Abstract
The Phlebovirus genus (family Bunyaviridae) is composed of a diverse group of arboviruses that cause disease syndromes ranging from mild febrile illness to hemorrhagic fever with high fatality. Although antigenically similar, these viruses differ by approximately 25% at the genome level, and their ecologies, including geographic ranges, preferred vector species, and hosts, vary considerably. In contrast to other ambisense viruses, where RNA hairpin structures which serve as transcription termination signals are frequently found separating the opposite-sense open reading frames, no evidence of predicted high-energy hairpin structures was found at the ambisense junctions of phlebovirus S RNA segments. However, a conserved sequence motif was identified on both negative and ambisense genome segments that functions as a transcription termination signal for the N, NSs, and GPC mRNAs in three diverse phleboviruses, namely, Rift Valley fever, sandfly Sicilian, and Toscana viruses. The exact termination of nascent virus mRNA molecules was determined by 3′ rapid amplification of cDNA ends. Surprisingly, analysis of the termini of mRNAs from both S and M segments of these three viruses revealed that transcription termination occurred immediately upstream of a conserved sequence motif with the general features 3′-C1-3GUCG/A-5′. In contrast, no corresponding sequence motif was found in the L segments, and analysis indicated a “runoff” transcript approach to L mRNA termination. The absolute requirement of the identified transcription termination motif was demonstrated by using a highly efficient Rift Valley fever virus reverse genetics system to generate live recombinant viruses with S segments lacking the termination signal motif for the NP or NSs mRNA and showing that these recombinant viruses generated mRNAs that failed to terminate correctly.
We present here an analysis of RNA transcription termination in three members of the genus Phlebovirus of the family Bunyaviridae, namely, Rift Valley fever virus (RVF virus), Sandfly fever Sicilian virus (SFS virus), and Toscana virus (TOS virus). Although grouped serologically, these viruses are quite distinct at the nucleotide level, with approximately 26 to 27% divergence, and have significantly different virus ecologies. Transmitted primarily by mosquitoes, RVF virus is a significant human and veterinary pathogen capable of causing explosive outbreaks of disease, ranging from mild febrile illness to hemorrhagic fever, throughout Africa and, more recently, the Arabian peninsula (3). TOS and SFS viruses are principally vectored by Phlebotomine sand flies and can cause mild febrile illness or encephalitic syndromes in humans (9, 23). While TOS virus is limited in geographic distribution to southern Europe, SFS virus is more widely spread and is found throughout Europe, the Mediterranean region, the Middle East, and northern Africa (6, 10).
These viruses all share similar genome organizations consisting of tripartite negative-sense single-stranded RNA molecules. The large (L) RNA segment encodes the virus RNA-dependent RNA polymerase, the medium (M) segment encodes the precursor glycoproteins, and the ambisense small (S) segment RNA encodes the nucleoprotein (N) in genomic sense and a nonstructural protein (NSs) in the antigenomic orientation. The mechanism of mRNA transcription from segmented negative-sense RNA virus genomes has been described widely in the literature and includes several features that are shared between viruses in the genus Phlebovirus (family Bunyaviridae) and viruses in the family Arenaviridae (5, 20, 21). Initiation of transcription in these viruses involves a similar pathway involving priming by capped host mRNAs (18, 22). However, mRNA transcription termination is less well characterized, and poly(A) tails are not generally found at the 3′ ends of mRNAs of viruses of either family. Both genome segments (S and L) are ambisense in the Arenaviridae, whereas only the S segment is ambisense in the phleboviruses. Transcription termination in arenaviruses clearly involves substantial high-energy hairpin RNA secondary structures at the intervening junction between the ambisense open reading frames (ORFs) (17, 19). The situation is less clear for phlebovirus S segments. Earlier studies utilizing Punta Toro virus and an oligonucleotide hybrid mapping approach indicated that the Punta Toro virus N and NSs mRNA 3′ ends were located about 40 nucleotides (nt) from one another (11). Secondary structure prediction analysis suggested that this intervening intergenic region may be capable of forming hairpin structures, although less convincingly than for those found in arenaviruses. Hybridization studies of Uukuniemi virus mRNAs demonstrated that although the intergenic region is approximately 70 nt long, the 3′ ends of the virus N and NSs mRNAs overlap by approximately 100 nt (i.e., each mRNA terminates within the end of the other ORF) (22). It was predicted that a short AU-rich hairpin may form within this region, although this potential structure was of relatively low energy. A similar overlap in the 3′ ends of N and NSs mRNAs was also approximately mapped for TOS virus (14, 15). Such data, together with the prediction of potential hairpin structures at the ambisense junctions of the S and M RNA segments of the plant viruses of the genus Tospovirus (family Bunyaviridae), led to the prevailing view that mRNA transcription termination of ambisense genome RNA segments of bunyaviruses likely involves secondary structure mechanisms similar to those described for arenaviruses (5, 8, 21, 24).
Transcription termination of the negative-strand L and M genome segments of phleboviruses and members of other genera within the family Bunyaviridae is generally considered to involve mechanisms other than RNA secondary structure terminators. Homopolymeric sequence elements have been found at or near the approximate locations of mRNA 3′ ends of several Bunyaviridae members, including members of the Phlebovirus genus (21). In particular, M segment mRNAs were found to terminate after a C-rich region in the virus templates of RVF virus (7) and TOS virus (15). Since RVF, SFS, and TOS viruses were also found to contain similar G-rich regions in the S segment (in the virus cRNA [vcRNA] sense), some investigators proposed that termination of N and NSs mRNAs might also be related to such sequence motifs (13).
Clear evidence of specific linear sequence motifs on negative-sense genome RNA segments serving as distinct termination signals was found for Bunyamwera virus (genus Orthobunyavirus) (1). Termination of mRNA transcription in this case was highly sensitive to the exact primary nucleotide sequence located in these motifs. Minor perturbations of these Bunyamwera virus sequence motifs greatly inhibited the functionality of the termination signal.
