Immune evasion strategies are thought to contribute to successful persistence of viruses in the infected immunocompetent host, delaying elimination of the infected cell long enough to enable the virus to replicate. Exemplary in this context are herpesviruses, which are carried as persistent infections by the vast majority of adults (65).
Immune evasion strategies have been identified for members of all three subfamilies, for example, herpes simplex virus (HSV; an alphaherpesvirus), human cytomegalovirus (HCMV; a betaherpesvirus), and Epstein-Barr virus (EBV; a gammaherpesvirus). In the infected cell, herpesviruses use general evasive approaches, such as blocking the induction of programmed cell death and shutting down host protein synthesis (65). In addition, herpesviruses specifically perturb recognition by virus-specific T cells, among others. To this end, herpesviruses appear to exploit a combination of characteristics. First, they are able to establish a latent state of infection, in which exposure of viral antigens to the immune system is limited due to an expression profile that is greatly restricted both in the amount and in the copy number of the viral genes expressed. This strategy forces antiviral CD4+ and CD8+ T cells directed against, for instance, EBV latent antigens (17) to always operate at their detection limit. Second, herpesviruses dedicate part of their genomes to functions that modulate immune recognition, suppressing antiviral detection and elimination mechanisms. Most specific immune evasion strategies described to date allow viruses to escape from major histocompatibility complex (MHC) class I-restricted cytotoxic T cells. As reviewed previously, every step of the class I processing and presentation pathway appears to be thwarted by one or more herpesviruses (4, 47, 80, 87, 89). In contrast, much less is known about viral interference with MHC class II-restricted immune responses; in the following sections, we will therefore discuss recent developments in this field, with an emphasis on the human herpesviruses.
INDICATIONS FOR HERPESVIRUS INTERFERENCE WITH HLA CLASS II PRESENTATION TO T-HELPER CELLS
Expression of MHC (HLA in humans) class II molecules is generally limited to cells with a specialized role in antigen presentation, although other cell types can be induced to express them—by gamma interferon (IFN-γ), for instance. At the cell surface, mature MHC class II complexes present peptide epitopes to T-cell receptors (TCR) on CD4+ T-helper cells (16). T-helper cells play a central role in antiviral immunity: they are essential for the induction and maintenance of effective CD8+ T-cell immunity (8), provide help for humoral immune responses, and can exert cytotoxic activity. Direct killing by CD4+ T cells has now been shown to play an important role in protective immunity against many herpesviruses (3, 6, 15, 20, 26, 27, 30, 45, 68, 70, 73, 85).
In view of the importance of MHC class II-restricted T cells, herpesviruses might also corrupt this part of the immune system. Indeed, the last years have revealed indications of downregulated HLA class II surface expression and/or reduced CD4+ T-cell recognition for cells expressing herpesvirus-encoded gene products, and these will be described in detail below.
Most HLA class II immunoevasins are expected to be expressed during productive infection, because transmission of herpesviruses to another host requires the production of infectious virions through a replicative cycle, involving the synthesis of a vast array of potential viral antigens (80 to 200 viral proteins are synthesized, depending on the herpesvirus species) (65). Studies on immune evasion strategies in full viral context are greatly influenced by the availability of an appropriate in vitro culture system that allows efficient viral infection. In this respect, there are major differences among members of the herpesvirus family. Infection of certain cell types with alpha- or betaherpesviruses, such as HSV and HCMV, can result in full lytic virus replication. This has led to the early observations of, for instance, reduced surface expression of HLA class II complexes on the surfaces of cells supporting productive infections with HCMV (11, 56, 57) and HSV type 1 (HSV-1) (81).
In contrast, gammaherpesvirus infection in vitro generally results in latent gene expression (with concomitant growth transformation). Recently, in vitro systems that enable analysis at the single-cell level and/or enrichment for cells that have entered the replicative cycle have been developed for two human gammaherpesviruses (22, 62). Using such systems, immune evasion strategies, including those targeting HLA class II-restricted T-cell activation, can now be explored for cells undergoing replication of these gammaherpesviruses. Thus, in cells supporting lytic EBV replication, a reduction in the surface display of HLA class I and class II complexes has become apparent (35, 62). Still, data have not always been consistent in that, for instance, some groups have not been able to confirm the abovementioned downregulation of HLA class II at the surfaces of cells expressing HCMV gene products (82) or productively infected by EBV (40). Inhibition of the synthesis or assembly of MHC class I and class II complexes does not quickly alter their surface levels. Still, the presentation of viral antigens by newly synthesized MHC molecules may be inhibited despite normal surface expression of these molecules. MHC molecules present at the cell surface, but dating from before virus infection, do not contain viral peptides and are therefore not recognized by virus-specific T cells. The use of cell lines that (stably) express the viral protein(s) of interest is more likely to present a clear phenotype and, accordingly, has been essential in elucidating the mechanisms underlying most known immunomodulatory functions of herpesviruses.
All in all, cellular expression systems are used differently in studies addressing viral immune evasion strategies, and this has an important impact on the results observed. Despite this fact, the combined data point toward specific viral modulation of antigen presentation in the context of HLA class II. The disruption of HLA class II-restricted antigen presentation to T-helper cells appears to occur at various levels (described in detail below). We will first discuss posttranslational interference with assembly and trafficking of newly synthesized class II complexes, as this affects both constitutive and IFN-γ-induced expression of HLA class II. Other mechanisms of herpesvirus-induced class II evasion will be dealt with thereafter.
