Abstract
Monocytes/macrophages are major targets of human immunodeficiency virus type 1 (HIV-1) infection. The viral preintegration complex (PIC) of HIV-1 enters the nuclei of monocyte-derived macrophages, but very little PIC migrates into the nuclei of immature monocytes. Vpr, one of the accessory gene products of HIV-1, is essential for the nuclear import of PIC in these cells, although the role of Vpr in the entry mechanism of PIC remains to be clarified. We have shown previously that Vpr is targeted to the nuclear envelope and then transported into the nucleus by importin α alone, in an importin β-independent manner. Here we demonstrate that the nuclear import of Vpr is strongly promoted by the addition of cytoplasmic extract from macrophages but not of that from monocytes and that the nuclear import activity is lost with immunodepletion of importin α from the cytoplasmic extract. Immunoblot analysis and real-time PCR demonstrate that immature monocytes express importin α at low levels, whereas the expression of three major importin α isoforms markedly increases upon their differentiation into macrophages, indicating that the expression of importin α is required for nuclear import of Vpr. Furthermore, interaction between importin α and the N-terminal α-helical domain of Vpr is indispensable, not only for the nuclear import of Vpr but also for HIV-1 replication in macrophages. This study suggests the possibility that the binding of Vpr to importin α, preceding a novel nuclear import process, is a potential target for therapeutic intervention.
Monocytes/macrophages are major targets of human immunodeficiency virus type 1 (HIV-1) and serve as a viral reservoir (12). Most tissue macrophages are permissive for the entry of macrophage-tropic viruses, and they release small amounts of viral particles in asymptomatic carriers (6). In AIDS patients with opportunistic infections, macrophages occasionally produce large quantities of viral particles (40). Recent studies suggest that HIV-1 in latently infected macrophages in some lymphoreticular tissues cannot be eradicated by highly active antiretroviral therapy and that residual cells may produce viral particles that can spread throughout the body (39). Although the extents of reverse transcription (RT) are similar in monocytes and macrophages after infection with an HIV-based vector, nuclear entry is blocked in monocytes (34). Therefore, it has been considered that active nuclear import of the viral preintegration complex (PIC) of HIV-1 is inefficient in monocytes. However, the precise mechanisms by which the nuclear import of PIC is controlled in monocytes and differentiated macrophages are not fully understood, although this distinction is critical for the design of antiviral strategies.
Nuclear import processes involve the nuclear pore complexes (NPCs) of the nuclear envelope and typically require nuclear localization signals (NLSs). The nuclear import of basic NLS-containing proteins is mediated by specific soluble factors composed of two essential components, importins α and β (13). The central portion of importin α, which contains armadillo repetitive motifs, recognizes the NLS, and its N-terminal basic region, termed the importin β-binding (IBB) domain, binds to importin β (13). The ternary complex docks at the NPC and is translocated into the nucleus. Therefore, importin α acts as an adapter molecule between cargo proteins and importin β, and it is importin β that actually conveys the cargo from the cytoplasm into the nucleus. In addition, several other factors participate in this transport system, including the small GTPase Ran and its binding protein, nuclear transport factor 2 (32). However, there are other pathways that mediate nuclear import; for example, transportin (transport factor of M9-containing cargo) and importin β are competent to transfer some cargo by themselves (41, 46). Moreover, it was recently reported that importin α can migrate into the nucleus in an importin β- and Ran-independent manner (29). In addition, importin α alone can escort Ca2+/calmodulin-dependent protein kinase type IV (CaMKIV) into the nucleus without utilizing the classical importin β-dependent transport system (23).