Using the 3′ rapid amplification of cDNA ends (3′RACE) technique, we were able to precisely determine the exact 3′ termini of the mRNAs of three diverse and medically important phleboviruses, namely, RVF, SFS, and TOS viruses. Surprisingly, a common core sequence motif was shown to represent a linear transcription termination signal both on the negative-sense M segments and on the ambisense S segments of these viruses. The absolute requirement of this transcription termination motif was demonstrated by successfully using a highly efficient RVF virus reverse genetics system (2, 12) to generate live recombinant RVF viruses with S segments lacking the termination signal motif for the NP or NSs mRNA and by showing that these recombinant viruses generated mRNAs which failed to terminate correctly. Interestingly, the mutant viruses were also found to have attenuated growth characteristics in cell culture. These findings provide a more complete understanding of the fundamental transcription mechanism of these viruses and suggest a mechanism for engineering additional S and M segment attenuation elements into live recombinant vaccines for these important diseases.
MATERIALS AND METHODS
Cells, viruses, and biosafety.
BSR-T7/5 cells which stably expressed T7 polymerase were a generous gift of K. Conzelmann (Max-von Pettenkofer-Institut, Munchen, Germany). These cells and Vero E6 cells were grown in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum and penicillin-streptomycin at the final concentration suggested by the manufacturer (Invitrogen). To maintain high expression of T7 polymerase, BSR-T7/5 cells were subcultivated and propagated once every other passage in the presence of 1 mg/ml of the selective agent G418 (Geneticin; Invitrogen). All manipulations that involved live RVF virus (wild-type [wt] or recombinant viruses) were performed within a biosafety level 4 (BSL-4) containment laboratory. All work that involved live SFS virus or TOS virus was completed within a BSL-3 laboratory. In parallel experiments, wt RVF virus strain ZH501, SFS virus strain Sabin, and TOS virus strain ISS Ph1.3 were propagated by infecting confluent monolayers of Vero E6 cells grown in 25-cm2 flasks at an approximate multiplicity of infection of 1. Infected Vero E6 cells were harvested for RNA extraction when a moderate cytopathic effect (CPE) was observed, at approximately 2 to 3 days postinfection. Total cellular and virus RNA was harvested by the addition of Tripure reagent (Roche) at a ratio of 5:1 and incubation at room temperature for 10 min. Tubes containing the resulting cell lysate were then decontaminated and transferred to a BSL-3 laboratory for RNA extraction utilizing an RNAid kit (QBiogene) according to the manufacturer's instructions.
Removal of putative signal motifs and generation of recombinant RVF viruses.
To confirm the absolute requirement of the putative transcription termination signal in transcription termination, we individually removed the two proposed signal motifs for both NP and NSs mRNAs on the RVF virus S segment. To remove each putative signal, a PCR-remote-cutter-enzyme strategy was employed. In brief, complementary PCR primers containing the following features were designed. Each primer contained the unique restriction site of BsmBI, a mutagenized RVF virus sequence containing a deletion of an 8-nt proposed motif (3′-CCCGTCGG-5′), and an additional ∼20-nt stretch that annealed within the RVF virus sequence flanking either of the proposed signal motifs, i.e., s1 or s2. After PCR amplification utilizing these primers and the full-length S segment plasmid, the resultant linear DNA amplification products were digested with 10 U of BsmBI and religated (details are available upon request). The resulting S segment plasmids had a perfect deletion of the 8-nt motif corresponding to either s1 or s2 and no residual restriction site sequences. These plasmids (pS-mut1 and pS-mut2) were used to rescue mutant 1 (S-mut1) and mutant 2 (S-mut2) recombinant RVF viruses, respectively.
To investigate the requirement of the C-rich region immediately upstream of the putative termination signal for proper function, we employed a similar strategy to introduce two separate modifications to a plasmid designed to express the RVF virus M segment (12). First, we generated a mutant plasmid (pM-mut1) that contained a deletion of a 14-nt (3′-CCCCACCACCCCAC-5′) tract that served as the template for the exact 3′ end of the GPC mRNA. We then generated a separate construct (pM-mut2) by removing the 6-nt motif (3′-CCGUCG-5′) that constituted the putative transcription termination signal in the M vRNA template while leaving intact the C-rich region.
Rescue of recombinant viruses was performed in a manner similar to that used successfully for Bunyamwera virus, utilizing three antigenomic (+)-sense plasmids (4). As described earlier (2, 12), rescues were accomplished by the liposome-mediated transfection of 1-μg quantities of the three antigenomic (+)-sense plasmids encoding the mutagenized or authentic S, M, and L segments, utilizing a liposome-mediated transfection reagent (LT-1; Mirus) at a ratio of 5:1, with subsequent transfer onto subconfluent (∼80%) BSR-T7/5 cells. Each of the plasmids contained a single virus cDNA (S, M, or L) and three common elements, namely, a T7 promoter element to allow T7 Pol-dependent transcription, the hepatitis delta virus ribozyme to generate authentic virus 3′ termini, and a T7 terminator element. CPE was typically noted at 3 days posttransfection, with complete lysis of the monolayer observed approximately 2 days later. Supernatants from transfected BSR-T7/5 cells were harvested at 3 to 5 days posttransfection, clarified by low-speed centrifugation, diluted 1:10 in Dulbecco's modified Eagle's medium, transferred onto a fresh monolayer of Vero E6 cells, and incubated at 37°C until moderate CPE was observed (3 to 12 days postinfection). Total RNA from infected Vero E6 cells was harvested as described above.
3′RACE amplification.