PROCESSING AND PRESENTATION OF VIRAL ANTIGENS IN THE CONTEXT OF HLA CLASS II
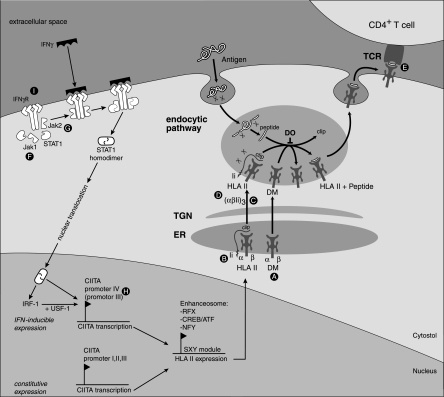
To permit an easy understanding of the specific mechanisms viruses have for perturbing HLA class II-restricted immune recognition, normal antigen processing and presentation has been summarized in Fig. 1. Mature HLA class II molecules are composed of αβ heterodimers loaded with peptide (16, 59). In the endoplasmic reticulum (ER), newly synthesized class II α and β chains associate with the invariant chains (Ii). Signals in the cytoplasmic tail of the Ii direct αβIi complexes from the trans-Golgi network toward acidic compartments. Proteases within these compartments cleave the Ii chain to enable peptide binding within the HLA class II peptide-binding groove. The process of peptide editing is aided by the class II-like chaperones HLA-DM and HLA-DO. Stable HLA class II αβ peptide complexes then proceed from the MHC class II loading compartment to the cell surface, where they can be recognized by specific CD4+ T-helper cells. Peptides presented in the context of HLA class II are generally derived from exogenous antigens that enter the endocytic pathway by phagocytosis or receptor-mediated endocytosis and are degraded by endolysosomal proteases. In this way, fragments of virus-infected cells are taken up by dendritic cells (DC) for cross-presentation. In addition, transmembrane and ER-lumenal/secreted proteins enter the sites of antigen processing for class II presentation. Viral envelope proteins, including the HCMV glycoprotein B (gB), which travels through the endosomal pathway as part of the virion assembly process, are examples of antigens that follow this route (19, 27).
FIG. 1.
Herpesvirus downregulation of HLA class II expression. Posttranscriptional interference: HCMV US2-mediated dislocation and subsequent degradation of HLA-DRα and DMα (A) results in decreased formation of HLA class II-Ii complexes in the ER and impaired loading of HLA class II with peptide downstream from the Golgi apparatus. Displacement of Ii by HSV gB (B) results in impaired peptide loading. HCMV US3 (C) causes retention of HLA class II-Ii complexes in the Golgi apparatus while HCMV pp65 (D) targets HLA class II complexes to abnormal perinuclear lysosomes. HLA class II-TCR interactions are inhibited by EBV gp42 (E). Other gene products of note are HSV γ34.5 and UL41, which act by an unknown mechanism (consequently, not shown). Pretranscriptional interference (affecting IFN-γ-induced expression only): interference with the function of IFN-γ-associated signaling molecules occurs during infection with HCMV (Jak1 [F]), HSV-1 (STAT1, STAT2, and Jak1 [F]), and also VZV (STAT1α and Jak2 [G]). Further inhibition occurs downstream at the level of CIITA promoter IV during HCMV infection (H). The EBV BZLF1 gene product decreases the levels of IFN-γ receptor α, thereby shutting down the entire IFN-γ-induced CIITA signaling cascade (I). HLA II, HLA class II; TGN, trans-Golgi network; RFX, regulatory factor x; ATF, activating transcription factor; IRF-1, IFN regulatory factor 1; USF-1, upstream stimulatory factor 1.
Finally, the routes by which access can be gained into the different HLA pathways are not so strict: peptides from newly synthesized cytosolic proteins can also enter the vesicular compartments to associate with HLA class II. Although this route of direct presentation is potentially less efficient, recent experimental data point toward effective HLA class II-restricted presentation of several virus-derived cytosolic antigens, such as HCMV IE1 (41) and EBV EBNA1 (45, 54, 86). Autophagy was presented to provide a mechanism through which cytosolic EBNA1 gains access to the endocytic HLA class II antigen presentation pathway (58).
Processing of viral proteins via the MHC class I and class II pathways ultimately results in the presentation of viral antigens to the immune system during latency and, in particular, during the replicative phase of herpesvirus infection. As a result, T-cell responses against a variety of herpesvirus-encoded proteins are observed. Nevertheless, these viruses cannot be eliminated from the host, indicating that they have acquired highly effective escape mechanisms to elude the immune response.
HERPESVIRUS GENE PRODUCTS THWARTING HLA CLASS II ASSEMBLY AND SURFACE EXPRESSION POSTTRANSLATIONALLY
HCMV US2, US3, and pp65 (UL83).
Research on the downregulation of HLA molecules on the surfaces of infected cells has revealed the involvement of HCMV gene products encoded in the US2-11 region (34). Among the viral proteins accounting for HLA class I downregulation is US2, which is expressed at early times of infection and was found to induce rapid proteasomal degradation of nascent HLA class I heavy chains following dislocation from the ER membrane into the cytoplasm (88). By expressing US2 in HLA class II+ cell lines, Tomazin et al. (79) found that US2 was also capable of inhibiting class II expression. Immunoprecipitation studies showed that the HLA-DR α-chain (HLA-DRα) was rapidly degraded by proteasomes in US2-expressing cells, while levels of DRβ and Ii remained largely unaffected. The C-terminal domain of US2 plays an essential role in the degradation process, possibly by recruiting the AAA ATPase complex cdc48/p97 into the ER-associated degradation complex (13, 14). Guided by the partial homology of HLA-DM chains with HLA-DR chains, DMα was also found to be degraded in US2-expressing cells, while DMβ was unaffected compared to the controls. It is somewhat surprising to find that one gene product can bind to structures which share only a limited number of structural features. The shared immunoglobulin domains can be excluded on the basis that they are also found in HLA-DRβ and β2-microglobulin. Therefore, the authors proposed that US2 binds the α1 domain distal to the membrane on HLA-DRα, -DMα, and class I heavy chains, in addition to the US2-binding region identified in the α2-α3 junction of class I heavy chains (21). Finally, IFN-γ production by T cells, specific for an exogenous antigen presented by HLA class II, was reduced to 20% to 30% when the antigen-presenting cells (APC) expressed US2 (79). The latter observation provides evidence that the disruption of HLA class II assembly by US2 has functional effects in vitro. The effects of US2 on HLA class II locus products were observed in a variety of cell lines, including biologically relevant epithelial cells (28).
Another HCMV gene product, US3, stably binds developing HLA class II αβ complexes in the ER, effecting the displacement of Ii, whose association with αβ complexes was reduced three- to fourfold in US3-expressing cells compared with US3-negative controls (29). Furthermore, US3 was not found in the αβIi complexes that did exist. The subsequent trafficking of HLA class II complexes is determined by various sequences present in Ii. Complexes binding US3 instead of Ii appeared to make normal progress to the Golgi apparatus but seemed to be unable to proceed effectively to the acidic loading compartments, indicating that the binding of US3 results in mislocalization of HLA class II molecules. In this way, US3 was demonstrated to cause downregulated expression of HLA class II at the cell surface.
