Abstract
Positive-strand RNA virus genome replication occurs in membrane-associated RNA replication complexes, whose assembly remains poorly understood. Here we show that prior to RNA replication, the multifunctional, transmembrane RNA replication protein A of the nodavirus flock house virus (FHV) recruits FHV genomic RNA1 to a membrane-associated state in both Drosophila melanogaster and Saccharomyces cerevisiae cells. Protein A has mitochondrial membrane-targeting, self-interaction, RNA-dependent RNA polymerase (RdRp), and RNA capping domains. In the absence of RdRp activity due to an active site mutation (AD692E), protein A stimulated RNA1 accumulation by increasing RNA1 stability. Protein AD692E stimulated RNA1 accumulation in wild-type cells and in xrn1− yeast defective in decapped RNA decay, showing that increased RNA1 stability was not due to protein A-mediated RNA1 recapping. Increased RNA1 stability was closely linked with protein A-induced membrane association of the stabilized RNA and was highly selective for RNA1. Substantial N- and C-proximal regions of protein A were dispensable for these activities. However, increased RNA1 accumulation was eliminated by deleting protein A amino acids (aa) 1 to 370 but was restored completely by adding back the transmembrane domain (aa 1 to 35) and partially by adding back peripheral membrane association sequences in aa 36 to 370. Moreover, although RNA polymerase activity was not required, even small deletions in or around the RdRp domain abolished increased RNA1 accumulation. These and other results show that prior to negative-strand RNA synthesis, multiple domains of mitochondrially targeted protein A cooperate to selectively recruit FHV genomic RNA to membranes where RNA replication complexes form.
All positive-strand RNA viruses replicate in association with intracellular membranes. The particular membrane(s) used during RNA replication varies, with many positive-strand RNA viruses replicating in association with the endoplasmic reticulum, while others use lysosomal, mitochondrial, peroxisomal, or other membranes (16, 17, 26, 28, 36, 42, 43, 45, 55, 56, 60). Some viral RNA replication proteins carry membrane-targeting signals and interact with other viral proteins to localize these to the sites of RNA replication complex formation (18, 56, 58, 62). In a few cases, interactions contributing to recruitment of viral RNA replication templates have also been identified. Brome mosaic virus (BMV) helicase-like protein 1a, e.g., induces formation of spherular endoplasmic reticulum membrane invaginations and selectively recruits BMV 2a polymerase and BMV genomic RNAs to these structures to form RNA replication complexes (1, 11, 12, 32, 62, 66). Similarly, tomato bushy stunt virus replication protein p33 binds defective interfering RNA (DI-RNA) in vitro, and the related cucumber necrosis virus p33 protein recruits such RNAs to replication complex sites in vivo (47, 48, 51, 54).
Flock house virus (FHV) is the best-studied member of the alphanodaviruses in the Nodaviridae family. The bipartite FHV genome consists of RNA1 (3.1 kb) and RNA2 (1.4 kb), which encode protein A, a multifunctional RNA replication factor, and the capsid precursor, respectively (7, 13, 21-23). Both genomic RNAs are capped, nonpolyadenylated, and copackaged into a nonenveloped icosahedral capsid with T=3 symmetry (61, 63). RNA1 also encodes a subgenomic RNA, RNA3, which translates B2, a 12-kDa RNA silencing inhibitor (20, 22, 27, 38).
FHV protein A (112 kDa) contains an RNA-dependent RNA polymerase (RdRp) domain, self-interaction domains, a putative RNA capping domain, and an N-terminal mitochondrial targeting and transmembrane domain that mediates protein A localization and insertion into the outer mitochondrial membrane, where FHV RNA replication occurs in association with spherular membrane invaginations strikingly similar to those of BMV (15, 33, 35, 44, 45, 62). Based on amino acid similarity within eight distinct RdRp motifs, protein A belongs to the positive-strand RNA RdRp supergroup I (34, 35). The sixth RdRp motif contains a GDD motif that is conserved among RdRps of all positive-strand RNA viruses and is required for metal ion coordination in the polymerase active site (4, 9, 34, 35). Mutation of the GDD motif inhibits polymerase activity in many viruses, such as hepatitis C virus, poliovirus, and FHV (9, 31).
FHV was originally isolated from the insect Costelytra zealandica and also productively infects Drosophila melanogaster cells. In addition, FHV can replicate in animal, plant, Caenorhabditis elegans, and Saccharomyces cerevisiae cells if RNA templates are provided by transfection or DNA expression (6, 21, 40, 52, 63, 64). Like Drosophila, the budding yeast Saccharomyces cerevisiae supports autonomous replication of RNA1, infectious virion production, RNA2 inhibition of RNA3 replication, distinct mitochondrial membrane alterations, and other features of natural FHV infection (39, 45, 46, 52, 53).
Here we show that FHV protein A selectively induces RNA1 association with membranes in the absence of negative-strand RNA1 synthesis. Simultaneously, protein A increases in vivo FHV RNA1 stability and accumulation. The protein A membrane association domain and regions surrounding and including the RdRp domain are required for these activities, while significant regions of the N and C termini and some protein-protein interaction domains are dispensable. Overall, the results imply that in a distinct step prior to negative-strand RNA synthesis, the mitochondrially localized transmembrane protein A recruits FHV RNA1 to the membrane-associated sites of RNA replication complex formation.
MATERIALS AND METHODS
Plasmids.
Standard molecular cloning techniques were used throughout (5, 59). All products generated by PCR were verified by sequencing. Detailed methods and primer sequences will be provided upon request. Numbering of FHV RNA1 sequences is based on GenBank accession number NC_004146. FHV RNA expression plasmids for RNA1fs (pF1fs) and protein Awt (pFA) have been described previously (39, 44, 46, 52, 53). pF1fs and pF1 are HIS3-selectable yeast centromeric plasmids that contain full-length RNA1 flanked by the GAL1 promoter and the hepatitis delta virus ribozyme (pF1 was provided courtesy of D. Miller). A 4-nucleotide (nt) insertion at nt 373 in pF1fs causes a frameshift in the protein A open reading frame (ORF). pFA is a LEU2-selectable yeast centromeric plasmid that contains protein A mRNA flanked by the GAL1 promoter and leader sequence at the 5′ end and a 3′ CYC1 polyadenylation signal.
(i) pFAD692E.
pFA was amplified with two sets of primers that yielded overlapping PCR products containing a T-to-A mutation at nt 2115. PCR products were mixed, amplified with bordering primers, digested with BlpI and RsrII, and ligated into common sites in pFA.
(ii) pFA-YFP and pFAD692E-YFP.
The C-terminal coding region of pFA and the yellow fluorescent protein (YFP) ORF of a protein A-YFP fusion-expressing plasmid (courtesy of B. Dye) were amplified by PCR with overlapping primers at the protein A mRNA-YFP mRNA junction. PCR products were mixed, amplified with bordering primers, digested with HindIII and BlpI, and ligated into common sites in pFA and pFAD692E to make pFA-YFP and pFAD692E-YFP, respectively. Both plasmids contain a stop codon after the protein A ORF.
(iii) pGlobin.
pGlobin, previously described as GAL-IRA or pMS99 (66), contains the β-globin ORF flanked by the GAL1 promoter and leader at the 5′ end and the alcohol dehydrogenase 1 (ADH1) 3′-untranslated region (UTR).
(iv) pYFP.
pYFP contains the YFP ORF flanked by the GAL1 promoter and the ADH1 3′UTR. The GAL1 promoter region and the YFP ORF of pA-YFP were amplified with primers that overlap at the GAL1 promoter-YFP mRNA junction. PCR products were mixed, amplified with bordering primers, digested with AgeI and XbaI, and ligated into common sites in pGlobin.
(v) YFP probe template.
The YFP ORF was amplified and ligated into pGEM-T with a Promega pGEM-T Easy Vector System I kit (Madison, WI). The YFP ORF was cut out of the pGEM-T vector with SacII and XhoI and ligated into common sites in pBluescript II KS(+) (Stratagene, La Jolla, CA).
(vi) Protein A deletions.
A two-step PCR process was used to make all protein A deletions. First, two protein A sequences that flanked the deletion region were amplified. The PCR products were then combined, amplified with bordering primers, digested, and ligated into common sites in PFAD692E or pFAD692E-YFP.
(vii) Drosophila expression plasmids.
All FHV expression plasmids were derivatives of pIE1hr/PA, which contains the baculovirus immediate-early 1 (IE1) promoter, the baculovirus transactivating hr5 enhancer, and a polyadenylation signal (10). pIE1-F1fs was made by amplifying RNA1 from pF1 and ligating the PstI- and SalI-digested product into common sites in pIE1hr/PA. pIE1-FA was made by amplifying the protein A ORF from pFA and ligating the HindIII- and SalI-digested product into common sites in pIE1hr/PA. pIE1-FAD692E was made by ligating the NcoI-digested protein AD692E insert into common sites in pIE1-FA.
Cells.
The haploid yeast strains YPH500 (MATα ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1) and YPH500 xrn1− (MATα ura3-52 lys2-801 ade2-101 trp1-Δ63 his3-Δ200 leu2-Δ1 xrn1Δ Bgl::URA3) were transformed by using an E-Z transformation kit (Zymo Research, Orange, CA) (44). Yeast cells were grown in 2% glucose selective medium for 2 days at 30°C. DNA-directed RNA expression was induced by growth of yeast in 2% galactose selective medium for 16 to 20 h, to mid-log phase (optical density at 600 nm, 0.75 to 1.0). Drosophila melanogaster S2 cells were grown at 28°C in Drosophila serum-free medium (SFM) supplemented with 17 mM l-glutamine (Gibco, Carlsbad, CA). For transfections, 25 μg of plasmid DNA in 500 μl SFM was mixed with an equal volume of SFM containing 160 μl of DOTAP-DOPE {N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium chloride salt and l-(phosphatidylethanolamine, dioleoyl)} liposomes for 30 min at room temperature (Sigma, St. Louis, MO). The DNA and DOTAP-DOPE mixture was then added to previously plated S2 cells (1 × 107 to 3 × 107 cells/T75 flask) in 3 ml of SFM.
