:229-242. [DOI] [PubMed] [Google Scholar]
- 40.Park, B.-H., L. Qiang, and S. R. Farmer. 2004. Phosphorylation of C/EBPβ at a consensus extracellular signal-regulated kinase/glycogen synthase kinase 3 site is required for the induction of adiponectin gene expression during the differentiation of mouse fibroblasts into adipocytes. Mol. Cell. Biol. 24:8671-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel, S., B. Doble, and J. R. Woodgett. 2004. Glycogen synthase kinase-3 in insulin and Wnt signalling: a double-edged sword? Biochem. Soc. Trans. 32:803-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piolot, T., M. Tramier, M. Coppey, J.-C. Nicolas, and V. Marechal. 2001. Close but distinct regions of human herpesvirus 8 latency-associated nuclear antigen 1 are responsible for nuclear targeting and binding to human mitotic chromosomes. J. Virol. 75:3948-3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 45.Renne, R., C. Barry, D. Dittmer, N. Compitello, P. O. Brown, and D. Ganem. 2001. Modulation of cellular and viral gene expression by the latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus. J. Virol. 75:458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson, M. J., S. A. Stippec, E. Goldsmith, M. A. White, and M. H. Cobb. 1998. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr. Biol. 8:1141-1150. [DOI] [PubMed] [Google Scholar]
- 47.Rosen, E. D., C. H. Hsu, X. Wang, S. Sakai, M. W. Freeman, F. J. Gonzalez, and B. M. Spiegelman. 2002. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 16:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosmarin, A. G., Z. Yang, and K. K. Resendes. 2005. Transcriptional regulation in myelopoiesis: Hematopoietic fate choice, myeloid differentiation, and leukemogenesis. Exp. Hematol. 33:131-143. [DOI] [PubMed] [Google Scholar]
- 49.Ross, S. E., R. L. Erickson, N. Hemati, and O. A. MacDougald. 1999. Glycogen synthase kinase 3 is an insulin-regulated C/EBPα kinase. Mol. Cell. Biol. 19:8433-8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sakakibara, S., K. Ueda, K. Nishimura, E. Do, E. Ohsaki, T. Okuno, and K. Yamanishi. 2004. Accumulation of heterochromatin components on the terminal repeat sequence of Kaposi's sarcoma-associated herpesvirus mediated by the latency-associated nuclear antigen. J. Virol. 78:7299-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarid, R., J. S. Wiezorek, P. S. Moore, and Y. Chang. 1999. Characterization and cell cycle regulation of the major Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) latent genes and their promoter. J. Virol. 73:1438-1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz, T. F. 2006. The pleiotropic effects of Kaposi's sarcoma herpesvirus. J. Pathol. 208:187-198. [DOI] [PubMed] [Google Scholar]
- 53.Schwam, D. R., R. L. Luciano, S. S. Mahajan, L. Wong, and A. C. Wilson. 2000. Carboxy terminus of human herpesvirus 8 latency-associated nuclear antigen mediates dimerization, transcriptional repression, and targeting to nuclear bodies. J. Virol. 74:8532-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shamay, M., A. Krithivas, J. Zhang, and S. D. Hayward. 2006. Recruitment of the de novo DNA methyltransferase Dnmt3a by Kaposi's sarcoma-associated herpesvirus LANA. Proc. Natl. Acad. Sci. USA 103:14554-14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, J. A., C. E. Poteet-Smith, K. Malarkey, and T. W. Sturgill. 1999. Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem. 274:2893-2898. [DOI] [PubMed] [Google Scholar]
- 56.Staudt, M. R., and D. P. Dittmer. 2006. Promoter switching allows simultaneous transcription of LANA and K14/vGPCR of Kaposi's sarcoma-associated herpesvirus. Virology 350:192-205. [DOI] [PubMed] [Google Scholar]
- 57.Talbot, S. J., R. A. Weiss, P. Kellam, and C. Boshoff. 1999. Transcriptional analysis of human herpesvirus-8 open reading frames 71, 72, 73, K14, and 74 in a primary effusion lymphoma cell line. Virology 257:84-94. [DOI] [PubMed] [Google Scholar]