The HCMV-encoded IE gene UL83 product pp65 has been identified as the third HLA class II-downregulating protein (11, 56, 57). Whereas late-stage inhibition of HLA class II (4 days postinfection) appeared to be dependent primarily on products of the HCMV US2-11 gene cassette, early inhibition (1 day postinfection) was found independent of viral replication, indicating that a structural component of the virus might be responsible (57). Odeberg et al. characterized the causative agent of the early phase of HLA class II downregulation as involving pp65 in cells infected with wild-type HCMV, because the effect was absent during infection with a pp65 knockout strain (56). Transcription of HLA-DR was unaffected, but the newly formed HLA class II complexes trafficked into perinuclear lysosomes, where the HLA-DRα chain was degraded (56). This result fits well with earlier observations that HCMV interferes with the trafficking of HLA class II molecules en route to the cell surface (11). Since Ii expression was not altered and colocalized with HLA class II in the infected cells, an HCMV-induced lesion in Ii-dependent trafficking was unlikely. Rather, HLA class II complexes remained sequestered in the peptide-loading compartments, and these compartments adopted an abnormal perinuclear distribution. Interestingly, in this context, HCMV was suggested to disrupt cytoskeletal trafficking of vesicles (18). Whether this altered cytoskeletal transport can indeed explain the unusual localization of the HLA class II-loading compartments merits specific investigation.
HSV-1 gB.
Trgovcich et al. (81) observed that productive infection with HSV-1 resulted in a twofold reduction of HLA class II surface expression compared to mock-infected cells. HSV-1 infection did not markedly affect the total amount of HLA class II proteins, but this is likely related to the long half-life of these molecules. Although the mechanism for class II downregulation for HSV-1-infected cells remains elusive, work using deletion mutants suggested involvement of the UL41 gene product and the γ134.5-infected cell protein (81). Some caution should, however, be taken in interpreting these results, since γ134.5 deletion mutants do not replicate well. Given the strong host shutoff function of UL41 in HSV-1 affecting cellular protein synthesis, UL41 could well account for a generalized block to class II presentation. A single report (55) suggests that HSV-1 gB specifically impairs peptide loading of HLA class II complexes but, in view of the substantial effects of HSV infection on the organization of cellular organelles and trafficking of membrane proteins, it is difficult to separate these generalized effects from potential specific effects on HLA class II.
EBV gp42.
Surface expression of HLA class II molecules has been shown to be downregulated on B cells that have entered the lytic cycle of EBV replication (35, 62). As yet, no molecular mechanism has been elucidated that explains this phenomenon at a molecular level.
Without affecting the levels of surface HLA class II expression, EBV gp42, encoded by the BZLF2 open reading frame, has been shown to interfere with HLA class II-restricted antigen presentation (63, 64, 75). As a viral envelope protein, gp42 was identified to act as a cofactor for B-cell infection by binding to its cellular receptor, HLA class II (43, 44, 75). Another function of EBV gp42 appears to be the impairment of antigen presentation in the context of HLA class II (63, 64, 75). Endogenous expression of gp42 in cells resulted in the formation of gp42:HLA-DRαβ peptide complexes and inhibition of CD4+ T-helper cells specific for various antigens. By using HLA-DR tetramers to directly visualize TCR-class II interactions, the mechanism of immune evasion mediated by EBV gp42 was demonstrated to rely on blockage of TCR engagement. A superimposition of the crystal structures of gp42:HLA-DR1 and TCR:HLA-DR1 revealed a steric clash between gp42 and the TCR Vα domain (63). Furthermore, in an analysis of the biosynthesis and maturation of gp42 in cells stably expressing the viral protein, gp42 was found in two forms: a full-length type II membrane protein and a truncated soluble form (64). The soluble form, s-gp42, is generated by proteolytic cleavage in the ER and is secreted. s-gp42 is sufficient to inhibit HLA class II-restricted antigen presentation to T cells. In an almost pure population of B cells in the lytic EBV cycle, both transmembrane and soluble forms of gp42 are detected. These results imply that s-gp42 is generated during lytic EBV infection and could contribute to undetected virus production by mediating evasion from T-cell immunity (64). The EBV gp42-mediated interference with TCR interactions represents a new theme in viral perturbation of HLA class II-restricted immune responses and merits further investigation with other members of the herpesvirus family.
VIRAL INTERFERENCE WITH IFN-γ-INDUCIBLE HLA CLASS II EXPRESSION
While professional APC, such as DC, macrophages, B cells, and thymic epithelial cells, constitutively express HLA class II molecules, other cell types, including epithelial and endothelial cells, may be stimulated to express HLA class II molecules by various cytokines, most notably IFN-γ. Expression of HLA class II molecules is exquisitely controlled at the transcriptional level, through the class II transactivator (CIITA) that exhibits cell-specific, cytokine-inducible, and differentiation-dependent expression (7, 78, 84) (Fig. 1).
Because CIITA is a master regulator of HLA class II gene transcription, it represents an ideal target for pathogens trying to evade the immune system. Indeed, HCMV, HSV-1, and varicella-zoster virus (VZV), among others, have evolved several pathways to alter the (IFN-γ-induced) HLA class II gene transcription, as summarized in Fig. 1 (1, 2, 12, 42, 48, 49, 69, 90). Although no single responsible viral gene product has been identified yet, these herpesviruses appear to affect more general target(s) upstream of CIITA expression and, thus, the effects will be far reaching, beyond HLA class II regulation.
So far, productive EBV infection has not been shown to directly corrupt inducible HLA class II gene transcription. However, cellular expression of a single EBV gene product, the IE transactivator BZLF1, disrupted the IFN-γ signaling pathway, thereby preventing IFN-γ-induced HLA class II surface expression (51). Mechanistically, BZLF1 was shown to decrease the cellular level of the ligand binding subunit of the IFN-γR (IFN-γRα), thereby abrogating cellular responses to IFN-γ in cells expressing BZLF1 (Fig. 1I). Since a transcriptional block to all IFN-γ-inducible HLA class II expression would shut down the activation of T-helper cells required to initiate an immune response, these results suggest a mechanism by which EBV may escape early antiviral immune responses during primary infection.
IL-10
Cellular interleukin 10 (IL-10) is known for its capacity to suppress expression of (among others) HLA class II complexes on human monocytes by preventing their progress from the loading compartment to the cell surface (36). A very interesting observation in herpesvirus immunobiology has been the identification of genes coding for viral IL-10 homologues within the genomes of HCMV, EBV, rhesus CMV, and murine CMV (MCMV) (38, 66). Indeed, the HCMV IL-10 homologue (cmvIL-10) is capable of instigating downregulated HLA class I and class II expression on monocytes (74). Similarly, the EBV BCRF-1 open reading frame product downregulates HLA class II expression (both constitutive and IFN-γ induced) as well as that of HLA-I, CD80/CD86, ICAM-1 (66), and TAP1 (91).