Mammals such as humans and mice possess at least six importin α isoforms (importin α1/Rch1, α3/Qip1, α4/hSRP1γ, α5/NPI1, α6, and α7 [8, 21, 22, 38, 44]). These isoforms can be divided into the following three major subfamilies according to their amino acid similarities: α1, α3 and α4, and α5 to α7 (22). Proteins in the three groups share about 50% overall amino acid identity. Many studies have shown that importin α isoforms differ in efficiency with respect to classical substrate-specific import (31, 45) and show unique expression patterns in various tissues. This suggests that importin α contributes primarily to tissue-specific nuclear transport. However, the expression patterns of importin α isoforms in human peripheral blood mononuclear cells (PBMC) are unknown.
The ability of HIV-1 to replicate in nondividing cells, such as macrophages, depends on the active nuclear import of the viral PIC (4). The HIV-1 PIC contains viral proteins such as reverse transcriptase, integrase (IN), nucleocapsid (NC), Vpr, and matrix (MA; p17) in addition to viral nucleic acids (5). MA, Vpr, and IN have all been implicated in the nuclear import of PIC, although their precise roles are controversial. Both MA and IN have functional NLSs that resemble the canonical NLS of the simian virus 40 (SV40) T antigen, and both utilize the classical nuclear import pathway that includes interaction with importins α and β (11). In contrast, despite the lack of an identifiable canonical NLS, Vpr displays karyophilic properties and is rapidly targeted to host cell nuclei after infection (26). Furthermore, it has been reported that the nuclear import of Vpr is mediated by an as-yet-unidentified pathway that is distinct from the classical NLS- and M9-dependent pathways (16). In this context, we have previously shown that Vpr traverses the NPC in an importin α-dependent manner (19). Vpr has also been implicated in the nuclear import of proviral DNA in macrophages (7, 11, 14), presumably by promoting interactions with the cellular machinery that regulates nucleocytoplasmic shuttling (10, 14, 25, 42, 43, 48). In addition to nuclear transport, Vpr functions in many processes, including the induction of cell cycle arrest at the G2 phase (17), the regulation of apoptosis (2, 3, 35-37), and splicing (24). The numerous biological activities of Vpr appear to be related to its interactions with a variety of cellular partners. Indeed, it has been suggested that importin α binds to Vpr (1, 42, 43, 48) to promote its passage through the NPC (19). We have previously shown that the region between residues 17 and 74 of Vpr, designated N17C74, is a bona fide NLS. In addition, Vpr seems to be targeted first to the NPC via interaction with the αH3 region, located between residues 46 and 74, and then enters the nucleus in a process that involves the αH1 region, located between residues 17 and 34 (19). However, it remains to be clarified whether Vpr is transported into the nucleus by importin α alone, without the need for other soluble factors, such as importin β.
In this investigation, we have studied the detailed mechanism of Vpr nuclear import and its correlation with HIV-1 infectivity in primary monocytes and macrophages. We demonstrate the following. (i) In digitonin-permeabilized cells, Vpr alone is targeted to the perinuclear region and then transported into the nucleus by importin α in an importin β-independent manner. (ii) The three major isoforms of importin α support the apparent nuclear import of Vpr. (iii) Primary monocytes exhibit a marked increase in the expression of importin α isoforms upon induction of differentiation to macrophages. (iv) The expression of importin α is essential for the nuclear import of Vpr in macrophages. (v) The interaction between importin α and Vpr is indispensable, not only for the importin α-mediated nuclear import of Vpr but also for the replication of HIV-1 in primary macrophages.
MATERIALS AND METHODS
Cell culture, cytoplasmic extract preparation, and RNA extraction.
Human cervical HeLa cells and African green monkey COS cells were cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum. Human PBMC were isolated on a Ficoll (Lymphosepal; IBL) gradient from a healthy HIV-1-seronegative donor. Monocytes were selected from the PBMC by a magnetic cell separation system, using microbeads coated with a CD14-specific monoclonal antibody (MAb), according to the manufacturer's instructions (MACS system; Miltenyi Biotech). Monocytes were plated at the desired density in 5-mm-diameter poly-l-lysine-coated glass-bottomed microwell dishes (Matek Corp.) and grown in RPMI medium (Invitrogen) containing 10% heat-inactivated fetal calf serum, 5% human serum, and 20 ng/ml macrophage colony-stimulating factor (M-CSF; PeproTech) for 1 week, until they spontaneously differentiated into mature macrophages (34).