Antibodies.
A rabbit polyclonal antibody against protein A was described previously (45). Mouse monoclonal antibodies against yeast 3-phosphoglycerate kinase (PGK) and mitochondrial porin were purchased from Molecular Probes (Eugene, OR). Rabbit polyclonal antibody against mitochondrial porin/voltage-dependent anion channel was obtained from Affinity Bioreagents (Golden, CO). Rabbit polyclonal antibody against actin was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Alkaline phosphate-conjugated secondary antibodies were purchased from Bio-Rad (Hercules, CA).
RNA extraction and Northern blot analysis.
Total RNA from yeast or D. melanogaster cells was isolated and prepared by the hot phenol method (39). Northern blotting was performed as previously described, except that 2 or 4 μg RNA was separated in 1% (wt/vol) agarose-MOPS (morpholinepropanesulfonic acid)-formaldehyde gels (39). Probes against globin and positive- and negative-strand RNA1 and RNA3 have been described (52, 66). Probes against 18S rRNA were derived from pTRI RNA 18S templates (Ambion, Austin, TX). Probe templates against the DEAD box protein 2 (DBP2), pyruvate kinase 1 (PYK1), and alcohol dehydrogenase 1 (ADH1) genes were made by amplifying 400 to 500 nt of their respective cDNAs (courtesy of D. Kushner) with an Ambion Lig'n Scribe kit (Austin, TX). The actin mRNA probe targeted a 315-nt fragment of actin mRNA expressed from pBluescript II KS(+) (Stratagene, La Jolla, CA). Probes were synthesized using either a Strip-EZ RNA kit from Ambion (Austin, TX) or an Epicenter Riboscribe probe synthesis kit (Madison, WI) with the appropriate enzyme, i.e., T7, T3, or SP6 polymerase. Northern blots were imaged on a Typhoon 9200 instrument (Amersham Biosciences, Piscataway, NJ). Band intensities were analyzed with ImageQuant software (Molecular Dynamics, Piscataway, NJ).
Protein extraction, Western blotting, and total protein analysis.
For Fig. 1, 2, 8, and 9, total protein was extracted from yeast as described previously (44). For Fig. 6B, to prevent the loss of material, yeast or Drosophila cells were resuspended in a 1% solution of β-mercaptoethanol and sodium dodecyl sulfate (SDS), boiled, vortexed, and supplemented with cracking buffer (10 ml total, containing 0.8 g SDS, 1.4 g Ficoll, 2 ml β-mercaptoethanol, 5 ml of 0.5 M Tris-HCl, pH 6.8, and bromphenol blue). Cell lysates were separated in 10% SDS-polyacrylamide gels and immunoblotted, and chemiluminescence was detected with a Boehringer Mannheim Lumi-Imager as described previously (45). The total protein concentration was determined with a reducing agent-compatible BCA protein assay kit (Pierce, Rockford, IL).
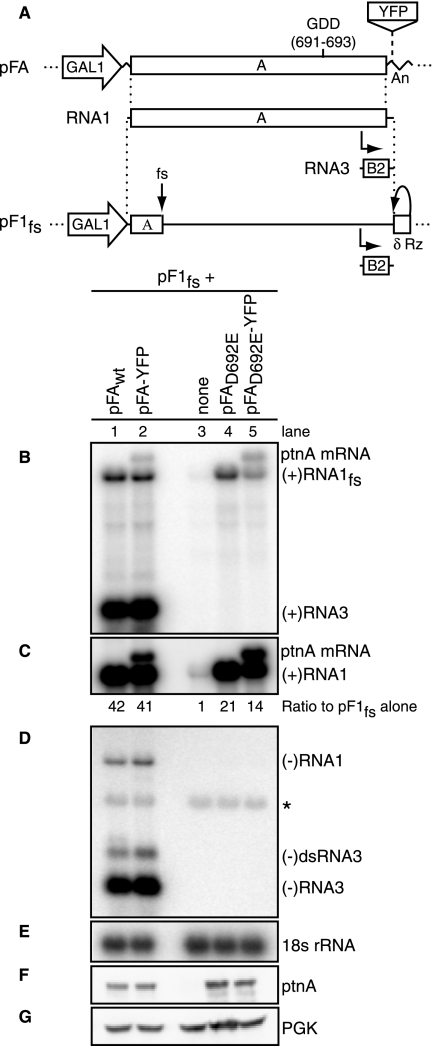
FIG. 1.
Protein A stimulates RNA1fs accumulation. (A) Schematic of plasmid-directed RNA1fs and protein A expression in yeast. pF1fs expresses RNA1fs from a GAL1 promoter, with a hepatitis delta virus ribozyme-mediated authentic 3′ end (δ Rz). Insertion of a 4-nt sequence causes a frameshift (fs) in RNA1 that prevents full-length protein A production. pFA encodes protein A mRNA flanked by a GAL1 leader sequence and a CYC1 polyadenylation signal (An). The protein A GDD polymerase motif occurs at aa 691 to 693. Insertion of YFP mRNA downstream of and separate from the protein A mRNA increases the size of protein A mRNA. (B to G) Representative data from seven independent experiments are shown. Total RNA and total protein were isolated from yeast cells transformed with pF1fs alone or together with pFA, pFA-YFP, pFAD692E, or pFAD692E-YFP. Total RNA (2 μg) was analyzed by Northern blotting with 32P-labeled cRNA probes for positive-strand RNA1fs and RNA3 (B and C), negative-strand RNA1 and RNA3 (D), and 18S rRNA (E). The blot in panel C was exposed 100 times longer than that in panel B to allow for visualization of positive-strand RNA1fs in lane 3. Panels C and D had the same exposure time. The asterisk represents a background band (D). Total protein was analyzed by Western blotting with antibodies against protein A (F) and the cytoplasmic yeast protein PGK (G).
FIG. 2.
Protein A stimulates RNA1fs stabilization. Yeast cells transformed with pF1fs alone (A) or together with pFAwt-YFP (B) or pFAD692E-YFP (C) were grown in selective galactose medium to mid-log phase and then switched to selective glucose medium. Total RNAs and total proteins were isolated from equal volumes of yeast cells collected 0 to 2 hours after the switch to selective glucose medium and were analyzed by Northern and Western blotting as described in the legend to Fig. 1. RNA levels, determined from five independent experiments, were graphed as the percentage of positive-strand RNA1fs remaining compared to that at time zero (D).
FIG. 8.
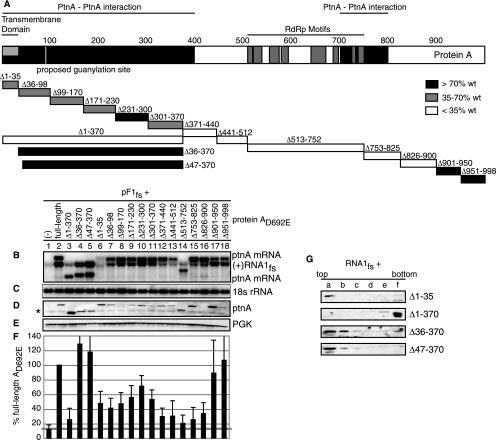
Membrane association and the RdRp region of protein A are required for RNA1fs accumulation. (A) Protein A map which identifies the transmembrane domain, protein A self-interaction domains, and eight conserved RdRp motifs. Sixteen deletions within protein AD692E were made and are indicated by boxes. Yeast cells were transformed with pF1fs alone or together with full-length or a deletion mutant of pFAD692E or pFAD692E-YFP. Total RNA and protein were extracted and analyzed as described in the legend to Fig. 1 for positive-strand RNA1fs (B), 18S rRNA (C), protein A (D), and PGK (E). The asterisk in panel D denotes a background band. Levels of RNA1fs accumulation, determined from at least four independent experiments after normalization to 18S rRNA, were expressed as percentages of RNA1fs accumulation in the presence of full-length pFAD692E-YFP (F) and are indicated by deletion box shading (A).
FIG. 9.
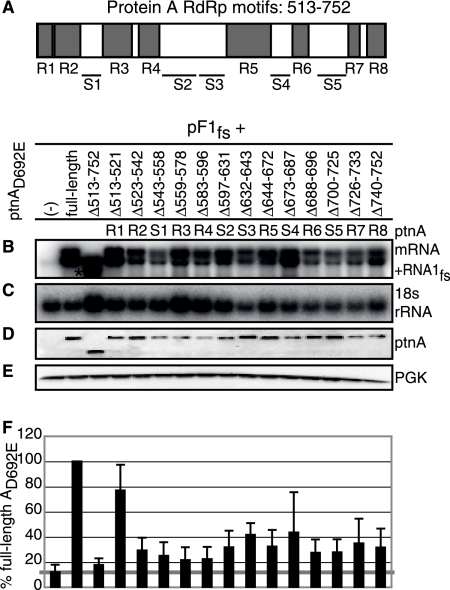
RNA1fs accumulation requires many RdRp motifs. (A) Map of eight conserved RdRp motifs in protein A. Deletions of RdRp motifs are labeled R1 through R8. Deletions of spaces between RdRp motifs are labeled S1 through S5. Yeast cells were transformed with pF1fs alone or together with full-length or deletion mutant pFAD692E-YFP. The asterisk in panel B denotes a protein A mRNA band. Total RNAs and proteins were extracted and analyzed as described in the legend to Fig. 7 (B to F). Averages from six independent experiments are shown.