In this context, considerable knowledge is available to date for MCMV. Downregulation of MHC class II expression in MCMV-infected macrophages is achieved at least in part by the generation of IL-10 (61). Within 24 h of infection, depletion of constitutive cell surface MHC class II was observed with murine macrophages both in vitro and in vivo. This result was supported by the observation that macrophages from IL-10-deficient mice displayed elevated levels of MHC class II expression, although it is important to realize that IL-10-deficient mice are known to also have higher serum levels of IFN-γ than normal mice (39). The immediacy of this observed effect is important. In normal immune responses, the production of IL-10 occurs late in infection, and it is generally thought that it functions to damp down the response and thus prevent the development of immunopathology. If, however, this powerful cytokine is induced almost immediately postinfection, it will tend to prevent the mounting of an effective immune response by acting on numerous components of the immune system, such as by inhibiting of the production of inflammatory cytokines.
All in all, the induction of (viral) IL-10 upon herpesvirus infection has broader implications than its effects on MHC class I and class II expression alone.
IMPLICATIONS FOR ANTIGEN PRESENTATION OF HOST PROTEIN SYNTHESIS SHUTOFF UPON HERPESVIRUS INFECTION
In addition to immune evasion strategies specifically affecting components of the cellular antigen processing and presentation machinery, more general mechanisms of preventing immune recognition may be encountered. Alphaherpesviruses, for example, encode “virion host shutoff” proteins causing a global shutdown of synthesis of host proteins (12, 23, 37, 72, 76, 77), including MHC molecules and proinflammatory cytokines, thereby blunting antiviral immunity. Only recently, de novo synthesis of host cell proteins has been found to also be inhibited by gammaherpesviruses (Kaposi's sarcoma-associated herpesvirus [22] and EBV [65a]). In contrast, betaherpesviruses so far appear to lack a generalized shutoff function (50, 92). Thus, interference with MHC-restricted T-cell recognition may rely in part on more broadly acting mechanisms, like the (virion) host shutoff that is present in alpha- and gammaherpesviruses.
HLA class II downregulation in context.
The gene products mentioned may represent just the tip of the iceberg that constitutes the array of herpesvirus gene products with significant effects on the immune response to infection. In addition, downregulation of HLA class II must not be considered in isolation. At the molecular level, HLA molecules form only one element in the immunological synapse formed between a T cell and an APC. Other players of this highly elaborate signaling complex include CD80 (B7.1), CD86 (B7.2), and adhesion molecules such as CD54 (ICAM-1). Unfortunately, studies that examine the effects of herpesvirus infection on these molecules are limited. CD80, CD86, and ICAM-1 appear to be downregulated by HSV-2, EBV, and HCMV (31, 33, 66). Morrow et al. (52) observed CD80/CD86 downregulation with VZV infection. Kaposi's sarcoma-associated herpesvirus K5 was capable of downregulating ICAM-1 and CD86 (32). Various trafficking defects, affecting not only viral proteins but also other vesicular transport systems, provide grounds for the hypothesis that members of the herpesvirus family might be capable of interfering with cytoskeleton formation and rearrangement, such as altering formation of the immunological synapse (18). This would provide a powerful mechanism by which the virus could interfere with an extremely wide range of immunological signaling interactions.
An essential interaction that is potentially affected by immune synapse disruption is that between the DC and naïve CD4+ T cells. Unfortunately, the available data on the permissiveness of DC to infection with different herpesviruses as well as the data on the phenotypes induced are, at times, contradictory. Maturation defects have been reported for DC infected by HCMV, EBV, and HSV-1 (25, 53, 60). Interestingly, the deletion of the host shutoff gene, UL41, from HSV-1 restored full maturation of DC upon infection with the mutant virus (67).
Improper DC-T-cell interactions can result in (functional) deletion of T cells, but also in immune deviation, since the immunological synapse between the DC and T cell instructs the differentiation pathway of a T-helper 0 (Th0) cell (46). In this respect, herpesvirus-encoded IL-10 homologues may take on a special importance. First, IL-10 production by DC could tend to promote Th2 immune deviation, which is counterproductive in herpes infections. Second, autocrine effects of viral IL-10 suppressing cytokine production by DC may cause regulatory T-cell responses to be induced (46).
Cross-presentation of viral antigen may overcome inhibitory effects on DC function induced endogenously upon herpesvirus infection. In this scenario, however, DC function can still be attenuated by secreted factors, such as IL-10 homologues and HCMV-induced TGF-β1 (5), inhibiting DC maturation. Along this line, HCMV-infected DC also exhibit depressed responses to the chemoattractant factors RANTES, MIP-1, and MIP-3β (24). Thus, modulation of DC function appears to represent an attractive strategy for viral evasion; still, the important targets may turn out to be host cytokines/chemokines or their receptors.
Why interfere with HLA class II antigen presentation?
Given the essential role of CD4+ T cells in antiviral immunity, combining the licensing of CD8+ T cells and B cells (8) with direct cytolytic potential (3, 6, 10, 15, 20, 26, 27, 30, 68, 70, 71, 73, 83, 85), it appears profitable for herpesviruses to (temporarily) impair HLA class II-restricted T-cell recognition.
To appreciate the relevance of HLA class II evasion, viral tropism should be considered. For instance, escape from CD4+ T-cell immunity is particularly relevant for viruses, such as EBV, that infect HLA class II+ host B cells. Likewise, HCMV infects cells that have class II constitutively expressed at high levels, such as monocytes and DC. HCMV and HSV-1, however, might not fully block antigen presentation in these professional APC. Yet these viruses also infect other cell types, such as epithelial cells and, in the case of HCMV, endothelial cells, in which class II expression can be induced. HCMV and HSV-1, among others, inhibit the induction of class II (12, 42, 48, 69, 90). In epithelial and endothelial cells, class II expression is generally lower than the constitutive expression on professional APC. Thus, evasion of class II presentation may be more effective in epithelial and endothelial cells, which are major cell types for infection in vivo.
In vivo evidence for a critical role of CD4+ T cells in the control of herpesvirus infection is provided by clinical experiences with AIDS and posttransplantation immunosuppression. Studies of renal transplant patients have revealed that while the CD8+ T cell is the dominant component in response to primary HCMV infection, it is the CD4+ T cell that is found most abundantly among long-term HCMV seropositive patients (71). Bitmansour et al. (9) proposed that there exists a threshold (determined by the number, function, and frequency of CD4+ clonotypes) below which the CD4+ T-cell response against HCMV is inadequate, leading to reactivation of latent disease foci and the development of end-organ disease.