- 58.Tang, J., G. M. Gordon, M. G. Müller, M. Dahiya, and K. E. Foreman. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen induces expression of the helix-loop-helix protein Id-1 in human endothelial cells. J. Virol. 77:5975-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang, Q. Q., M. Gronborg, H. Huang, J. W. Kim, T. C. Otto, A. Pandey, and M. D. Lane. 2005. Sequential phosphorylation of CCAAT enhancer-binding protein beta by MAPK and glycogen synthase kinase 3beta is required for adipogenesis. Proc. Natl. Acad. Sci. USA 102:9766-9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tang, Q. Q., T. C. Otto, and M. D. Lane. 2003. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 100:850-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verma, S. C., S. Borah, and E. S. Robertson. 2004. Latency-associated nuclear antigen of Kaposi's sarcoma-associated herpesvirus up-regulates transcription of human telomerase reverse transcriptase promoter through interaction with transcription factor Sp1. J. Virol. 78:10348-10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viejo-Borbolla, A., M. Ottinger, E. Brüning, A. Bürger, R. König, E. Kati, J. A. Sheldon, and T. F. Schulz. 2005. Brd2/RING3 interacts with a chromatin-binding domain in the Kaposi's Sarcoma-associated herpesvirus latency-associated nuclear antigen 1 (LANA-1) that is required for multiple functions of LANA-1. J. Virol. 79:13618-13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 64.Wang, S. E., F. Y. Wu, Y. Yu, and G. S. Hayward. 2003. CCAAT/enhancer-binding protein-α is induced during the early stages of Kaposi's sarcoma-associated herpesvirus (KSHV) lytic cycle reactivation and together with the KSHV replication and transcription activator (RTA) cooperatively stimulates the viral RTA, MTA, and PAN promoters. J. Virol. 77:9590-9612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe, T., M. Sugaya, A. M. Atkins, E. A. Aquilino, A. Yang, D. L. Borris, J. Brady, and A. Blauvelt. 2003. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen prolongs the life span of primary human umbilical vein endothelial cells. J. Virol. 77:6188-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watcharasit, P., G. N. Bijur, L. Song, J. Zhu, X. Chen, and R. S. Jope. 2003. Glycogen synthase kinase-3beta (GSK3beta) binds to and promotes the actions of p53. J. Biol. Chem. 278:48872-48879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Watcharasit, P., G. N. Bijur, J. W. Zmijewski, L. Song, A. Zmijewska, X. Chen, G. V. Johnson, and R. S. Jope. 2002. Direct, activating interaction between glycogen synthase kinase-3beta and p53 after DNA damage. Proc. Natl. Acad. Sci. USA 99:7951-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wei, W., J. Jin, S. Schlisio, J. W. Harper, and W. G. Kaelin, Jr. 2005. The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 8:25-33. [DOI] [PubMed] [Google Scholar]
- 69.Welcker, M., A. Orian, J. Jin, J. E. Grim, J. W. Harper, R. N. Eisenman, and B. E. Clurman. 2004. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc. Natl. Acad. Sci. USA 101:9085-9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Welcker, M., J. Singer, K. R. Loeb, J. Grim, A. Bloecher, M. Gurien-West, B. E. Clurman, and J. M. Roberts. 2003. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell 12:381-392. [DOI] [PubMed] [Google Scholar]
- 71.Woodgett, J. R. 2005. Recent advances in the protein kinase B signaling pathway. Curr. Opin. Cell Biol. 17:150-157. [DOI] [PubMed] [Google Scholar]
- 72.Wu, F. Y., Q. Q. Tang, H. Chen, C. ApRhys, C. Farrell, J. Chen, M. Fujimuro, M. D. Lane, and G. S. Hayward. 2002. Lytic replication-associated protein (RAP) encoded by Kaposi sarcoma-associated herpesvirus causes p21CIP-1-mediated G1 cell cycle arrest through CCAAT/enhancer-binding protein-alpha. Proc. Natl. Acad. Sci. USA 99:10683-10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.You, J., V. Srinivasan, G. V. Denis, W. J. Harrington, Jr., M. E. Ballestas, K. M. Kaye, and P. M. Howley. 2006. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen interacts with bromodomain protein Brd4 on host mitotic chromosomes. J. Virol. 80:8909-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zmijewski, J. W., and R. S. Jope. 2004. Nuclear accumulation of glycogen synthase kinase-3 during replicative senescence of human fibroblasts. Aging Cell 3:309-317. [DOI] [PMC free article] [PubMed] [Google Scholar]