FIG. 6.
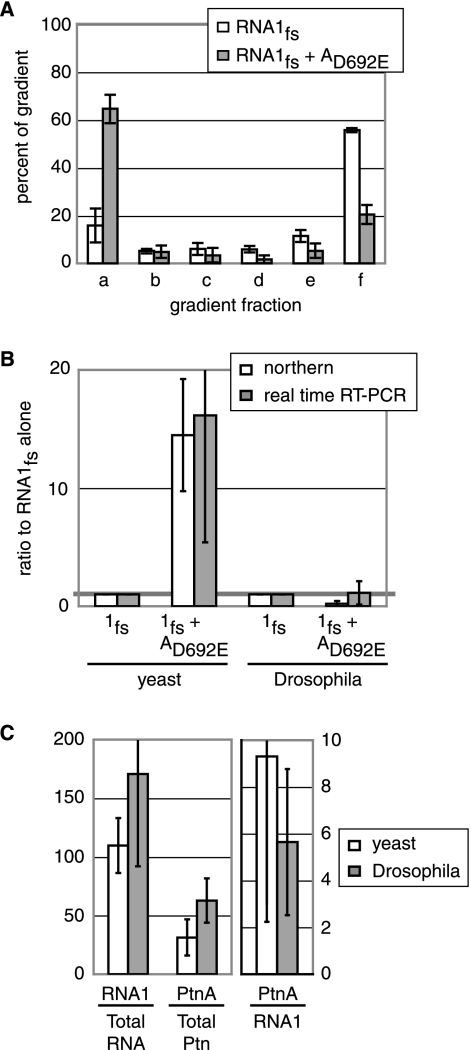
Protein A induces RNA1fs membrane association in Drosophila. D. melanogaster cells expressing RNA1fs alone or together with protein AD692E were collected 2 days after transfection. (A) Flotation and RNA extraction were done as described in the legend to Fig. 4. One-step real-time RT-PCR was performed on 5 ng of RNA, using primers and a probe specific for either RNA1 or actin mRNA. The RNA1fs amount in each gradient fraction, determined from four independent experiments, was graphed as the percentage of RNA1fs in the entire gradient. (B) Total RNA was extracted and analyzed by Northern blotting as described in the legend to Fig. 1 or by real-time RT-PCR as described above. Data from at least three independent experiments are shown. (C) Protein and RNA were extracted from equal cell volumes of yeast or Drosophila expressing RNA1fs and protein AD692E and were analyzed by Western blotting for protein A as described in the legend to Fig. 1 and by real-time RT-PCR for positive-strand RNA1 as described above, except that 75 ng of total RNA was used. The ratios of protein A Boehringer light units to μg of total protein (Ptn A/total Ptn), positive-strand RNA1 molecules per ng of RNA loaded to ng of total RNA (RNA1/total RNA), and protein A/total protein to RNA1/total RNA (Ptn A/RNA1) are graphed on two different scales. Averages from three independent experiments are shown.
RNA half-life.
Yeast cells were grown and induced as described above and then resuspended at an optical density at 600 nm of 1.0 in 2% dextrose selective medium. Aliquots (1 ml) were taken in duplicate 0, 5, 15, 30, 60, and 120 min after resuspension (66) and frozen on dry ice before RNA and protein extraction as described above. Equal RNA concentrations and protein lysate volumes were analyzed by Northern and Western blotting, respectively. Drosophila cells were transfected and grown as described above. Two days after transfection, cell suspensions were treated with actinomycin D at 20 μg/ml and incubated on a rotator at room temperature. Aliquots (100 μl) were taken 0, 15, 30, 60, 90, 120, 150, 180, 210, and 240 min after treatment and frozen on dry ice before RNA extraction and subsequent analysis by Northern blotting as described above.
Membrane flotation.
Yeast cells were prepared for membrane flotation, spheroplast formation, and lysis by Dounce homogenization as described previously (44). Drosophila cells were collected 2 days after transfection and incubated in flotation buffer [50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES), pH 7.4, 50 mM KCl, 5 mM EDTA, 2 mM MgCl2, 1 mM dithiothreitol, 4 mM ribonucleoside vanadyl complex (New England Biolabs, Beverly, MA), and protease inhibitor cocktail (Sigma, St. Louis, MO)] for 10 min at 4°C before lysis by 100 strokes with a Dounce homogenizer. Cell debris, unlysed cells, and nuclei were extracted from either yeast or Drosophila cells by centrifugation at 500 × g for 5 min. Cell lysates were mixed with Nycodenz to a final concentration of 37.5%, loaded under a 5 to 35% Nycodenz discontinuous gradient, and centrifuged at 100,000 × g for 4 to 5 h in a Beckman TLS-55 swing-bucket rotor. Six equal fractions were collected from each gradient. RNAs were isolated from half of each fraction by hot phenol RNA extraction as described above. Equal amounts of RNA, based on the bottom fraction's concentration, were analyzed by Northern blotting or real-time reverse transcription-PCR (RT-PCR), as described above and below, respectively. Half of each fraction was mixed with 3× protein loading buffer for protein analysis by Western blotting.
RNA analysis by real-time RT-PCR.
RNAs from membrane flotation assays in Drosophila cells or from total RNA assays in yeast and Drosophila cells were treated with DNase by use of an Ambion Turbo DNA-free kit (Austin, TX). Five nanograms of RNA (5 ng), based on the bottom fraction concentration, or total RNA (75 ng) was analyzed by real-time RT-PCR for positive-strand RNA1fs or actin mRNA with a Taqman one-step RT-PCR master mix reagent kit (Applied Biosystems, Foster City, CA) in an Applied Biosystems 7900 machine at a 9600 emulation setting (Foster City, CA). The conditions were as follows: 1× master mix without uracil-N-glycosylase (AmpliTaq gold enzyme, deoxynucleoside triphosphates, ROX, and buffer), 0.25 units/μl Multiscribe and RNase inhibitor mix, a 700 μM concentration of each primer, 350 μM probe, and water to 10 μl. The PCR conditions were as follows: 48°C for 30 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. Primer and probe pairs were designed with the Primer Express program (Applied Biosystems, Foster City, CA), and their sequences are available upon request. The Drosophila actin probe was described previously (50). The RNA1 forward primer, reverse primer, and probe initiated at nt 23, 153, and 110, respectively. The Drosophila actin forward and reverse primers initiated at nt 507 and 748, respectively.
Cell fractionation.
Spheroplasted yeast cells were divided into two fractions and lysed by vigorous pipetting in lysis buffer (50 mM Tris-Cl, pH 7.4, 10 mM ribonucleoside vanadyl complex [New England Biolabs, Beverly, MA]). Centrifugation of half of each sample at 20,000 × g for 5 min separated the membrane-enriched pellet (P) from the supernatant (S) fraction. RNAs were extracted from the pellet, supernatant, and unspun (total [T]) fractions by successive treatments with acidic phenol, phenol-chloroform, and 100% ethanol with sodium acetate. Equal RNA amounts, based on the total RNA concentration, were analyzed by Northern blotting.
RESULTS
Protein A stimulates RNA1fs accumulation.
To study the effects of protein A on RNA1 prior to virus-induced RNA synthesis, a previously documented, polymerase-inactivating D-to-E mutation was made at the first aspartate (amino acid [aa] 692) in the conserved GDD motif of the polymerase domain of protein A (9, 31). The resulting mutant and wild-type (wt) protein A were expressed from plasmids pFAD692E and pFA, respectively (Fig. 1A). These plasmids contain the protein A ORF but lack the 5′- and 3′-noncoding regions of RNA1, including cis-acting signals essential for RNA1 replication (39). Accordingly, the resulting transcripts of pFA and pFAD692E did not serve as replication templates for protein A. For a replication template, we used the previously characterized RNA1 derivative RNA1fs, which bears the full-length sequence of RNA1 together with a frameshift that blocks protein A expression (Fig. 1A) (39). This trans-replication system separates the cis- and trans-acting RNA replication functions of RNA1 onto separate plasmids for independent manipulation (39).
In yeast cells, viral RNAs were under the control of the GAL1 promoter. cis cleavage by a flanking hepatitis delta virus antigenomic ribozyme produced a wt 3′ end on RNA1fs, while the addition of a CYC1 poly(A) tail protected protein A mRNA (Fig. 1A). Total RNA was collected, and 2 μg of total RNA was analyzed by Northern blotting. All experiments were repeated at least three times, and representative results are shown. Expression of pF1fs alone in yeast yielded very low levels of positive-strand RNA1fs because its only source was cellular transcription from the GAL1 promoter (Fig. 1B and C, lanes 3). However, when pF1fs and pFA were coexpressed, trans-replication of RNA1fs occurred, as seen by the presence of negative-strand RNA1fs and positive- and negative-strand RNA3 (Fig. 1B to D, lanes 1). As expected, positive-strand RNA1fs and RNA3 levels were about 25-fold higher than negative-strand RNA1 and RNA3 levels. (Fig. 1C and D had the same exposure time, which was 100 times longer than that for Fig. 1B, and the positive- and negative-strand RNA1 and RNA3 probes had comparable relative radioactivities).
To independently follow the effects of protein A on its mRNA and RNA1fs, the YFP ORF was inserted into pFA downstream of and separated from the protein A ORF (pFA-YFP) (Fig. 1A). This allowed wt protein A expression but increased the size of protein A mRNA by ∼700 nucleotides. Expression of pFA-YFP and pF1fs produced a second band above the positive-strand RNA1fs band that corresponded to the YFP ORF-augmented protein A mRNA (Fig. 1B and C, lanes 2). Protein A levels were equivalent in the presence of either pFA or pFA-YFP (Fig. 1F).