Extracted total RNA from infected Vero E6 cells was polyadenylated in vitro by using an A-Plus poly(A) polymerase tailing kit (Epicenter Biotechnologies) following the manufacturer's instructions and subsequently purified using an RNeasy kit (QIAGEN) following standard protocols. Ten microliters of in vitro polyadenylated RNA was used as the template for reverse transcription-PCRs (RT-PCRs), using the SuperScript III One-Step RT-PCR system with Platinum Taq High Fidelity (Invitrogen) following the manufacturer's protocol. An oligo(dT)-containing primer (3′RACE-AP; Invitrogen) was included in all RT-PCRs in combination with a second primer specific for RVF virus, SFS virus, or TOS virus. All primers were designed according to sequences available in GenBank. To detect RVF virus L, M, NSs, and N mRNAs, we used primers for each segment corresponding to the following respective positions: L, 6208 to 6226; M, 3010 to 3032 and 3570 to 3592; S, 511 to 532; and S, 1099 to 1117. To detect SFS virus M mRNA, we used a forward primer corresponding to positions 3983 to 4002. SFS virus NSs and N mRNAs were amplified with a forward primer (positions 468 to 488) and a reverse primer (positions 1203 to 1223) for the S segment. To detect TOS virus L and M mRNAs, we used forward primers corresponding to positions 6185 to 6205 and 3812 to 3831, respectively. For TOS virus NSs and N mRNAs, we used a forward primer (positions 570 to 588) and a reverse primer (positions 1405 to 1423) for the S RNA. The resulting RT-PCR products were analyzed by agarose electrophoresis, and DNA bands of the correct sizes were recovered and purified using a GFX PCR DNA and gel band purification kit (Amersham). Purified DNA products were automatically sequenced using standard protocols (ABI).
Sequence analysis.
Sequence contigs and chromatogram profiles were generated within Sequencher, version 4.2. (GeneCodes Corporation). Sequence alignment and comparisons were done with BioEdit (5.0.6; North Carolina State University, Raleigh, NC). Prediction of RNA secondary structures were done using Mfold, version 3.2 (http://www.bioinfo.rpi.edu/applications/mfold/rna/form1.cgi).
RESULTS
Termination of RVF virus L and M mRNAs.
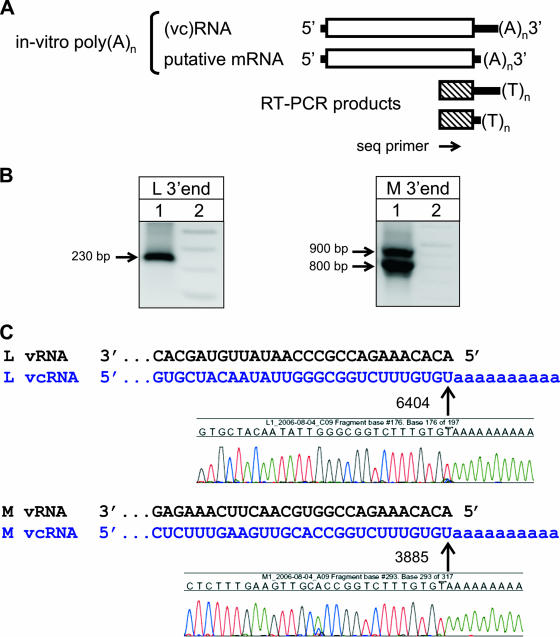
Total RNA from RVF virus (ZH501 strain)-infected cells was extracted at 2 to 3 days postinfection, added to a polyadenylation reaction mixture, purified, and used in two parallel RT-PCRs designed to detect the 3′ ends of L and M vcRNAs and mRNAs (Fig. 1A). A single unique L-derived DNA band was amplified (Fig. 1B, left panel), which corresponded in size (∼230 bp) to the expected size of the 3′-terminal amplicon of the full-length L segment vcRNA. No smaller 3′RACE products corresponding to a smaller putative L mRNA molecule were observed during several independent replicates of this experiment.
FIG. 1.
(A) Diagram depicting the 3′RACE strategy employed throughout these studies for detection of L and M segment mRNAs. (B) Agarose gel results depicting the 3′RACE amplification of RVF virus L (left) and M (right) segment RNA species. Lane 1, virus-specific amplicons; lane 2, size marker. Full-length replication products (vcRNA) were detected for both L and M segments. However, in contrast to the case with the M segment, no smaller fragments corresponding to L segment mRNA species were observed. Similar results were obtained with both TOS and SFS viruses (data not shown). (C) Chromatogram sequence data indicating the exact sites of in vitro polyadenylation of L and M segment full-length vcRNA replication products, as indicated by arrows. Note that polyadenylation of the RVF virus L segment occurred only after the last genomic nucleotide at position 6404, indicating the lack of upstream mRNA termination. All nucleotide numbering is relative to the virus GenBank entry.
In marked contrast, results utilizing primers specific for the 3′ termini of RVF virus M segment RNA demonstrated two distinct 3′RACE products (Fig. 1B, right panel). The larger of the two bands corresponded in size (∼900 bp) to the expected product for the 3′ terminus of the full-length M segment vcRNA. The smaller product (∼800 bp) was consistent with a shorter mRNA molecule derived from the M segment. These 3′RACE amplification fragments were extracted, purified, and sequenced. The resulting chromatogram profiles and RVF virus reference template sequences were used to deduce the exact 3′ termini of these products relative to the site of in vitro polyadenylation (Fig. 1C).
Analysis of the single L and the large M segment amplification products provided the exact identification of the expected 3′ termini of full-length L and M vcRNAs (Fig. 1C, upper and lower panels). Additionally, the smaller M amplification product indicated the location of a major site of mRNA termination, at nt 3774 (position numbered relative to the GenBank entry, i.e., in the antigenomic sense) (Fig. 2A, upper panel). While nt 3774 appeared to serve as the primary site of M segment transcription termination (as determined by the detection of in vitro polyadenylation), it was apparent from the chromatogram data that a smaller proportion of mRNA molecules also terminated at positions 3775 and 3776. These sites occur well within the 3′-untranslated region (UTR) of the RVF virus M segment, which stretches from positions 3614 to 3885 (respective to GenBank numbering) (Fig. 2B).
FIG. 2.
(A) Alignment of virus-sense M segment genome and 3′RACE-detected mRNA species of RVF, SFS, and TOS viruses. The initial position of in vitro polyadenylation is indicated by a bold arrow and can be visualized directly in each respective 3′RACE amplification product sequence chromatogram. The putative M segment transcription signal motifs are boxed and depicted in red. (B) Diagram depicting the nucleotide positions of the glycoprotein precursor molecule translation initiation and stop codons (boxed area), the site of transcription termination (bold arrow), and the last genomic nucleotide (thin arrow) on the M segments of RVF, SFS, and TOS viruses. All nucleotide numbering is relative to the virus GenBank entry.
Termination of SFS virus M and TOS virus L and M mRNAs.