Studies using mice show the critical role for CD4+ T cells in controlling chronic infection with murine gammaherpesvirus 68 (10, 15, 73). A similar function for human CD4+ T cells in maintaining anti-EBV immunity is suggested by the increased EBV-related morbidity observed for AIDS patients with reduced CD4+ T-cell counts (83). Several recent studies underscore the relevance of cytolytic CD4+ T cells in the control of EBV infection (3, 30). In conclusion, HLA class II-restricted CD4+ T-cell immunity forms an important antiviral response that herpesviruses need to withstand.
CONCLUSION
In summary, recent studies have demonstrated the capacity of HSV-1, HCMV, EBV, and other herpesviruses to downregulate surface expression of HLA class II molecules. A number of gene products responsible for specific effects have been identified. The mechanisms by which this downregulation is achieved are diverse and embrace both constitutive and IFN-γ-induced HLA class II expression. In addition, general strategies including host shutoff have effects on T-helper cell immunity. Together, these strategies are likely to aid the escape of herpesviruses from direct CD4+ T-cell recognition and also to attenuate antiviral CD8+ T-cell immunity and antibody responses by preventing stimulation of the HLA class II-restricted T-helper cell subset.
Acknowledgments
We thank Peter van den Elsen for critically reading the manuscript.
Footnotes
Published ahead of print on 1 November 2006.
REFERENCES
- 1.Abendroth, A., B. Slobedman, E. Lee, E. Mellins, M. Wallace, and A. M. Arvin. 2000. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J. Virol. 74:1900-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Accolla, R. S., A. D. Barbaro, S. Mazza, C. Casoli, A. De Maria, and G. Tosi. 2001. The MHC class II transactivator: prey and hunter in infectious diseases. Trends Immunol. 22:560-563. [DOI] [PubMed] [Google Scholar]
- 3.Adhikary, D., U. Behrends, A. Moosmann, K. Witter, G. W. Bornkamm, and J. Mautner. 2006. Control of Epstein-Barr virus infection in vitro by T helper cells specific for virion glycoproteins. J. Exp. Med. 203:995-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcami, A., and U. H. Koszinowski. 2000. Viral mechanisms of immune evasion. Immunol. Today 21:447-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrode, G., C. Boccaccio, J. P. Abastado, and C. Davrinche. 2002. Cross-presentation of human cytomegalovirus pp65 (UL83) to CD8+ T cells is regulated by virus-induced, soluble-mediator-dependent maturation of dendritic cells. J. Virol. 76:142-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvin, A. M., M. Sharp, S. Smith, C. M. Koropchak, P. S. Diaz, P. Kinchington, W. Ruyechan, and J. Hay. 1991. Equivalent recognition of a varicella-zoster virus immediate early protein (Ie62) and glycoprotein I by cytotoxic lymphocytes of either Cd4+ or Cd8+ phenotype. J. Immunol. 146:257-264. [PubMed] [Google Scholar]
- 7.Bach, E. A., M. Aguet, and R. D. Schreiber. 1997. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu. Rev. Immunol. 15:563-591. [DOI] [PubMed] [Google Scholar]
- 8.Bevan, M. J. 2004. Helping the CD8+ T-cell response. Nat. Rev. Immunol. 4:595-602. [DOI] [PubMed] [Google Scholar]
- 9.Bitmansour, A. D., S. L. Waldrop, C. J. Pitcher, E. Khatamzas, F. Kern, V. C. Maino, and L. J. Picker. 2001. Clonotypic structure of the human CD4+ memory T cell response to cytomegalovirus. J. Immunol. 167:1151-1163. [DOI] [PubMed] [Google Scholar]
- 10.Cardin, R. D., J. W. Brooks, S. R. Sarawar, and P. C. Doherty. 1996. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J. Exp. Med. 184:863-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cebulla, C. M., D. M. Miller, Y. Zhang, B. M. Rahill, P. Zimmerman, J. M. Robinson, and D. D. Sedmak. 2002. Human cytomegalovirus disrupts constitutive MHC class II expression. J. Immunol. 169:167-176. [DOI] [PubMed] [Google Scholar]
- 12.Chee, A. V., and B. Roizman. 2004. Herpes simplex virus 1 gene products occlude the interferon signaling pathway at multiple sites. J. Virol. 78:4185-4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevalier, M. S., G. M. Daniels, and D. C. Johnson. 2002. Binding of human cytomegalovirus US2 to major histocompatibility complex class I and II proteins is not sufficient for their degradation. J. Virol. 76:8265-8275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevalier, M. S., and D. C. Johnson. 2003. Human cytomegalovirus US3 chimeras containing US2 cytosolic residues acquire major histocompatibility class I and II protein degradation properties. J. Virol. 77:4731-4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen, J. P., R. D. Cardin, K. C. Branum, and P. C. Doherty. 1999. CD4+ T cell-mediated control of a γ-herpesvirus in B cell-deficient mice is mediated by IFN-γ. Proc. Natl. Acad. Sci. USA 96:5135-5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cresswell, P. 1994. Assembly, transport, and function of MHC class II molecules. Annu. Rev. Immunol. 12:259-293. [DOI] [PubMed] [Google Scholar]
- 17.Crotzer, V. L., R. E. Christian, J. M. Brooks, J. Shabanowitz, R. E. Settlage, J. A. Marto, F. M. White, A. B. Rickinson, D. F. Hunt, and V. H. Engelhard. 2000. Immunodominance among EBV-derived epitopes restricted by HLA-B27 does not correlate with epitope abundance in EBV-transformed B-lymphoblastoid cell lines. J. Immunol. 164:6120-6129. [DOI] [PubMed] [Google Scholar]
- 18.Fish, K. N., W. Britt, and J. A. Nelson. 1996. A novel mechanism for persistence of human cytomegalovirus in macrophages. J. Virol. 70:1855-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gamadia, L. E., E. B. M. Remmerswaal, J. F. Weel, F. Bemelman, R. A. W. van Lier, and I. J. M. ten Berge. 2003. Primary immune responses to human CMV: a critical role for IFN-gamma-producing CD4+ T cells in protection against CMV disease. Blood 101:2686-2692. [DOI] [PubMed] [Google Scholar]