As expected, coexpression of pF1fs and pFAD692E produced neither negative-strand RNA1fs nor positive- or negative-strand RNA3 (Fig. 1B to D, lanes 4). However, even in the absence of protein A polymerase activity, pFAD692E markedly stimulated positive-strand RNA1fs accumulation (Fig. 1B and C, lanes 4). To isolate protein A's effects on its own mRNA from those on RNA1fs prior to RNA synthesis, pFAD692E-YFP was used. Expression of pFAD692E-YFP and pF1fs caused a 14-fold increase in RNA1fs accumulation over that from pF1fs alone (Fig. 1B and C, lanes 5). The sum of the protein A mRNA and RNA1fs bands in Fig. 1B, lanes 2 and 5, was equivalent to the positive-strand RNA1 band intensities in Fig. 1B, lanes 1 and 4, respectively, which contained both protein A mRNA and RNA1fs. Similar protein A levels were expressed from pFAD692E and pFAD692E-YFP (Fig. 1F). Protein A did not affect the 18S rRNA or PGK protein level (Fig. 1E and G).
Protein A stimulates RNA1fs stabilization.
Protein AD692E-stimulated RNA1 accumulation in the absence of protein A polymerase activity might result from stimulating plasmid-directed RNA1 transcription or inhibiting RNA1 degradation. To investigate the first possibility, run-on transcription assays were performed (32). Protein AD692E did not increase the DNA-dependent transcription of RNA1, other cellular mRNAs such as actin, or other nonyeast genes, such as globin or YFP, introduced via plasmid transformation (data not shown).
To analyze protein A effects on FHV RNA1 stability by half-life analysis, we took advantage of the tightly regulated galactose-inducible, glucose-repressible GAL1 promoter. Yeast cells transformed with the appropriate viral plasmids were grown in selective galactose medium overnight to induce RNA1fs and protein A mRNA transcription. Viral RNA transcription via the GAL1 promoter was shut off by switching yeast cells grown to mid-log phase to selective glucose medium. Yeast cells were collected at six time points over a 2-hour period after this glucose-induced shutoff, and equal amounts of total RNA were isolated and analyzed by Northern blotting. When expressed alone, RNA1fs rapidly degraded, with a half-life of less than 5 minutes, while 18S rRNA and PGK protein levels remained constant (Fig. 2A). The entire blot intensity for pF1fs alone (Fig. 2A, top panel) was substantially enhanced relative to that for Fig. 2B and C to allow for visualization of the time zero RNA1fs band. The actual relative band intensities of RNA1fs alone compared to RNA1fs with protein AD692E-YFP under these conditions are shown in Fig. 1B, lanes 3 and 5, respectively. Time course analysis (Fig. 2D) was used to calculate the RNA1fs half-life. Dissecting the pF1fs curve revealed two pools of RNA1fs. One pool, containing >90% of the RNA1fs transcripts, degraded rapidly, with a half-life of about 4 minutes. In contrast, about 5% of the RNA1fs transcripts persisted with a much longer half-life throughout the time course studied, suggesting a second, minor pool, such as nuclear transcripts.
Coexpressing wt protein A from pFAwt-YFP and pF1fs maintained a nearly constant level of RNA1fs per cell after glucose-induced shutoff (Fig. 2B), representing the steady-state level of RNA1fs maintained by RNA replication in the rapidly dividing yeast cells (Fig. 1B and D). In the absence of protein A polymerase activity, protein AD692E-YFP dramatically enhanced RNA1fs stability to a half-life of ∼66 min (Fig. 2C and D). Since the PGK protein is an abundantly expressed cytoplasmic protein in yeast and the PGK1 mRNA is very stable (half-life is 45 min), the PGK protein was used as a control (Fig. 2) (29). Despite occasional fluctuations in individual measurements, 18S rRNA, PGK protein, and protein A (where expressed) levels were constant, on average, at all time points (Fig. 2A to C).
Increased RNA1fs accumulation is not due to protein A-mediated RNA recapping.
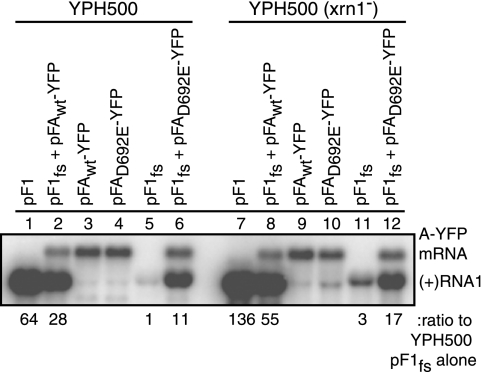
RNA1 is sufficient for viral replication, and protein A contains a proposed guanylylation site that is suggestive of guanylyltransferase activity (33). Consequently, protein A likely adds the 5′ 7-methylguanosine caps found on FHV genomic RNAs (33). In yeast, the major mechanism of mRNA decay occurs by decapping followed by Xrn1p-mediated 5′-to-3′ degradation (37, 65, 67). Because selective recapping of decapped RNA1fs by protein A could increase RNA1fs stability, we examined RNA1fs levels in wt and xrn1− mutant yeast. If protein A-stimulated RNA1fs accumulation was due only to RNA1fs recapping by protein A, thus inhibiting RNA1fs degradation, then protein AD692E should have no effect on RNA1fs levels in xrn1− yeast. Furthermore, the equal RNA1fs levels in xrn1− yeast with or without protein AD692E should match the high RNA1fs levels in wt yeast expressing protein AD692E. Instead, we found that protein A still stimulated RNA1fs accumulation in xrn1− yeast approximately sixfold (Fig. 3, lanes 11 and 12). Additionally, RNA1fs levels in xrn1− yeast lacking protein AD692E were approximately fourfold lower than RNA1fs levels in wt yeast expressing protein AD692E (Fig. 3, lanes 6 and 11). Thus, a protein A function or functions other than RNA1fs recapping caused increased RNA1fs accumulation prior to negative-strand RNA synthesis.
FIG. 3.
Protein A increases RNA1fs accumulation in yeast defective in the mRNA decay pathway (xrn1−). YPH500 cells (wt or xrn1−) were transformed with pF1, pF1fs plus pFAwt-YFP, pFAwt-YFP, pFAD692E-YFP, pF1fs, or pF1fs plus pFAD692E-YFP. Total RNA was isolated and analyzed by Northern blotting as described in the legend to Fig. 1. Representative Northern blots from four independent experiments are shown.
Protein A selectively induces RNA1fs membrane association.
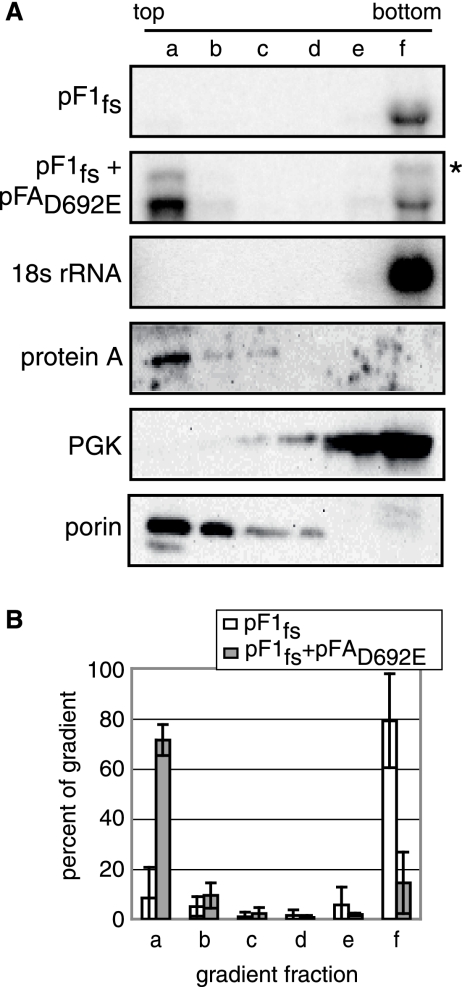
FHV replicates in association with the outer mitochondrial membrane (45). Consequently, FHV genomic RNA must localize to the outer mitochondrial membrane before or during RNA synthesis. To test if protein A-stabilized RNA1fs was membrane associated, we used a Nycodenz gradient membrane flotation assay. As expected, 18S rRNA and the cytoplasmic yeast protein PGK were recovered only in the soluble, cytoplasmic fraction at the bottom of the gradient, while protein A and a yeast mitochondrial porin protein were present in the membrane-associated fraction at the top of the gradient, as seen by Northern and Western blotting (Fig. 4A). In the absence of protein A, ∼80% of RNA1fs was present at the bottom of the gradient, while in the presence of pFAD692E-YFP, RNA1fs accumulation increased and ∼70% of RNA1fs was present at the top of the gradient (Fig. 4A and B). As described for Fig. 2, in Fig. 4A the entire blot intensity for pF1fs alone was increased relative to that for the pF1fs-plus-pFAD692E-YFP blot to visualize the fraction f band. See Fig. 1B, lanes 3 and 5, for the actual relative band intensities of RNA1fs in the absence or presence of pFAD692E under these conditions.
FIG. 4.
Protein A induces RNA1 membrane association in yeast. Yeast cells transformed with pF1fs alone or together with pFAD692E-YFP were grown in galactose medium to mid-log phase. Equal amounts of yeast were loaded under 5 to 35% discontinuous Nycodenz gradients and centrifuged for 4 to 5 hours at 40,000 rpm. Total RNA and protein were isolated from six equal-volume fractions from each gradient and analyzed by Northern blotting as described in the legend to Fig. 1 or by Western blotting with antibodies against protein A, PGK, and the yeast mitochondrial porin protein. The asterisk marks the protein A mRNA band (A). The amount of RNA1fs in each gradient fraction, determined from four independent experiments, was graphed as the percentage of RNA1fs in the entire gradient (B).