In order to determine if this mRNA termination pattern was specific to RVF virus or was found more broadly throughout the genus Phlebovirus, we performed similar analyses utilizing two additional diverse phleboviruses, the SFS and TOS viruses. Similar to the results obtained with RVF virus, TOS virus generated a single L-derived amplification product corresponding in size to the expected full-length L segment vcRNA and two M segment-derived amplification products. Analysis of the SFS virus L segment could not be completed due to the lack of availability of sequence data in GenBank. The SFS virus M segment terminal products corresponded in size to the expected full-length M vcRNA and to a putative M mRNA molecule. Sequence analysis of the 3′RACE amplification products confirmed the expected 3′ termini of the TOS virus full-length L and TOS and SFS virus M vcRNAs (data not shown). Like the case with RVF virus, the nucleotide sequences of the smaller M segment-derived amplicons indicated that the major sites of mRNA termination were precise and occurred within the 3′ UTRs of both the SFS and TOS viruses (Fig. 2A, middle and bottom panels). The major sites of termination were at position 4242 for SFS virus and 4090 for TOS virus. Similar to RVF virus, a small proportion of M segment mRNA molecules appeared to terminate 1 nt beyond the major site, at nt 4243 and 4091 for SFS and TOS viruses, respectively. Both virus M segment mRNA molecules were found to terminate well within their 3′ UTRs, which stretch between positions 4044 and 4402 for SFS virus and 4037 to 4215 for TOS virus (Fig. 2B). Taken together, these data indicate that mRNA transcription termination patterns for RVF, SFS, and TOS virus L and M segments were grossly similar, in that RVF and TOS virus L mRNAs appeared to terminate at the end of the segment template, whereas RVF, SFS, and TOS virus M segment mRNAs terminated 111 to 160 nt prior to the end of the template (Fig. 2B).
Identification of a common putative mRNA transcription termination signal on RVF, SFS, and TOS virus M RNA segments.
Comparative sequence analysis of the exact locations of M segment mRNA termination sites among the three viruses revealed strikingly similar patterns. In each case, the virus template region involved in mRNA termination consists of a C-rich region (11 or 12 bases) followed by a highly conserved sequence motif of 5 or 6 nt (Fig. 2A). The high nucleotide identity of these core signal elements was surprising given the high nucleotide diversity (approximately 75%) seen between these three viruses in their M segment 3′ UTRs. The homologous motifs located in the respective virus 3′ UTRs were 3′-CCGUCG-5′ for RVF virus, 3′-CGUCG-5′ for SFS virus, and 3′-CCGUC-5′ for TOS virus.
Termination of RVF, SFS, and TOS virus NSs and N mRNAs.
Given the striking similarity of RVF, SFS, and TOS virus M segment mRNA transcription termination, we attempted to analyze the S segments of these viruses by using a similar 3′RACE approach (Fig. 3A), starting with RVF virus. Utilizing gene-specific primers and a poly(T) primer, we were able to generate both terminal amplification products for the RVF virus full-length S segment vcRNA (∼1,130 bp) and smaller putative mRNA 3′-terminal products representing the N (∼280 bp) and NSs (400 bp) mRNAs (Fig. 3B). The relative intensities of the amplification products corresponded with the relative abundance of these RNA species, as detected by Northern blot analyses (data not shown). As expected, sequence analyses of the larger full-length products derived from S segment vcRNA showed perfect identity with the expected 3′-terminal RVF virus genomic sequences (data not shown). Analysis of the smaller amplification products indicated the locations of the major transcription termination sites for NSs and N mRNAs, at positions 902 and 850 (numbered relative to the GenBank entry, i.e., in the vRNA sense), respectively (Fig. 3C). Similar to our findings with the M segments of these viruses, a small proportion of mRNA molecules, as judged by sequence chromatogram data, appeared to terminate 1 or 2 nt upstream of the major site of termination. Both the NSs and N mRNAs appeared to terminate within the approximately 52-nt S segment intergenic region and in close proximity to the translation stop codon of the opposite ORF (Fig. 3C).
FIG. 3.
(A) Schematic of the 3′RACE strategy employed to detect ambisense S segment vcRNA and mRNA species for NSs and N. (B) Agarose gel results depicting 3′RACE amplification of RVF virus S segment full-length vRNA (left panel, left lane) and full-length vcRNA (left panel, right lane) and NSs and N mRNAs (right panel). Lane L, size marker. (C) Chromatogram sequence data indicating the nucleotide positions of NSs (902) and N (850) mRNA termination. Underlined nucleotides indicate the positions of stop codons for N (915) and NSs (832), with the thin blunt arrows indicating the relative position and direction of each ORF. Note that in each panel the experimentally identified transcription termination signal is boxed and that both NSs and N mRNA species terminate prior to the opposite ambisense ORF. All nucleotide numbering is relative to the virus GenBank entry.
Based on the success with analysis of RVF N and NSs transcript termination, a similar approach was applied to SFS and TOS viruses. Sequence analyses of S-derived amplicons of SFS virus indicated the locations of the major termination sites for NSs and N mRNAs, at positions 952 and 838, respectively (Fig. 4A). Both SFS virus S segment termination sites were located in the S RNA intergenic region, which stretches from positions 823 to 967, and in proximity to the translation stop codons of the opposite ORF (Fig. 4A). The TOS virus NSs and N mRNA major transcription termination sites were found at positions 1062 and 983, respectively. Since the TOS virus S segment intergenic region stretches from positions 1007 to 1070, this means that unlike the case for RVF and SFS viruses, the TOS virus N mRNA terminates inside the NSs ORF (Fig. 4B).
FIG. 4.
(A) Alignment of SFS virus S segment vcRNA or vRNA and the respective N or NSs mRNA species. Sequence chromatograms indicate the sites for transcription termination of N and NSs mRNAs, at positions 838 and 952, respectively. (B) Alignment of TOS virus S segment vcRNA or vRNA and the respective NSs or N mRNA species. Sequence chromatograms indicate the sites for transcription termination of N and NSs mRNAs, at positions 983 and 1062, respectively. Note that in each panel the putative transcription termination signal is boxed, underlined nucleotides indicate the relative positions of stop codons, and a thin blunt arrow indicates the relative position and direction of each respective ORF. All nucleotide numbering is relative to the virus GenBank entry.