- 21.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, H. L. Ploegh, and D. C. Wiley. 2001. Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA 98:6794-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13:713-723. [DOI] [PubMed] [Google Scholar]
- 23.Glaunsinger, B. A., and D. E. Ganem. 2006. Messenger RNA turnover and its regulation in herpesviral infection. Adv. Virus Res. 66:337-394. [DOI] [PubMed] [Google Scholar]
- 24.Gredmark, S., and C. Soderberg-Naucler. 2003. Human cytomegalovirus inhibits differentiation of monocytes into dendritic cells with the consequence of depressed immunological functions. J. Virol. 77:10943-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guerreiro-Cacais, A. O., L. Li, D. Donati, M. T. Bejarano, A. Morgan, M. G. Masucci, L. Hutt-Fletcher, and V. Levitsky. 2004. Capacity of Epstein-Barr virus to infect monocytes and inhibit their development into dendritic cells is affected by the cell type supporting virus replication. J. Gen. Virol. 85:2767-2778. [DOI] [PubMed] [Google Scholar]
- 26.Gyulai, Z., V. Endresz, K. Burian, S. Pincus, J. Toldy, W. I. Cox, C. Meric, S. Plotkin, E. Gonczol, and K. Berencsi. 2000. Cytotoxic T lymphocyte (CTL) responses to human cytomegalovirus pp65, IE1-exon4, gB, pp150, and pp28 in healthy individuals: reevaluation of prevalence of IE1-specific CTLs. J. Infect. Dis. 181:1537-1546. [DOI] [PubMed] [Google Scholar]
- 27.Hegde, N. R., C. Dunn, D. M. Lewinsohn, M. A. Jarvis, J. A. Nelson, and D. C. Johnson. 2005. Endogenous human cytomegalovirus gB is presented efficiently by MHC class II molecules to CD4+ CTL. J. Exp. Med. 202:1109-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegde, N. R., and D. C. Johnson. 2003. Human cytomegalovirus US2 causes similar effects on both major histocompatibility complex class I and II proteins in epithelial and glial cells. J. Virol. 77:9287-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde, N. R., R. A. Tomazin, T. W. Wisner, C. Dunn, J. M. Boname, D. M. Lewinsohn, and D. C. Johnson. 2002. Inhibition of HLA-DR assembly, transport, and loading by human cytomegalovirus glycoprotein US3: a novel mechanism for evading major histocompatibility complex class II antigen presentation. J. Virol. 76:10929-10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heller, K. N., C. Gurer, and C. Munz. 2006. Virus-specific CD4+ T cells: ready for direct attack. J. Exp. Med. 203:805-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hertel, L., V. G. Lacaille, H. Strobl, E. D. Mellins, and E. S. Mocarski. 2003. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J. Virol. 77:7563-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishido, S., J. K. Choi, B. S. Lee, C. Wang, M. DeMaria, R. P. Johnson, G. B. Cohen, and J. U. Jung. 2000. Inhibition of natural killer cell-mediated cytotoxicity by Kaposi's sarcoma-associated herpesvirus K5 protein. Immunity 13:365-374. [DOI] [PubMed] [Google Scholar]
- 33.Jones, C. A., M. Fernandez, K. Herc, L. Bosnjak, M. Miranda-Saksena, R. A. Boadle, and A. Cunningham. 2003. Herpes simplex virus type 2 induces rapid cell death and functional impairment of murine dendritic cells in vitro. J. Virol. 77:11139-11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones, T. R., L. K. Hanson, L. Sun, J. S. Slater, R. M. Stenberg, and A. E. Campbell. 1995. Multiple independent loci within the human cytomegalovirus unique short region down-regulate expression of major histocompatibility complex class I heavy chains. J. Virol. 69:4830-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keating, S., S. Prince, M. Jones, and M. Rowe. 2002. The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules. J. Virol. 76:8179-8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koppelman, B., J. J. Neefjes, J. E. De Vries, and M. R. de Waal. 1997. Interleukin-10 down-regulates MHC class II αβ peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7:861-871. [DOI] [PubMed] [Google Scholar]
- 37.Koppers-Lalic, D., F. A. Rijsewijk, S. B. Verschuren, van Gaans-Van den Brink, J. A., A. Neisig, M. E. Ressing, J. Neefjes, and E. J. Wiertz. 2001. The UL41-encoded virion host shutoff (vhs) protein and vhs-independent mechanisms are responsible for down-regulation of MHC class I molecules by bovine herpesvirus 1. J. Gen. Virol. 82:2071-2081. [DOI] [PubMed] [Google Scholar]
- 38.Kotenko, S. V., S. Saccani, L. S. Izotova, O. V. Mirochnitchenko, and S. Pestka. 2000. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc. Natl. Acad. Sci. USA 97:1695-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhn, R., J. Lohler, D. Rennick, K. Rajewsky, and W. Muller. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263-274. [DOI] [PubMed] [Google Scholar]
- 40.Laichalk, L. L., and D. A. Thorley-Lawson. 2005. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J. Virol. 79:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Roy, E., M. Baron, W. Faigle, D. Clement, D. M. Lewinsohn, D. N. Streblow, J. A. Nelson, S. Amigorena, and J. L. Davignon. 2002. Infection of APC by human cytomegalovirus controlled through recognition of endogenous nuclear immediate early protein 1 by specific CD4+ T lymphocytes. J. Immunol. 169:1293-1301. [DOI] [PubMed] [Google Scholar]
- 42.Le Roy, E., A. Muhlethaler-Mottet, C. Davrinche, B. Mach, and J. L. Davignon. 1999. Escape of human cytomegalovirus from HLA-DR-restricted CD4+ T-cell response is mediated by repression of gamma interferon-induced class II transactivator expression. J. Virol. 73:6582-6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, Q., M. K. Spriggs, S. Kovats, S. M. Turk, M. R. Comeau, B. Nepom, and L. M. Hutt-Fletcher. 1997. Epstein-Barr virus uses HLA class II as a cofactor for infection of B lymphocytes. J. Virol. 71:4657-4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long, H. M., T. A. Haigh, N. H. Gudgeon, A. M. Leen, C. W. Tsang, J. Brooks, E. Landais, E. Houssaint, S. P. Lee, A. B. Rickinson, and G. S. Taylor. 2005. CD4+ T-cell responses to Epstein-Barr virus (EBV) latent-cycle antigens and the recognition of EBV-transformed lymphoblastoid cell lines. J. Virol. 79:4896-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz, M. B., and G. Schuler. 2002. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 23:445-449. [DOI] [PubMed] [Google Scholar]