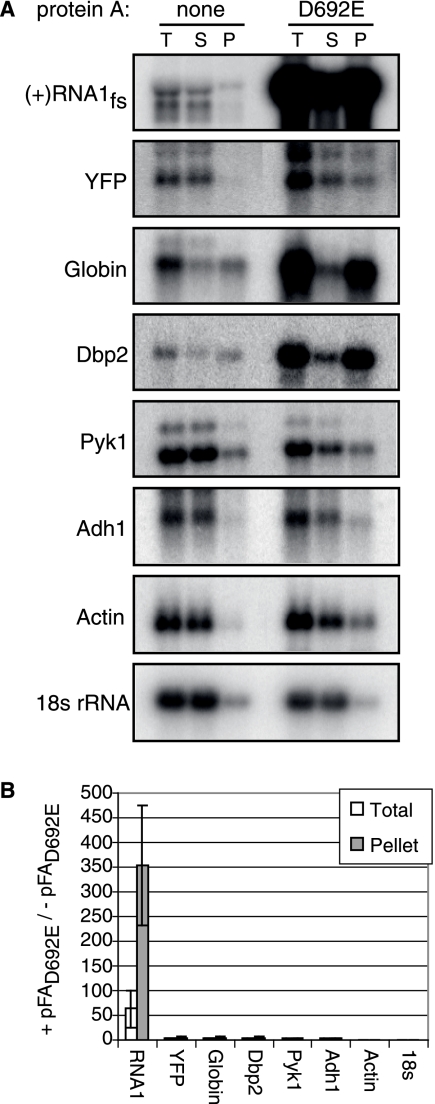
To determine if protein A preferentially increased the accumulation and membrane association of RNA1fs relative to those of nonviral RNAs, we used a cell fractionation assay. Centrifugation of a cell lysate created a membrane-enriched pellet(P) fraction and a membrane-depleted, cytoplasmic supernatant (S) fraction. Total RNA was isolated from the S and P fractions and an unfractionated yeast aliquot (total [T]). Equal fractions of yeast expressing no plasmids or the selectable plasmid pF1fs, pGlobin, or pYFP in the presence or absence of pFAD692E or pFAD692E-YFP were analyzed by Northern blotting with 32P-labeled RNA probes against positive-strand RNA1, selected cellular mRNAs, globin mRNA, or YFP mRNA. pF1fs alone yielded a very low level of RNA1fs accumulation that was mostly localized to the S fraction (Fig. 5A). However, when pF1fs and pFAD692E-YFP were coexpressed, the RNA1fs pool in the S fraction was joined by a larger pool of RNA1fs in the P fraction (Fig. 5A). Protein A increased RNA1fs levels ∼60-fold and ∼350-fold in the T and P fractions, respectively (Fig. 5B). The level of protein A-stimulated total RNA1fs accumulation determined in this cell fractionation assay was higher than the 14-fold increase shown in Fig. 1. This difference was likely due to minor assay differences, such as the cell lysis and subsequent fractionation time prior to RNA extraction and necessary differences in the RNA extraction methods themselves (see Materials and Methods), and likely primarily reflected preferential decay of RNA1fs in the absence of protein A. Despite these differences, both assays show that protein A greatly increases RNA1fs accumulation. In contrast, protein A had no detectable effect on PYK1 mRNA, ADH1 mRNA, actin mRNA, and 18S rRNA (Fig. 5). Interestingly, protein A also increased the accumulation of globin mRNA, YFP mRNA, and DBP2 mRNA (Fig. 5A). However, the effects of protein A on these cellular RNAs were dramatically less (2- to 4-fold and 3- to 5-fold increases in the T and P fractions, respectively) than the 60- to 350-fold increase for RNA1fs (Fig. 5A and B).
FIG. 5.
Protein A selectively induces FHV RNA1fs membrane association. (A) Yeast cells expressing no plasmids or a selectable plasmid (pGlobin, pYFP, or pF1fs) in the presence or absence of protein AD692E were grown in galactose medium to mid-log phase and spheroplasted. Half of each sample was centrifuged for 5 minutes at 20,000 rpm to separate the membrane-enriched P fraction from the cytosolic S fraction, and half of each sample was untreated (T). Total RNA was isolated from each fraction, and equal amounts of RNA (2 μg of T) were analyzed by Northern blotting with [32P]cRNA probes against positive-strand RNA1, globin mRNA, YFP mRNA, DBP2 mRNA, PYK1 mRNA, ADH1 mRNA, actin mRNA, and 18S rRNA. (B) RNA levels in the T or P fractions in the presence and absence of pFAD692E, determined from three independent experiments.
Protein A stimulates RNA1fs membrane association in Drosophila melanogaster.
Since FHV naturally infects insects, we next analyzed the ability of protein A to stimulate membrane association in Drosophila melanogaster cells, which support productive FHV infection. To analyze RNA1fs levels in the absence and presence of protein AD692E in Drosophila cells, we transfected these cells with plasmid pIE1-F1fs in the absence or presence of pIE1-FAD692E, which expressed RNA1fs and protein AD692E, respectively, from the baculovirus IE1 protein promoter. Two days after transfection, cells were harvested, lysed by Dounce homogenization, and assayed by flotation as described for Fig. 4. Since plasmid transfection levels in D. melanogaster cells are low and RNA1fs levels were further diluted by fractionation in flotation gradients, Northern blotting was insufficiently sensitive to reliably analyze RNA1fs levels in each gradient fraction. Accordingly, real-time RT-PCR was performed on DNase-treated RNA (5 ng) samples, using primer sets that selected for the wt FHV 5′ UTR of RNA1fs, thus avoiding coamplification of protein AD692E mRNA. In parallel, Drosophila actin mRNA was analyzed by RT-PCR to verify assay functionality and provide an internal control.
When RNA1fs was expressed alone in D. melanogaster, 16% and 56% of RNA1fs were present at the top and bottom of the gradient, respectively (Fig. 6A). However, when protein AD692E was expressed with RNA1fs in D. melanogaster, the distribution reversed, with 62% and 20% of RNA1fs present at the top and bottom of the gradient, respectively (Fig. 6A). As expected, Western blotting showed that protein A and the voltage-dependent anion-channel mitochondrial porin protein localized to the top of the gradient, while actin protein was found at the bottom (data not shown). Thus, the behavior of RNA1fs and the effect of protein AD692E on RNA1fs association with membranes were equivalent in Drosophila cells (Fig. 6A) and yeast (Fig. 4). In contrast, the presence of protein AD692E did not increase total RNA1fs levels in Drosophila cells, as determined by Northern blotting and quantitative real-time RT-PCR (Fig. 6B). This result differs from the 14-fold protein A-stimulated RNA1fs accumulation effect seen in yeast (Fig. 1).
Conceivably, this difference in protein A-mediated RNA1fs accumulation in yeast and Drosophila cells might result from greater protein A expression in yeast than that in Drosophila. Therefore, we determined the ratio of protein A to RNA1fs in both yeast and Drosophila cells expressing RNA1fs and protein AD692E (Fig. 6C). Protein A levels and total RNA (75 ng) were analyzed by Western blotting and real-time RT-PCR, respectively, as shown in Fig. 1 and described above. Total protein levels were determined using a modified Bradford assay that was compatible with reducing agents. The assays revealed that there were no significant differences between yeast and Drosophila cells in the ratios of protein A to total protein, RNA1fs to total RNA, and thus, protein A to RNA1fs (Fig. 6C). Consequently, differences in protein A expression levels did not explain the differences in protein A-mediated RNA1fs accumulation in yeast and Drosophila cells.
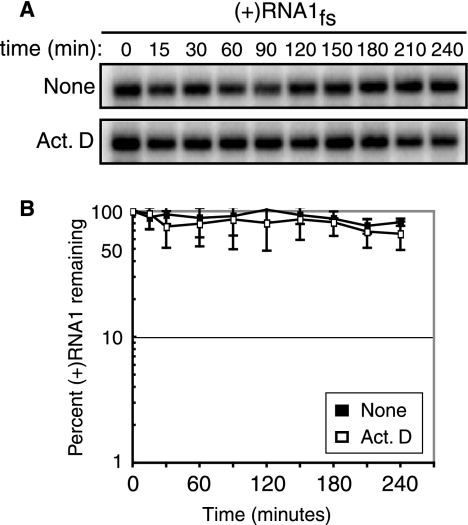
Differences in RNA1fs stability in the absence of protein A could also affect protein A-mediated RNA1fs accumulation levels. In these experiments with Drosophila cells, cellular transcription of plasmid pIE1-F1fs was the only source of RNA1fs. Consequently, to measure RNA1fs stability, we treated Drosophila cells with actinomycin D at 20 μg/ml 2 days after transfection with pIE1-F1fs to inhibit cellular transcription. Cells were collected at 10 time points over a 4-hour time period after actinomycin D treatment, and total RNA (4 μg) was isolated and analyzed by Northern blotting. Cellular transcription was inhibited within 30 min of actinomycin D treatment, as seen by labeling with [32P]UTP for 15 min prior to each time point (data not shown). However, even 4 hours after actinomycin D treatment, RNA1fs levels were indistinguishable from RNA1fs levels in untreated Drosophila cells (Fig. 7) or in Drosophila cells expressing protein AD692E. Consequently, the half-life of RNA1fs in the absence of protein A in Drosophila cells was at least greater than 3 hours, and thus dramatically higher than the approximately 4-min half-life of RNA1fs in yeast (Fig. 2 and 7). Thus, differing RNA1fs intrinsic stabilities in yeast and Drosophila cells lacking protein A appear to account for the difference between the abilities of protein AD692E to stimulate RNA1fs accumulation in yeast and Drosophila cells.