Identification of a common putative mRNA transcription termination signal for RVF, SFS, and TOS virus N and NSs mRNAs.
Given the high nucleotide diversity of the RVF, SFS, and TOS virus S segment intergenic regions and the fact that the N and NSs mRNAs are templated by opposite-sense RNA templates (N mRNA from vRNA and NSs mRNA from vcRNA), we did not anticipate finding common sequence signals involved in termination of these transcripts. Instead, we expected that we might uncover potential RNA hairpin secondary structures to explain transcript termination at the determined locations. However, extensive analysis using secondary structure prediction algorithms failed to identify any likely high-energy structures for RVF, SFS, or TOS virus (data not shown). Interestingly, similar to our observations with the M segment mRNA, the RVF virus N mRNA terminated following a C-rich region (12 or 13 Cs in a total stretch of 19 nt) and just prior to the putative termination signal on the vRNA template. In contrast, due to the ambisense character of the S segment, the RVF virus NSs mRNA terminated following the template G-rich region (13 Gs in a total stretch of 18 nt) prior to the termination signal motif on the vcRNA template. Surprisingly, given the overall lack of nucleotide homology in the intergenic region, the intergenic regions in both the RVF virus S segment vRNA and vcRNA contained the same 8-nt sequence motif (3′-CCCGUCGG-5′) approximately 2 nt downstream of where the N and NSs mRNAs terminated (Fig. 3C). When the transcription termination of SFS and TOS virus N and NSs mRNAs was investigated, a similar pattern to that observed with RVF virus was found. In all three viruses, termination of N mRNA occurs after a C-rich region, while NSs mRNA termination occurs just downstream of a G-rich region on the respective virus templates. More importantly, however, alignments of the mRNAs, vRNAs, and vcRNAs of these three viruses revealed a homologous linear sequence motif immediately downstream of the major sites of N, NSs, and GPC mRNA transcription termination. These were C/UCCGUCGG/U for RVF virus, U/CCGUCGC/U for SFS virus, and U/ACCGUCG/A for TOS virus (Fig. 5A). Interestingly, the RVF virus consensus motif is not found elsewhere in the RVF virus M and S RNA template RNAs, the SFS virus consensus motif is not found elsewhere in the SFS virus M and S RNA templates, and the TOS virus consensus motif is not found elsewhere in the TOS virus M and S RNA templates. Although these transcription termination motifs have slightly different lengths, they share a common core element of 3′-C1-3GUCG-5′, except for the TOS virus M segment, where the 5′ G is replaced by a single A residue. Thus, our proposed consensus motif for these three viruses is 3′-C1-3GUCG/A-5′. While experimental data are lacking, it is interesting that similar sequence motifs can be found in Punta Toro and Uukuniemi virus M and S segment UTRs in the proximity of where GPC, N, and NSs mRNA termination may occur (Fig. 5B).
FIG. 5.
(A) Summary alignment of M and S segment vRNA and vcRNA genomes of RVF, SFS, and TOS viruses. Note that in each panel, the experimentally identified putative transcription termination signal is boxed. (B) Summary alignment of Punta Toro (PT) and Uukuniemi (UUK) virus M and S segment vRNA and vcRNA genomes indicating the presence of predicted sequence motifs (boxed) found in appropriate genomic locations downstream of each respective stop codon that may play a role in mRNA transcription termination. Note the high nucleotide sequence identity of these predicted motifs with experimentally determined putative transcription termination signals found in RVF, SFS, and TOS viruses. All nucleotide numbering is relative to the virus GenBank entry.
Removal of the transcription termination motifs in RVF virus S segment RNA abolishes correct N and NSs mRNA termination.
The finding of a conserved linear sequence motif immediately downstream of the S and M segment mRNA transcription termination points in all three of these viruses was unexpected. To validate the proposed functional role of the consensus motif, a recently developed RVF virus reverse genetics system (2, 12) was utilized to examine the absolute requirement of these motifs in N and NSs mRNA transcription termination. Due to the ambisense nature of the RVF virus S RNA, it contains two copies of the proposed motif in opposite orientations, with one (s1) occurring 1 or 2 nt downstream of the termination site of the NSs mRNA and the other (s2) found 1 or 2 nt downstream of the site of mRNA termination of the N mRNA (Fig. 6A). To investigate the requirement of these signals in NSs or N mRNA termination, we constructed two S segment-derived recombinant cDNA clones (pS-mut 1 and pS-mut 2) in which only one copy (either genomic or antigenomic) of the 8-nt motif was removed (Fig. 6B).
FIG. 6.
(A) Schematic drawing of the wt RVF virus S segment depicting NSs mRNA (left) and N mRNA (right) and the relative position of each putative transcription termination signal. (B) Diagram depicting the deletion of each transcription termination signal, with S-mut 1 containing a deletion of the putative NSs signal s1 and S-mut 2 containing a deletion of the putative N signal s2. (C) Agarose gel results depicting the 3′RACE amplification of vRNA (top left), vcRNA (top right), NSs mRNA (bottom left), and N mRNA (bottom right). Lanes wt, m1, and m2 contain amplification products from wt, S-mut 1, and S-mut 2 viruses, respectively; lane L, size marker. The top right panel indicates that both mutant viruses were able to complete full-length viral complementary S segment replication despite the fact that each putative transcription termination signal was individually removed. Note that the S-mut 1 virus did not generate discrete amplification products for NSs mRNA (bottom left, lane m1) and that the S-mut 2 virus did not generate discrete amplification products for N mRNA (bottom right, lane m2).
Briefly, we rescued these recombinant mutant viruses by transfecting BSR-T7/5 cells with plasmids designed to express full-length antigenomic (+)-sense copies of L, M, and S (wt, S-mut 1, or S-mut 2) RNAs. After significant CPE was observed in the transfected monolayer, the supernatant was harvested, clarified by low-speed centrifugation, diluted, and passaged on confluent monolayers of Vero E6 cells. After approximately 10 to 12 days, when moderate CPE was observed, total RNA from infected cells was harvested and used in 3′RACE reactions designed to detect RVF virus NSs and N mRNAs, similar to those described above.