- 47.Lybarger, L., X. Wang, M. Harris, and T. H. Hansen. 2005. Viral immune evasion molecules attack the ER peptide-loading complex and exploit ER-associated degradation pathways. Curr. Opin. Immunol. 17:71-78. [DOI] [PubMed] [Google Scholar]
- 48.Miller, D. M., B. M. Rahill, J. M. Boss, M. D. Lairmore, J. E. Durbin, J. W. Waldman, and D. D. Sedmak. 1998. Human cytomegalovirus inhibits major histocompatibility complex class II expression by disruption of the Jak/Stat pathway. J. Exp. Med. 187:675-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller, D. M., Y. X. Zhang, B. M. Rahill, K. Kazor, S. Rofagha, J. J. Eckel, and D. D. Sedmak. 2000. Human cytomegalovirus blocks interferon-gamma stimulated up-regulation of major histocompatibility complex class I expression and the class I antigen processing machinery. Transplantation 69:687-690. [DOI] [PubMed] [Google Scholar]
- 50.Mocarski, E. S., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2674. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 51.Morrison, T. E., A. Mauser, A. Wong, J. P. Ting, and S. C. Kenney. 2001. Inhibition of IFN-gamma signaling by an Epstein-Barr virus immediate-early protein. Immunity 15:787-799. [DOI] [PubMed] [Google Scholar]
- 52.Morrow, G., B. Slobedman, A. L. Cunningham, and A. Abendroth. 2003. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J. Virol. 77:4950-4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moutaftsi, M., A. M. Mehl, L. K. Borysiewicz, and Z. Tabi. 2002. Human cytomegalovirus inhibits maturation and impairs function of monocyte-derived dendritic cells. Blood 99:2913-2921. [DOI] [PubMed] [Google Scholar]
- 54.Munz, C., K. L. Bickham, M. Subklewe, M. L. Tsang, A. Chahroudi, M. G. Kurilla, D. Zhang, M. O'Donnell, and R. M. Steinman. 2000. Human CD4+ T lymphocytes consistently respond to the latent Epstein-Barr virus nuclear antigen EBNA1. J. Exp. Med. 191:1649-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Neumann, J., A. M. Eis-Hubinger, and N. Koch. 2003. Herpes simplex virus type 1 targets the MHC class II processing pathway for immune evasion. J. Immunol. 171:3075-3083. [DOI] [PubMed] [Google Scholar]
- 56.Odeberg, J., B. Plachter, L. Branden, and C. Soderberg-Naucler. 2003. Human cytomegalovirus protein pp65 mediates accumulation of HLA-DR in lysosomes and destruction of the HLA-DR alpha-chain. Blood 101:4870-4877. [DOI] [PubMed] [Google Scholar]
- 57.Odeberg, J., and C. Soderberg-Naucler. 2001. Reduced expression of HLA class II molecules and interleukin-10 and transforming growth factor beta1-independent suppression of T-cell proliferation in human cytomegalovirus-infected macrophage cultures. J. Virol. 75:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paludan, C., D. Schmid, M. Landthaler, M. Vockerodt, D. Kube, T. Tuschl, and C. Munz. 2005. Endogenous MHC class II processing of a viral nuclear antigen after autophagy. Science 307:593-596. [DOI] [PubMed] [Google Scholar]
- 59.Pieters, J. 1997. MHC class II restricted antigen presentation. Curr. Opin. Immunol. 9:89-96. [DOI] [PubMed] [Google Scholar]
- 60.Pollara, G., K. Speidel, L. Samady, M. Rajpopat, Y. McGrath, J. Ledermann, R. S. Coffin, D. R. Katz, and B. Chain. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165-178. [DOI] [PubMed] [Google Scholar]
- 61.Redpath, S., A. Angulo, N. R. Gascoigne, and P. Ghazal. 1999. Murine cytomegalovirus infection down-regulates MHC class II expression on macrophages by induction of IL-10. J. Immunol. 162:6701-6707. [PubMed] [Google Scholar]
- 62.Ressing, M. E., S. E. Keating, D. van Leeuwen, D. Koppers-Lalic, I. Y. Pappworth, E. J. Wiertz, and M. Rowe. 2005. Impaired transporter associated with antigen processing-dependent peptide transport during productive EBV infection. J. Immunol. 174:6829-6838. [DOI] [PubMed] [Google Scholar]
- 63.Ressing, M. E., D. van Leeuwen, F. A. Verreck, R. Gomez, B. Heemskerk, M. Toebes, M. M. Mullen, T. S. Jardetzky, R. Longnecker, M. W. Schilham, T. H. Ottenhoff, J. Neefjes, T. N. Schumacher, L. M. Hutt-Fletcher, and E. J. Wiertz. 2003. Interference with T cell receptor-HLA-DR interactions by Epstein-Barr virus gp42 results in reduced T helper cell recognition. Proc. Natl. Acad. Sci. USA 100:11583-11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ressing, M. E., D. van Leeuwen, F. A. Verreck, S. Keating, R. Gomez, K. L. Franken, T. H. Ottenhoff, M. Spriggs, T. N. Schumacher, L. M. Hutt-Fletcher, M. Rowe, and E. J. Wiertz. 2005. Epstein-Barr virus gp42 is posttranslationally modified to produce soluble gp42 that mediates HLA class II immune evasion. J. Virol. 79:841-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roizman, B., and P. E. Pellet. 2001. The family Herpesviridae: a brief introduction, p. 2381-2397. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 65a.Rowe, M., B. Glaunsinger, D. van Leeuwen, J. Zuo, D. Sweetman, D. Ganem, J. Middeldorp, E. J. H. J. Wiertz, and M. E. Ressing. 2007. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc. Natl. Acad. Sci. USA 104:3366-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salek-Ardakani, S., J. R. Arrand, and M. Mackett. 2002. Epstein-Barr virus encoded interleukin-10 inhibits HLA-class I, ICAM-1, and B7 expression on human monocytes: implications for immune evasion by EBV. Virology 304:342-351. [DOI] [PubMed] [Google Scholar]