FIG. 7.
RNA1fs is stable in Drosophila cells. D. melanogaster cells expressing RNA1fs alone were treated with actinomycin D or dimethyl sulfoxide (None) 2 days after transfection. (A) Total RNA was extracted from equal cell aliquots collected 0 to 4 hours after treatment and analyzed by Northern blotting as described in the legend to Fig. 1 (except that 4 μg of RNA was used). (B) RNA levels determined from three independent experiments were graphed as the percentage of positive-strand RNA1fs remaining compared to that at time zero, using the same scale as that in Fig. 2B.
Protein A region(s) required for RNA1fs recruitment.
Protein A is a multifunctional protein, and different regions of protein A are required for its many functions. A transmembrane domain and additional mitochondrial targeting signals are encompassed in the N-terminal 35 and 47 aa of protein A, respectively (44). Regions within and including aa 1 to 400 and 700 to 800 of protein A self-interact in vivo (15). In addition, the eight conserved RdRp motifs of protein A are located between aa 513 and 752 (35). We have shown here that another function of protein A is to recruit RNA1 to a membrane-associated state (Fig. 1 to 7). To determine the region(s) of protein A required for this new function, we made 29 in-frame deletion mutants that spanned the length of protein A (Fig. 8A and 9A). Yeast cells expressing RNA1fs and protein AD692E deletion mutants were assayed for total RNA1fs accumulation and graphed as the percentage of RNA1fs accumulation when RNA1fs and full-length protein AD692E were expressed (Fig. 8A and F). Although minor variations in accumulation of the various protein A derivatives occurred in some experiments (Fig. 8D and 9D), over many experiments the average levels of the various protein A deletion mutants and full-length protein AD692E were similar. Consequently, any differences in average RNA1fs accumulation were due to differences in the specific activities, not expression, of each protein A deletion mutant.
Multiple results implied that membrane association of protein A was required for RNA1fs accumulation. Eliminating protein A membrane association by deleting the N-terminal 370 aa (Δ1-370) (Fig. 8A and G) reduced RNA1fs accumulation nearly fourfold (Fig. 8B, lane 3). Furthermore, adding back just 35 to 47 aa of protein A sequence sufficient for mitochondrial localization, including the transmembrane domain (aa 17 to 35), completely restored protein A membrane association and RNA1fs accumulation (Δ36-370 and Δ47-370) (Fig. 8B, lanes 4 and 5, and G) (44, 46). Despite occasional small fluctuations in individual RNA1fs accumulation measurements, deleting the protein A transmembrane domain (Δ1-35) caused a milder, on average approximately twofold decrease, in RNA1fs accumulation (Fig. 8B and F, lanes 6) but maintained protein A membrane association (Fig. 8G). Despite the fact that the entire region of aa 36 to 370 was dispensable for protein A-mediated RNA1fs accumulation (Δ36-370) (Fig. 8B, lane 4), various partial deletions of 60 to 70 aa between aa 35 and 370 of protein A also caused milder reductions in protein A-mediated RNA1fs accumulation (Δ36-98, Δ99-170, Δ171-230, Δ231-300, and Δ301-370) (Fig. 8B, lanes 7 to 11), suggesting a perturbation of protein folding, interaction, or similar functions.
The C-terminal 100 aa of protein A (Δ901-950 and Δ951-998) were also dispensable for protein A-stimulated RNA1fs accumulation (Fig. 8, lanes 17 and 18). In contrast, although protein A RdRp activity was not required for RNA1fs accumulation (Fig. 1), deletion of the RdRp region (Δ513-752) significantly reduced RNA1fs accumulation (Fig. 8B, lane 14, and 9B). To explore this further, we made separate deletions of each RdRp motif, which ranged in size from 9 to 35 aa, and the spaces between these motifs (Fig. 9A). The first RdRp motif (R1 [Δ513-752]) was dispensable for RNA1fs accumulation (Fig. 9B, lane R1). In contrast, deletion of all other RdRp motifs and spaces between RdRp motifs reduced RNA1fs accumulation to approximately threefold lower than RNA1fs levels expressed in the presence of full-length protein AD692E-YFP, although deletions of the third and fourth spaces between RdRp motifs were the least defective (Fig. 9). RNA1fs accumulation also required protein A regions immediately surrounding the RdRp motifs, since deletions of approximately 70 aa on either side of the RdRp motifs (Δ371-440, Δ441-512, Δ753-825, and Δ826-900) significantly reduced RNA1fs accumulation (Fig. 8B, lanes 12, 13, 15, and 16).
These results show that efficient protein A-mediated RNA1 recruitment requires protein A membrane association and the regions surrounding and including RdRp motifs. However, with the exception of the transmembrane domain, the N-terminal 370 aa of protein A, including some protein A self-interaction domains, and the C-terminal 100 aa of protein A are dispensable for protein A-mediated RNA1 recruitment.
DISCUSSION
In this study, we used FHV to analyze selected aspects of positive-strand RNA virus replication, revealing a new function for FHV protein A in recruiting genomic RNA1 to a membrane-associated state. As discussed below, our results imply that during an early stage of replication prior to negative-strand RNA synthesis, multiple regions of protein A, including regions required for other protein A functions, act together to recruit RNA1 to membranes for FHV RNA replication complex formation.
Protein A recruits RNA1 to a membrane-associated state.
In both Drosophila and yeast cells, FHV protein A induced otherwise cytoplasmic RNA1 to become largely membrane associated, causing similar near-inversions of the distribution of RNA1 between soluble and membrane-associated fractions in both cell types (Fig. 4 and 6). This strong membrane localization was induced by protein A even when viral RNA synthesis was blocked by an RdRp active site mutation and was strongly selective for FHV RNA, increasing the accumulation of RNA1fs in the membrane-associated pellet fraction 70- to >300-fold more than that of diverse nonviral mRNAs (Fig. 5). Since protein A is the sole FHV protein required for RNA replication and is a transmembrane protein localized almost exclusively to outer mitochondrial membranes, the results imply that protein A recruits RNA1 prior to RNA replication to the outer mitochondrial membranes, where FHV RNA replication complexes form (44, 45).
However, in yeast, this protein A-induced membrane association was linked with a >10-fold increase in RNA1 accumulation, while in Drosophila cells no significant increase in RNA1 accumulation occurred (Fig. 1 and 6B). This difference in protein A-mediated RNA1 accumulation was not caused by differences in the amount of protein A available in each cell type to confer RNA1 stability, since the ratios of protein A to RNA1 in yeast and Drosophila cells were similar (Fig. 6C). Rather, in yeast cells, which are widely diverged from FHV's natural insect host cells, the >10-fold increase reflected the fact that RNA1 was stable in the presence of protein A (>60-min half-life) but had low basal stability and accumulation in the absence of protein A (4-min half-life) (Fig. 2). In contrast, in Drosophila cells, which support productive FHV infection (8, 63), the intrinsic stability (>3-hour half-life) of RNA1 was much higher than that in yeast (Fig. 7). Accordingly, the accumulation of RNA1 without protein A was close to that with protein A (Fig. 6B). Similarly, the BMV 1a protein, which recruits viral RNA replication templates to a membrane-associated, highly stable state for RNA replication, increased BMV genomic RNA3 stability and accumulation ∼10-fold in yeast, but little or not at all in Nicotiana benthamiana cells, apparently because the basal stability of plant-adapted BMV RNA in the absence of 1a is much higher in plant cells than in yeast cells (25, 32, 66).
Nevertheless, the short, ∼5-min half-life of BMV and FHV RNAs in yeast cells in the absence of viral factors has been useful as a marker to reveal and follow the 1a- and protein A-induced changes in the state of viral RNA. For BMV, multiple additional studies have revealed that the 1a-induced increase in viral RNA stability reflects important effects associated with recruitment to a membrane-associated state, including translation inhibition and sequestration of the RNA in a nuclease-resistant compartment (1, 32, 62, 68). Since BMV and FHV replicate their RNAs in similar, virus-induced membrane invaginations (45, 62), similar effects appear likely to underlie the related protein A-induced changes in the state of FHV RNA1. The increase in RNA1 stability, and thus accumulation, was not significantly due to the predicted ability of protein A to cap FHV RNAs (33), since protein A increased RNA1fs accumulation sixfold even in yeast lacking the Xrn1p 5′-to-3′ exonuclease that degrades uncapped RNAs (Fig. 3) (37, 65, 67). A lack of RNA1 recapping by protein A is consistent with the fact that cellular decapping enzymes produce 5′-monophosphate ends (67), while known positive-strand RNA virus capping pathways require 5′-diphosphate substrates (2, 3, 14, 30, 41), and with continuing stimulation of RNA1 accumulation after deleting the predicted guanylyltransferase active site (aa 91 to 93) from protein A (Δ36-370 and Δ47-370) (Fig. 8, lanes 4 and 5) (33).
Protein A regions required for RNA1 recruitment.