The 3′RACE amplification products from wt RVF virus strain ZH501 and each mutant virus were analyzed, and products corresponding to the expected sizes of S segment vRNA and vcRNA (Fig. 6C, upper panel) and the expected sizes of N and NSs mRNAs (Fig. 5C, lower panels) were obtained. While all viruses generated full-length vcRNA, it was clear that the recombinant virus S-mut 1 generated correctly terminated N mRNA but not NSs mRNA and that recombinant virus S-mut 2 only generated correctly terminated NSs mRNA, not N mRNA (Fig. 6C, lower panel). Furthermore, the deletion of either s1 or s2 in these mutant viruses could be confirmed by direct sequencing of the 3′RACE amplification products derived from the N or NSs mRNA, respectively (data not shown). These results, taken together, conclusively demonstrate the absolute requirement of the identified homologous sequence motif (3′-CCCGUCGG-5′) for RVF virus S segment mRNA transcription termination. Additionally, the N and NSs mRNA termination motifs, s1 and s2, which are present on opposite-sense template RNAs, appear to function independently (Fig. 6C). Despite their complementarity, there was no detectable cooperation between them (such as panhandle formation) needed for correct NSs or N mRNA termination.
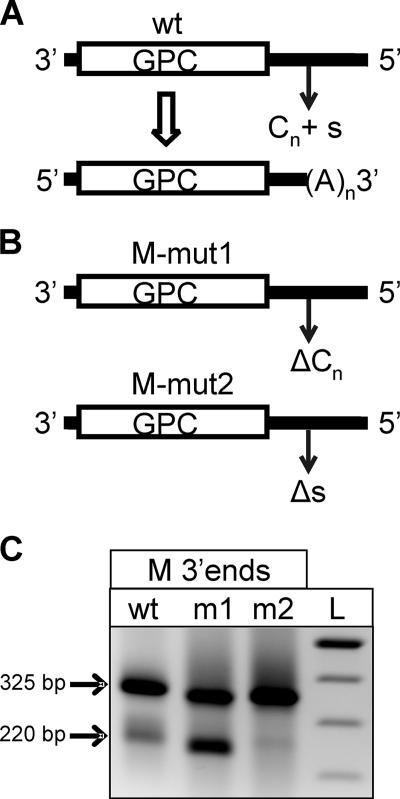
The C-rich RNA template region preceding the RVF virus GPC mRNA transcription termination signal is not part of the termination signal.
The RVF, SFS, and TOS virus GPC and N mRNA transcription termination motifs are all preceded by a C-rich RNA template region. In addition, the transcription termination motif of the ambisense NSs mRNA of each of these viruses is preceded by a G-rich RNA template region. This leads one to pose the question of whether this conserved feature plays a role in the mRNA transcription termination process. Conceivably, the virus transcriptase complex needs to transit this C-rich sequence (in the case of GPC and N mRNAs) to allow recognition of the specific transcription termination motif, or this C-rich region could perhaps form an RNA secondary structure with some G-rich sequence elsewhere in the RNA template to play a role in pausing the transcriptase in this region. To address this issue, RVF M segment-expressing plasmids were constructed where either the GPC mRNA transcription termination signal (M-mut 2) or the C-rich RNA template region preceding the signal (M-mut 1) was deleted (Fig. 7A). Similar to the findings described above for the S segment, deletion of the transcription termination motif disrupted specific mRNA termination (Fig. 7B, lane m2). However, deletion of the preceding C-rich template region caused no decrease in specific termination at the transcription termination motif (Fig. 7B, lane m1). These findings rule out a secondary structure involving this region or some other role for this C-rich sequence in mRNA transcription termination.
FIG. 7.
(A) Schematic drawing of wt RVF virus M segment depicting the relative locations of the GPC ORF, stop codon, poly(C) region, and transcription termination signal and the resulting M segment mRNA molecule. (B) Schematic drawings of RVF virus M segment cDNA plasmid mutants. The M-mut1 (upper panel) construct contained a deletion of the 14-nt C-rich region (ΔCn) located immediately upstream of the putative transcription termination signal only. The M-mut2 (lower panel) construct contained a deletion of the 6-nt putative transcription termination signal (Δs [3′-CCGUCG-5′]) only. (C) Results of 3′RACE amplification to detect vcRNA replication products and mRNA species of RVF virus M segment wt, M-mut 1 (m1), and M-mut 2 (m2) constructs. Note that deletion of the 14-nt C-rich region alone (m1) did not disrupt authentic transcription termination compared to that in the wt, whereas deletion of the 6-nt putative signal alone (m2) abolished the production of shorter mRNA species.
DISCUSSION
Here we have presented a comprehensive analysis of mRNA transcription termination in the S, M, and L segments of the phleboviruses RVF virus, SFS virus, and TOS virus and successfully identified the exact sites of termination to the nucleotide level. Our data revealed some surprising aspects of the mRNA transcription termination mechanism employed by these important pathogens. With regard to the L segments, we expected that the L mRNA may terminate within the UTR prior to the end of the genome template. However, despite the high resolution of the 3′RACE approach, RVF and TOS viruses yielded only single L-specific products, which represented an exact copy of each entire genome terminus. Careful analysis of the resulting L product chromatograms gave no indication of mixed base peaks suggestive of shorter mRNA products within the single L band. In addition, analysis of the RVF and TOS virus L genome segment 5′ UTRs revealed no sequence motif similar to that identified as the transcription termination signal for the S and M segment mRNAs of these viruses. Taken together, these data indicate that the L mRNAs of these viruses terminate in a manner similar to that for vcRNA and that termination occurs simply by “runoff” of the RNA template. This may be a general feature of L segments of viruses of the family Bunyaviridae, as similar findings have been reported for viruses in other genera (16).