- 67.Samady, L., E. Costigliola, L. MacCormac, Y. McGrath, S. Cleverley, C. E. Lilley, J. Smith, D. S. Latchman, B. Chain, and R. S. Coffin. 2003. Deletion of the virion host shutoff protein (vhs) from herpes simplex virus (HSV) relieves the viral block to dendritic cell activation: Potential of vhs− HSV vectors for dendritic cell-mediated immunotherapy. J. Virol. 77:3768-3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schmid, D. S. 1988. The human MHC-restricted cellular-response to herpes simplex virus type 1 is mediated by CD4+, CD8− T cells and is restricted to the DR region of the MHC complex. J. Immunol. 140:3610-3616. [PubMed] [Google Scholar]
- 69.Sedmak, D. D., A. M. Guglielmo, D. A. Knight, D. J. Birmingham, E. H. Huang, and W. J. Waldman. 1994. Cytomegalovirus inhibits major histocompatibility class II expression on infected endothelial cells. Am. J. Pathol. 144:683-692. [PMC free article] [PubMed] [Google Scholar]
- 70.Sester, M., U. Sester, B. Gartner, G. Heine, M. Girndt, N. Mueller-Lantzsch, A. Meyerhans, and H. Kohler. 2001. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation 71:1287-1294. [DOI] [PubMed] [Google Scholar]
- 71.Sester, M., U. Sester, B. C. Gartner, M. Girndt, A. Meyerhans, and H. Kohler. 2002. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J. Am. Soc. Nephrol. 13:2577-2584. [DOI] [PubMed] [Google Scholar]
- 72.Smiley, J. R. 2004. Herpes simplex virus virion host shutoff protein: immune evasion mediated by a viral RNase? J. Virol. 78:1063-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sparks-Thissen, R. L., D. C. Braaten, S. Kreher, S. H. Speck, and H. W. Virgin. 2004. An optimized CD4 T-cell response can control productive and latent gammaherpesvirus infection. J. Virol. 78:6827-6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spencer, J. V., K. M. Lockridge, P. A. Barry, G. Lin, M. Tsang, M. E. Penfold, and T. J. Schall. 2002. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J. Virol. 76:1285-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spriggs, M. K., R. J. Armitage, M. R. Comeau, L. Strockbine, T. Farrah, B. Macduff, D. Ulrich, M. R. Alderson, J. Mullberg, and J. I. Cohen. 1996. The extracellular domain of the Epstein-Barr virus BZLF2 protein binds the HLA-DR β chain and inhibits antigen presentation. J. Virol. 70:5557-5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Taddeo, B., W. R. Zhang, and B. Roizman. 2006. The UL41 protein of herpes simplex virus 1 degrades RNA by endonucleolytic cleavage in absence of other cellular or viral proteins. Proc. Natl. Acad. Sci. USA 103:2827-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tigges, M. A., S. Leng, D. C. Johnson, and R. L. Burke. 1996. Human herpes simplex virus (HSV)-specific CD8+ CTL clones recognize HSV-2-infected fibroblasts after treatment with IFN-gamma or when virion host shutoff functions are disabled. J. Immunol. 156:3901-3910. [PubMed] [Google Scholar]
- 78.Ting, J. P. Y., and J. Trowsdale. 2002. Genetic control of MHC class II expression. Cell 109:S21-S33. [DOI] [PubMed] [Google Scholar]
- 79.Tomazin, R., J. Boname, N. R. Hegde, D. M. Lewinsohn, Y. Altschuler, T. R. Jones, P. Cresswell, J. A. Nelson, S. R. Riddell, and D. C. Johnson. 1999. Cytomegalovirus US2 destroys two components of the MHC class II pathway, preventing recognition by CD4+ T cells. Nat. Med. 5:1039-1043. [DOI] [PubMed] [Google Scholar]
- 80.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 81.Trgovcich, J., D. Johnson, and B. Roizman. 2002. Cell surface major histocompatibility complex class II proteins are regulated by the products of the γ134.5 and UL41 genes of herpes simplex virus 1. J. Virol. 76:6974-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ustinov, J. A., R. J. Loginov, C. A. Bruggeman, P. H. van der Meide, P. J. Hayry, and I. T. Lautenschlager. 1993. Cytomegalovirus induces class II expression in rat heart endothelial cells. J. Heart Lung Transplant. 12:644-651. [PubMed] [Google Scholar]
- 83.van Baarle, D., E. Hovenkamp, M. F. Callan, K. C. Wolthers, S. Kostense, L. C. Tan, H. G. Niesters, A. D. Osterhaus, A. J. McMichael, M. H. van Oers, and F. Miedema. 2001. Dysfunctional Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes and increased EBV load in HIV-1 infected individuals progressing to AIDS-related non-Hodgkin lymphoma. Blood 98:146-155. [DOI] [PubMed] [Google Scholar]
- 84.van den Elsen, P. J., T. M. Holling, H. F. Kuipers, and N. van der Stoep. 2004. Transcriptional regulation of antigen presentation. Curr. Opin. Immunol. 16:67-75. [DOI] [PubMed] [Google Scholar]
- 85.van Leeuwen, E. M. M., E. B. A. Remmerswaal, M. T. M. Vossen, A. T. Rowshani, P. M. E. Wertheim-van Dillen, R. A. W. van Lier, and I. J. M. ten Berge. 2004. Emergence of a CD4+CD28− granzyme B+, cytomegalovirus-specific T cell subset after recovery of primary cytomegalovirus infection. J. Immunol. 173:1834-1841. [DOI] [PubMed] [Google Scholar]
- 86.Voo, K. S., T. H. Fu, H. E. Heslop, M. K. Brenner, C. M. Rooney, and R. F. Wang. 2002. Identification of HLA-DP3-restricted peptides from EBNA1 recognized by CD4+ T cells. Cancer Res. 62:7195-7199. [PubMed] [Google Scholar]
- 87.Vossen, M. T., E. M. Westerhout, C. Soderberg-Naucler, and E. J. Wiertz. 2002. Viral immune evasion: a masterpiece of evolution. Immunogenetics 54:527-542. [DOI] [PubMed] [Google Scholar]
- 88.Wiertz, E. J. H. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 89.Yewdell, J. W., and A. B. Hill. 2002. Viral interference with antigen presentation. Nat. Immunol. 3:1019-1025. [DOI] [PubMed] [Google Scholar]
- 90.Yokota, S., N. Yokosawa, T. Kubota, T. Suzutani, I. Yoshida, S. Miura, K. Jimbow, and N. Fujii. 2001. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology 286:119-124. [DOI] [PubMed] [Google Scholar]
- 91.Zeidler, R., G. Eissner, P. Meissner, S. Uebel, R. Tampe, S. Lazis, and W. Hammerschmidt. 1997. Downregulation of TAP1 in B lymphocytes by cellular and Epstein-Barr virus-encoded interleukin-10. Blood 90:2390-2397. [PubMed] [Google Scholar]
- 92.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]