Protein A was the only viral protein required for FHV genomic RNA1 recruitment to membranes, implying that protein A interacts directly or indirectly (via host proteins) with FHV RNA1 to recruit it to a membrane-associated state. Recruiting FHV genomic RNAs required both membrane-targeting domains and the RdRp domain of protein A (Fig. 8 and 9). This association of RNA recruitment with the FHV RdRp domain differs from the case for BMV, cucumber necrosis tombusvirus, and tomato bushy stunt tombusvirus, for which genomic RNA recruitment to membranes requires only viral protein 1a or p33, respectively, which contain membrane-targeting and other domains but lack the RdRp domains carried by other proteins of these viruses (47, 51, 54, 55, 62). Use of the FHV protein A RdRp domain for template recruitment as well as polymerization may reflect appropriate or essential economy for a virus with a single RNA replication protein. Moreover, like the case for FHV, carnation Italian ringspot tombusvirus recruitment of DI-RNAs to a stabilized state is significantly enhanced by the presence of both membrane-targeting and RdRp domains, carried by the viral p36 and p95 proteins, respectively, although either p36 or p95 confers some DI-RNA stabilization (35, 49, 56, 57). Additionally, the poliovirus protein 3CD, containing protease and RdRp domains, is required to bind a 5′ cloverleaf in poliovirus RNA to mediate the switch from translation to viral RNA replication, a step that is at least a precursor to recruiting viral RNA to the replication complex (24). When expressed in vitro, 3CD and 3C, but not 3D, can bind the 5′ cloverleaf (70). However, mutations in both the 3C and 3D regions of 3CD rescue complementary changes in the 5′-cloverleaf structure, suggesting that regions in both proteins are important for RNA binding (69).
While active site mutation showed that polymerase activity was not required, nearly all deletions within or flanking the conserved RdRp motifs of FHV protein A dramatically suppressed protein A-stimulated RNA1 accumulation (Fig. 8 and 9). The strong effect of even small deletions in this region suggests that proper folding of the RdRp domain may be important for RNA1 recruitment. Such folding might be required whether the RdRp domain contributes to recruitment by RNA or to protein interactions that are the same or distinct from those in RNA polymerization. For example, multiple regions surrounding and including the conserved RdRp motifs of foot-and-mouth disease virus 3D polymerase are involved in binding RNA templates, as seen by X-ray crystallography (19), and tomato bushy stunt virus p92 contains multiple RNA binding sites flanking and overlapping its conserved RdRp motifs (54).
Our deletion analysis revealed a parallel between the membrane association of protein A derivatives and their ability to stimulate RNA1fs accumulation. Deleting the N-terminal 370 aa of protein A (Δ1-370) eliminated protein A-membrane association and increased RNA1fs recruitment, while retaining protein A sequences sufficient for membrane association and mitochondrial targeting (Δ36-370 and Δ47-370) fully restored protein A stimulation of RNA1fs accumulation (Fig. 8). Deleting the transmembrane domain (Δ1-35) significantly inhibited but did not completely eliminate increased RNA1fs accumulation (Fig. 8). However, this derivative remained largely membrane associated (Fig. 8) (46), showing that protein A has more than one mode of membrane association and explaining the remaining RNA1 recruitment activity without violating the membrane association correlation.
Several functions may contribute to the link between protein A-membrane association and increased RNA1 stability/accumulation in yeast. Membrane association might alter the protein A conformation and would increase the local protein A concentration, promoting protein A multimerization and potentially cooperative binding to RNA1. Protein A forms multimers in vivo, and this ability is linked to successful RNA replication (15). While some N-proximal protein A self-interaction domains were dispensable for increased RNA1 accumulation, a self-interaction domain overlapping the RdRp region was required and might represent a significant contribution of the RdRp domain (Fig. 8) (15). Similarly, self-interaction of tomato bushy stunt virus p33 is required for DI-RNA recruitment to the membrane sites of replication complex formation (47, 51). As found for BMV (62), protein A-membrane binding and the FHV-specific outer mitochondrial membrane invaginations associated with RNA replication (45) may be important for compartmentalizing RNA1 away from cellular translation and RNA decay pathways. We also cannot rule out that membrane association may be important for protein A or RNA1 association with host factors.
The results reported here for FHV in both D. melanogaster cells and S. cerevisiae cells show some strong parallels with BMV and with recent emerging results for tombusviruses. In all cases, a viral replication protein recruits genomic RNA to membranes prior to negative-strand RNA synthesis (1, 47, 51, 62). Such recruitment is often accompanied by an increase in genomic RNA stability and accumulation that is tightly linked to RNA membrane association (12, 32, 62, 66). Membrane-targeting domains and, in some cases, RdRp domains and self-interaction domains within the viral replication proteins are required for these effects on genomic RNA (12, 47, 49, 51, 54, 66). Given the many other substantial differences between FHV, BMV, and the tombusviruses, these related features suggest that some of the major principles involved in the RNA recruitment processes revealed here may be applicable to a range of positive-strand RNA viruses.
Acknowledgments
We thank Paul Friesen, Ben Kopek, and Billy Dye for helpful comments and David Miller, David Kushner, Billy Dye, Erik Settles, and Paul Friesen for various plasmids.
This work was supported by NIH grant GM35072. P.A. is an investigator of the Howard Hughes Medical Institute. P.M.V. gratefully acknowledges support from a Howard Hughes Medical Institute predoctoral fellowship and an NIH Predoctoral Training grant in Molecular Biosciences (T32 GM07215).
Footnotes
Published ahead of print on 14 February 2007.
REFERENCES
- 1.Ahlquist, P. 2006. Parallels among positive-strand RNA viruses, reverse-transcribing viruses and double-stranded RNA viruses. Nat. Rev. Microbiol. 4:371-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahola, T., and P. Ahlquist. 1999. Putative RNA capping activities encoded by brome mosaic virus: methylation and covalent binding of guanylate by replicase protein 1a. J. Virol. 73:10061-10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahola, T., and L. Kaariainen. 1995. Reaction in alphavirus mRNA capping: formation of a covalent complex of nonstructural protein nsP1 with 7-methyl-GMP. Proc. Natl. Acad. Sci. USA 92:507-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argos, P. 1988. A sequence motif in many polymerases. Nucleic Acids Res. 16:9909-9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, NY.
- 6.Ball, L. A. 1992. Cellular expression of a functional nodavirus RNA replicon from vaccinia virus vectors. J. Virol. 66:2335-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball, L. A. 1995. Requirements for the self-directed replication of flock house virus RNA 1. J. Virol. 69:720-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ball, L. A., and K. L. Johnson. 1998. Nodaviruses of insects, p. 225-267. In L. K. Miller and L. A. Ball (ed.), The insect viruses. Plenum Publishing Corporation, New York, NY.
- 9.Benzaghou, I., I. Bougie, and M. Bisaillon. 2004. Effect of metal ion binding on the structural stability of the hepatitis C virus RNA polymerase. J. Biol. Chem. 279:49755-49761. [DOI] [PubMed] [Google Scholar]
- 10.Cartier, J. L., P. A. Hershberger, and P. D. Friesen. 1994. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: dominant interference by N-terminal sequences p35(1-76). J. Virol. 68:7728-7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen, J., A. Noueiry, and P. Ahlquist. 2001. Brome mosaic virus protein 1a recruits viral RNA2 to RNA replication through a 5′-proximal RNA2 signal. J. Virol. 75:3207-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dasgupta, R., A. Ghosh, B. Dasmahapatra, L. A. Guarino, and P. Kaesberg. 1984. Primary and secondary structure of black beetle virus RNA2, the genomic messenger for BBV coat protein precursor. Nucleic Acids Res. 12:7215-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunigan, D. D., and M. Zaitlin. 1990. Capping of tobacco mosaic virus RNA. Analysis of viral-coded guanylyltransferase-like activity. J. Biol. Chem. 265:7779-7786. [PubMed] [Google Scholar]
- 15.Dye, B. T., D. J. Miller, and P. Ahlquist. 2005. In vivo self-interaction of nodavirus RNA replicase protein A revealed by fluorescence resonance energy transfer. J. Virol. 79:8909-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egger, D., and K. Bienz. 2005. Intracellular location and translocation of silent and active poliovirus replication complexes. J. Gen. Virol. 86:707-718. [DOI] [PubMed] [Google Scholar]
- 17.Egger, D., B. Wolk, R. Gosert, L. Bianchi, H. E. Blum, D. Moradpour, and K. Bienz. 2002. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J. Virol. 76:5974-5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elazar, M., P. Liu, C. M. Rice, and J. S. Glenn. 2004. An N-terminal amphipathic helix in hepatitis C virus (HCV) NS4B mediates membrane association, correct localization of replication complex proteins, and HCV RNA replication. J. Virol. 78:11393-11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrer-Orta, C., A. Arias, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2004. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J. Biol. Chem. 279:47212-47221. [DOI] [PubMed] [Google Scholar]