Thorough analysis of the 3′ termini of the M segment mRNAs of RVF, SFS, and TOS viruses demonstrated that each terminated at a specific transcription termination motif immediately following a C-rich RNA template region. These findings are in agreement with earlier less precise studies (done by nuclease mapping) analyzing the M segment mRNA 3′ ends of RVF and TOS viruses (7, 14, 15). It appears that the virus transcriptase reads through this C-rich region of the template and then terminates mRNA transcription when it encounters the identified conserved motif. Despite earlier suggestions of possible involvement of this C-rich region in transcript termination (13) and the conservation of this region among these viruses, we demonstrated that deletion of this template region did not reduce specific termination of mRNA transcripts at the transcription termination motif. These findings suggest that this C-rich region may play some other role, such as influencing mRNA stability. Comparison of the M RNA genome segment sequences of RVF, SFS, and TOS viruses shows that this conserved transcription termination motif, 3′-C1-3GUCG/A-5′, lies in a highly divergent region of the segment, suggesting evolutionary pressure to maintain this important domain in this location. These data clearly indicate that these viruses employ quite different mechanisms of mRNA transcript termination on their L and M RNA segments.
The 3′RACE analysis of the N and NSs mRNAs of these viruses provided several surprising results. The ambisense S segments of these viruses contain intergenic regions which are C-rich (in the vRNA sense) in sequence and 63 to 144 nt long (defined as the distance between the N and NSs ORF stop codons). The 3′ ends of these mRNAs were found to be very precise and to contain much of the intergenic region, such that their 3′ ends actually overlap. This overlap means that similar to the situation with M segment mRNA termination, the virus transcriptase reading the vRNA template transits a C-rich region prior to N mRNA termination at the transcription termination motif. However, the virus transcriptase reading the vcRNA transits the complementary G-rich region prior to NSs mRNA termination at the transcription termination motif. Unlike the other virus N and NSs mRNAs, the N mRNA of TOS virus was found to actually terminate 24 nt upstream of the translation stop codon within the opposing NSs ORF. By analogy with the C-rich sequence region of virus M segments, it is likely that these C-rich (prior to N mRNA termination) and G-rich template regions are not part of the transcription termination signal but perhaps play a role in templating homopolymer regions at the mRNA 3′ ends which may influence their stability.
Alignment of the UTRs of RVF, SFS, and TOS viruses is difficult because they vary in length and are highly diverse, so sequence analysis provides little evolutionary insight into their precise function. Given such diversity, it was remarkable that nearly identical linear sequence motifs were identified in the S segment templates immediately downstream of the mapped N and NSs mRNA 3′ termini of all three viruses. Given the overlap of the N and NSs mRNA 3′ termini, two copies of the 3′-C1-3GUCG/A-5′ transcription termination motif were found, with one on the vRNA template and one on the vcRNA template (referred to as s1 and s2 in Fig. 6). The finding of the 3′-C1-3GUCG/A-5′ motif in RNA templates immediately downstream of the N, NSs, and M segment mRNA transcript termination points of RVF, SFS, and TOS viruses made a strong argument for these viruses sharing a common mechanism of transcription termination and this sequence motif representing a bona fide transcription termination signal. Extensive RNA secondary structure prediction analysis of the S segment intergenic regions and M segment UTRs of these viruses failed to predict convincing high-energy hairpin structures which could be involved in the transcription termination process. Possible hairpin structures had been suggested previously for Punta Toro and Uukuniemi virus S RNA segment junctions (11, 22), but on closer inspection, these too are not similar to those known to exist for arenavirus ambisense RNA templates and are of such low energy or high complexity as to be unlikely to form.
One possibility that was difficult to rule out was that since the ambisense RVF, SFS, and TOS virus S segments contain two copies of the termination signal motif that are perfectly complementary, these motifs might function to create the stem of a large loop structure containing the entire S segment intergenic region. It seemed unlikely that such a secondary structure would be part of the mRNA transcript termination mechanism, as only one copy of the 3′-C1-3GUCG/A-5′ signal exists in the virus M segments. However, to address this issue and to gain direct evidence that the 3′-C1-3GUCG/A-5′ motif functioned as a transcription termination signal, we utilized a newly developed RVF virus high-efficiency reverse genetics system to generate recombinant viruses lacking such sites (2, 12).
The results of the RVF virus reverse genetics experiments clearly demonstrated that deletion of the 3′-CCCGUCGG-5′ motif from the s1 or s2 position (Fig. 6) destroyed correct termination of the NSs or N mRNA, but in each case the other signal remained functional. These data showed that the N and NSs termination signals function independently and do not form stem-loop structures. We were surprised by the ability to rescue infectious recombinant viruses which failed to correctly terminate their N or NSm mRNA due to deletion of the respective transcription termination signal. However, these viruses grew less successfully upon subsequent passage on Vero E6 cells. Both S-mut 1 (NSs defect) and S-mut 2 (N defect) recombinant RVF viruses caused complete CPE after approximately 12 days, in contrast to similar dilutions of wt RVF virus, which caused complete CPE by approximately 5 days postinfection. This delay suggests that the lack of correct termination of the N or NSs mRNA may result in a protein synthesis imbalance which reduces virus replication efficiency. Further studies will be required to more precisely determine the attenuated phenotype of these viruses.
Recent work elucidating the complete genome sequences of 33 diverse RVF virus strains collected from throughout the virus's known geographic range and spanning 56 years demonstrated that the two transcription termination motifs in the S segment and the single motif in the M segment were completely conserved in all RVF virus strains analyzed (3). This was to be expected given the high conservation of these motifs among the three diverse phleboviruses examined in detail in this study. Similar motifs could also be seen in other phleboviruses, such as Punta Toro virus and the more distantly related Uukuniemi virus (Fig. 5B). It is also interesting that the core of the 3′-C1-3GUCG/A-5′ motif identified here (shown in bold) is similar to those of the 3′-GUCGAC-5′ and 3′-UGUCG-5′ mRNA transcription termination motifs identified for the S RNA segment of the prototypic Bunyamwera virus (1).
In addition to increasing our understanding of RNA virus mRNA transcription mechanisms, these findings may also have practical implications. The observation of delayed growth characteristics in cell culture for the S mut1 and S mut2 viruses suggests a possible target that may be included in future rationally designed vaccine candidates. Identification of the mRNA transcription termination motifs, together with our success in using a highly efficient RVF virus reverse genetics system to generate live recombinant RVF viruses with precise knockouts of these termination signals, suggests a direct mechanism to engineer S and M segment attenuation elements into live attenuated recombinant vaccines for these important diseases.