- 20.Friesen, P. D., and R. R. Rueckert. 1982. Black beetle virus: messenger for protein B is a subgenomic viral RNA. J. Virol. 42:986-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friesen, P. D., and R. R. Rueckert. 1981. Synthesis of black beetle virus proteins in cultured Drosophila cells: differential expression of RNAs 1 and 2. J. Virol. 37:876-886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallagher, T. M., P. D. Friesen, and R. R. Rueckert. 1983. Autonomous replication and expression of RNA 1 from black beetle virus. J. Virol. 46:481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallagher, T. M., and R. R. Rueckert. 1988. Assembly-dependent maturation cleavage in provirions of a small icosahedral insect ribovirus. J. Virol. 62:3399-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamarnik, A. V., and R. Andino. 1998. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 12:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopinath, K., B. Dragnea, and C. Kao. 2005. Interaction between brome mosaic virus proteins and RNAs: effects on RNA replication, protein expression, and RNA stability. J. Virol. 79:14222-14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosert, R., D. Egger, V. Lohmann, R. Bartenschlager, H. E. Blum, K. Bienz, and D. Moradpour. 2003. Identification of the hepatitis C virus RNA replication complex in Huh-7 cells harboring subgenomic replicons. J. Virol. 77:5487-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guarino, L. A., A. Ghosh, B. Dasmahapatra, R. Dasgupta, and P. Kaesberg. 1984. Sequence of the black beetle virus subgenomic RNA and its location in the viral genome. Virology 139:199-203. [DOI] [PubMed] [Google Scholar]
- 28.Heinlein, M., H. S. Padgett, J. S. Gens, B. G. Pickard, S. J. Casper, B. L. Epel, and R. N. Beachy. 1998. Changing patterns of localization of the tobacco mosaic virus movement protein and replicase to the endoplasmic reticulum and microtubules during infection. Plant Cell 10:1107-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrick, D., R. Parker, and A. Jacobson. 1990. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol. Cell. Biol. 10:2269-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang, Y. L., Y. H. Hsu, Y. T. Han, and M. Meng. 2005. mRNA guanylation catalyzed by the S-adenosylmethionine-dependent guanylyltransferase of bamboo mosaic virus. J. Biol. Chem. 280:13153-13162. [DOI] [PubMed] [Google Scholar]
- 31.Jablonski, S. A., and C. D. Morrow. 1995. Mutation of the aspartic acid residues of the GDD sequence motif of poliovirus RNA-dependent RNA polymerase results in enzymes with altered metal ion requirements for activity. J. Virol. 69:1532-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janda, M., and P. Ahlquist. 1998. Brome mosaic virus RNA replication protein 1a dramatically increases in vivo stability but not translation of viral genomic RNA3. Proc. Natl. Acad. Sci. USA 95:2227-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson, K. N., K. L. Johnson, R. Dasgupta, T. Gratsch, and L. A. Ball. 2001. Comparisons among the larger genome segments of six nodaviruses and their encoded RNA replicases. J. Gen. Virol. 82:1855-1866. [DOI] [PubMed] [Google Scholar]
- 34.Kamer, G., and P. Argos. 1984. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 12:7269-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koonin, E. V. 1991. The phylogeny of RNA-dependent RNA polymerases of positive-strand RNA viruses. J. Gen. Virol. 72:2197-2206. [DOI] [PubMed] [Google Scholar]
- 36.Kujala, P., A. Ikaheimonen, N. Ehsani, H. Vihinen, P. Auvinen, and L. Kaariainen. 2001. Biogenesis of the Semliki Forest virus RNA replication complex. J. Virol. 75:3873-3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larimer, F. W., C. L. Hsu, M. K. Maupin, and A. Stevens. 1992. Characterization of the XRN1 gene encoding a 5′→3′ exoribonuclease: sequence data and analysis of disparate protein and mRNA levels of gene-disrupted yeast cells. Gene 120:51-57. [DOI] [PubMed] [Google Scholar]
- 38.Li, H., W. X. Li, and S. W. Ding. 2002. Induction and suppression of RNA silencing by an animal virus. Science 296:1319-1321. [DOI] [PubMed] [Google Scholar]
- 39.Lindenbach, B. D., J. Y. Sgro, and P. Ahlquist. 2002. Long-distance base pairing in flock house virus RNA1 regulates subgenomic RNA3 synthesis and RNA2 replication. J. Virol. 76:3905-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu, R., M. Maduro, F. Li, H. W. Li, G. Broitman-Maduro, W. X. Li, and S. W. Ding. 2005. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 436:1040-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Magden, J., N. Takeda, T. Li, P. Auvinen, T. Ahola, T. Miyamura, A. Merits, and L. Kaariainen. 2001. Virus-specific mRNA capping enzyme encoded by hepatitis E virus. J. Virol. 75:6249-6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magliano, D., J. A. Marshall, D. S. Bowden, N. Vardaxis, J. Meanger, and J. Y. Lee. 1998. Rubella virus replication complexes are virus-modified lysosomes. Virology 240:57-63. [DOI] [PubMed] [Google Scholar]
- 43.Mas, P., and R. N. Beachy. 1999. Replication of tobacco mosaic virus on endoplasmic reticulum and role of the cytoskeleton and virus movement protein in intracellular distribution of viral RNA. J. Cell Biol. 147:945-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, D. J., and P. Ahlquist. 2002. Flock house virus RNA polymerase is a transmembrane protein with amino-terminal sequences sufficient for mitochondrial localization and membrane insertion. J. Virol. 76:9856-9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, D. J., M. D. Schwartz, and P. Ahlquist. 2001. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells. J. Virol. 75:11664-11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller, D. J., M. D. Schwartz, B. T. Dye, and P. Ahlquist. 2003. Engineered retargeting of viral RNA replication complexes to an alternative intracellular membrane. J. Virol. 77:12193-12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Panavas, T., C. M. Hawkins, Z. Panaviene, and P. D. Nagy. 2005. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of cucumber necrosis tombusvirus. Virology 338:81-95. [DOI] [PubMed] [Google Scholar]
- 48.Panaviene, Z., T. Panavas, and P. D. Nagy. 2005. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J. Virol. 79:10608-10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pantaleo, V., L. Rubino, and M. Russo. 2004. The p36 and p95 replicase proteins of carnation Italian ringspot virus cooperate in stabilizing defective interfering RNA. J. Gen. Virol. 85:2429-2433. [DOI] [PubMed] [Google Scholar]
- 50.Perrigoue, J. G., J. A. den Boon, A. Friedl, M. A. Newton, P. Ahlquist, and B. Sugden. 2005. Lack of association between EBV and breast carcinoma. Cancer Epidemiol. Biomarkers Prev. 14:809-814. [DOI] [PubMed] [Google Scholar]
- 51.Pogany, J., K. A. White, and P. D. Nagy. 2005. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J. Virol. 79:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Price, B. D., R. R. Rueckert, and P. Ahlquist. 1996. Complete replication of an animal virus and maintenance of expression vectors derived from it in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93:9465-9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Price, B. D., M. Roeder, and P. Ahlquist. 2000. DNA-directed expression of functional flock house virus RNA1 derivatives in Saccharomyces cerevisiae, heterologous gene expression, and selective effects on subgenomic mRNA synthesis. J. Virol. 74:11724-11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rajendran, K. S., and P. D. Nagy. 2003. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J. Virol. 77:9244-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Restrepo-Hartwig, M. A., and P. Ahlquist. 1996. Brome mosaic virus helicase- and polymerase-like proteins colocalize on the endoplasmic reticulum at sites of viral RNA synthesis. J. Virol. 70:8908-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rubino, L., and M. Russo. 1998. Membrane targeting sequences in tombusvirus infections. Virology 252:431-437. [DOI] [PubMed] [Google Scholar]
- 57.Rubino, L., F. Weber-Lotfi, A. Dietrich, C. Stussi-Garaud, and M. Russo. 2001. The open reading frame 1-encoded (′36K') protein of carnation Italian ringspot virus localizes to mitochondria. J. Gen. Virol. 82:29-34. [DOI] [PubMed] [Google Scholar]
- 58.Salonen, A., T. Ahola, and L. Kaariainen. 2005. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 285:139-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sambrook, J., and D. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 60.Schlegel, A., T. H. Giddings, Jr., M. S. Ladinsky, and K. Kirkegaard. 1996. Cellular origin and ultrastructure of membranes induced during poliovirus infection. J. Virol. 70:6576-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schneemann, A., V. Reddy, and J. E. Johnson. 1998. The structure and function of nodavirus particles: a paradigm for understanding chemical biology. Adv. Virus Res. 50:381-446. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz, M., J. Chen, M. Janda, M. Sullivan, J. den Boon, and P. Ahlquist. 2002. A positive-strand RNA virus replication complex parallels form and function of retrovirus capsids. Mol. Cell 9:505-514. [DOI] [PubMed] [Google Scholar]
- 63.Scotti, P. D., S. Dearing, and D. W. Mossop. 1983. Flock house virus: a nodavirus isolated from Costelytra zealandica (White) (Coleoptera: Scarabaeidae). Arch. Virol. 75:181-189. [DOI] [PubMed] [Google Scholar]
- 64.Selling, B. H., R. F. Allison, and P. Kaesberg. 1990. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc. Natl. Acad. Sci. USA 87:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevens, A. 1980. Purification and characterization of a Saccharomyces cerevisiae exoribonuclease which yields 5′-mononucleotides by a 5′ leads to 3′ mode of hydrolysis. J. Biol. Chem. 255:3080-3085. [PubMed] [Google Scholar]
- 66.Sullivan, M. L., and P. Ahlquist. 1999. A brome mosaic virus intergenic RNA3 replication signal functions with viral replication protein 1a to dramatically stabilize RNA in vivo. J. Virol. 73:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tucker, M., and R. Parker. 2000. Mechanisms and control of mRNA decapping in Saccharomyces cerevisiae. Annu. Rev. Biochem. 69:571-595. [DOI] [PubMed] [Google Scholar]
- 68.Wang, X., W. M. Lee, T. Watanabe, M. Schwartz, M. Janda, and P. Ahlquist. 2005. Brome mosaic virus 1a nucleoside triphosphatase/helicase domain plays crucial roles in recruiting RNA replication templates. J. Virol. 79:13747-13758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang, Y., R. Rijnbrand, S. Watowich, and S. M. Lemon. 2004. Genetic evidence for an interaction between a picornaviral cis-acting RNA replication element and 3CD protein. J. Biol. Chem. 279:12659-12667. [DOI] [PubMed] [Google Scholar]
- 70.Yin, J., A. V. Paul, E. Wimmer, and E. Rieder. 2003. Functional dissection of a poliovirus cis-acting replication element [PV-cre(2C)]: analysis of single- and dual-cre viral genomes and proteins that bind specifically to PV-cre RNA. J. Virol. 77:5152-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]