Acknowledgments
We thank Thomas G. Ksiazek and Pierre Rollin for their helpful comments and support during the completion of these studies.
B.B. was supported during the completion of these studies by the Veterinary Scientist Training Program (VSTP), by a Students Training in Advanced Research fellowship of the University of California, Davis, School of Veterinary Medicine, and by the Oak Ridge Institute for Science and Education (ORISE), Oak Ridge, TN.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the funding agencies.
Footnotes
Published ahead of print on 28 February 2007.
REFERENCES
- 1.Barr, J. N., J. W. Rodgers, and G. W. Wertz. 2006. Identification of the Bunyamwera bunyavirus transcription termination signal. J. Gen. Virol. 87:189-198. [DOI] [PubMed] [Google Scholar]
- 2.Bird, B. H., C. G. Albarino, and S. T. Nichol. Rift Valley fever virus lacking the NSm proteins retains high virulence in vivo and may provide a model of human delayed onset neurologic disease. Virology, in press. [DOI] [PubMed]
- 3.Bird, B. H., M. L. Khristova, P. E. Rollin, T. G. Ksiazek, and S. T. Nichol. 2007. Complete genome analysis of 33 ecologically and biologically diverse Rift Valley fever virus strains reveals widespread virus movement and low genetic diversity due to recent common ancestry. J. Virol. 81:2805-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bridgen, A., and R. M. Elliott. 1996. Rescue of a segmented negative-strand RNA virus entirely from cloned complementary DNAs. Proc. Natl. Acad. Sci. USA 93:15400-15404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, p. 1635-1668. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 6.Charrel, R. N., P. Gallian, J. M. Navarro-Mari, L. Nicoletti, A. Papa, M. P. Sanchez-Seco, A. Tenorio, and X. de Lamballerie. 2005. Emergence of Toscana virus in Europe. Emerg. Infect. Dis. 11:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collett, M. S. 1986. Messenger RNA of the M segment RNA of Rift Valley fever virus. Virology 151:151-156. [DOI] [PubMed] [Google Scholar]
- 8.de Haan, P., L. Wagemakers, D. Peters, and R. Goldbach. 1990. The S RNA segment of tomato spotted wilt virus has an ambisense character. J. Gen. Virol. 71:1001-1007. [DOI] [PubMed] [Google Scholar]
- 9.Di Nicuolo, G., P. Pagliano, S. Battisti, M. Starace, V. Mininni, V. Attanasio, and F. S. Faella. 2005. Toscana virus central nervous system infections in southern Italy. J. Clin. Microbiol. 43:6186-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dionisio, D., F. Esperti, A. Vivarelli, and M. Valassina. 2003. Epidemiological, clinical and laboratory aspects of sandfly fever. Curr. Opin. Infect. Dis. 16:383-388. [DOI] [PubMed] [Google Scholar]
- 11.Emery, V. C., and D. H. Bishop. 1987. Characterization of Punta Toro S mRNA species and identification of an inverted complementary sequence in the intergenic region of Punta Toro phlebovirus ambisense S RNA that is involved in mRNA transcription termination. Virology 156:1-11. [DOI] [PubMed] [Google Scholar]
- 12.Gerrard, S. R., B. H. Bird, C. G. Albarino, and S. T. Nichol. 2007. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology 359:459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giorgi, C., L. Accardi, L. Nicoletti, M. C. Gro, K. Takehara, C. Hilditch, S. Morikawa, and D. H. Bishop. 1991. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian Sandfly fever, and Uukuniemi viruses. Virology 180:738-753. [DOI] [PubMed] [Google Scholar]
- 14.Gro, M. C., P. Di Bonito, L. Accardi, and C. Giorgi. 1992. Analysis of 3′ and 5′ ends of N and NSs messenger RNAs of Toscana phlebovirus. Virology 191:435-438. [DOI] [PubMed] [Google Scholar]
- 15.Gro, M. C., P. Di Bonito, D. Fortini, S. Mochi, and C. Giorgi. 1997. Completion of molecular characterization of Toscana phlebovirus genome: nucleotide sequence, coding strategy of M genomic segment and its amino acid sequence comparison to other phleboviruses. Virus Res. 51:81-91. [DOI] [PubMed] [Google Scholar]
- 16.Hutchinson, K. L., C. J. Peters, and S. T. Nichol. 1996. Sin Nombre virus mRNA synthesis. Virology 224:139-149. [DOI] [PubMed] [Google Scholar]
- 17.Iapalucci, S., N. Lopez, and M. T. Franze-Fernandez. 1991. The 3′ end termini of the Tacaribe arenavirus subgenomic RNAs. Virology 182:269-278. [DOI] [PubMed] [Google Scholar]
- 18.Jin, H., and R. M. Elliott. 1993. Non-viral sequences at the 5′ ends of Dugbe nairovirus S mRNAs. J. Gen. Virol. 74:2293-2297. [DOI] [PubMed] [Google Scholar]
- 19.Lopez, N., and M. T. Franze-Fernandez. 2007. A single stem-loop structure in Tacaribe arenavirus intergenic region is essential for transcription termination but is not required for a correct initiation of transcription and replication. Virus Res. 124:237-244. [DOI] [PubMed] [Google Scholar]
- 20.Nichol, S. T. 2001. Bunyaviruses, p. 1603-1633. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 21.Schmaljohn, C. S., and J. W. Hooper. 2001. Bunyaviridae: the viruses and their replication, p. 1581-1602. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 22.Simons, J. F., and R. F. Pettersson. 1991. Host-derived 5′ ends and overlapping complementary 3′ ends of the two mRNAs transcribed from the ambisense S segment of Uukuniemi virus. J. Virol. 65:4741-4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valassina, M., M. G. Cusi, and P. E. Valensin. 2003. A Mediterranean arbovirus: the Toscana virus. J. Neurovirol. 9:577-583. [DOI] [PubMed] [Google Scholar]
- 24.van Knippenberg, I., R. Goldbach, and R. Kormelink. 2005. Tomato spotted wilt virus S-segment mRNAs have overlapping 3′-ends containing a predicted stem-loop structure and conserved sequence motif. Virus Res. 110:125-131. [DOI] [PubMed] [Google Scholar]