Cells were lysed in cold, hypotonic buffer (10 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 3 mM CaCl2, 0.3 M sucrose, 1 mM dithiothreitol, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride). The cytoplasmic extracts were clarified by centrifugation at 8,000 × g for 30 s, and the supernatants were subjected to Western blotting analysis and an in vitro nuclear transport assay.
Total cellular RNAs were isolated from monocytes and differentiated primary macrophages by using TRIzol reagent (Invitrogen).
Plasmid constructions.
Construction of glutathione S-transferase (GST)- and green fluorescent protein (GFP)-tagged mutant forms of Vpr, named N17C74-GFP and GST-αLA/N17C74-GFP, was done as previously described (15, 19). For construction of GST-αH1-GFP and GST-αLA/αH1-GFP, fragments encoding the sequences were prepared from pGFP-FαH1 and pGFP-FαLA/αH1, respectively, by digestion with EcoRV and NotI (18). Each fragment was subcloned into BamHI/NotI-digested pGEX-6P3 (GE Healthcare). The BamHI site was blunted with KOD DNA polymerase (Toyobo) for ligation with an EcoRV site. GST-tagged human importin α1, α3, and α5 isoforms were constructed as follows. Insert fragments were isolated from pGEX-2T/importin α1, pGEX-2T/importin α5, and pGEX-2T/importin α3 and subcloned into pGEX-6P3 (30). This vector includes the GST coding region and a Flag tag, at the N and C termini of the multicloning site, respectively. For the importin α1 deletion mutant that lacked the IBB domain, the ΔIBB importin α fragment was amplified with the primers 5′-TATGGATCCAGCTCCTTTCCTGAT-3′ and 5′-GGCCTCGAGGTAAAAGTTAAAGGTCCCAGG-3′, using pGEX-6P3/importin α1 as the template. GST- and hemagglutinin-tagged human importin β was cloned into pGEX-2T at the BamHI and KpnI sites. This fragment was then subcloned into pGEX-6P3 at the BamHI and XhoI sites. The GST-tagged SV40 NLS-GFP construct was made as previously described (19). An infectious molecular clone, HIV-1 pNF462, a clone that encoded a Vpr-negative ATG mutant (ΔVpr), and a clone that encoded a substitution mutant of Vpr designated αLA were constructed as previously described (15).
Expression and purification of recombinant proteins.
GST- and GFP-tagged mutant forms of Vpr and GST-tagged importin α1, α3, α5, and β were expressed in Escherichia coli strain NovaBlue (Novagen) or BL21 CodonPlus (DE3)-RIL (Stratagene) and purified as described elsewhere (15, 19). GST-tagged SV40 NLS-GFP (18) and Ran/TC4 (28) were also expressed in E. coli and purified as described previously (15, 19).
Western blotting.
Cell lysates were examined by immunoblotting with a MAb against importin α1 (BD Biosciences), a MAb against importin α3 (MBL), a MAb against importin α5/7 (MBL), a MAb against importin β (BD Biosciences), a MAb against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; BD Biosciences), an anti-HIV-1 Vpr rabbit serum (NIH AIDS Research and Reference Reagent Program), or a MAb against HIV-1 Gag (p24) (15), followed by horseradish peroxidase-linked sheep antibodies against mouse or rabbit immunoglobulin G (IgG; GE Healthcare), as previously described (37).
Quantitative real-time PCR.
Approximately 600 ng total RNA was used for the RT reaction, which was performed with the Superscript preamplification system (Invitrogen). The RT product was used for real-time quantitative PCR amplification of three importin α isoforms in a LightCycler system (Roche) in the presence of LightCycler FastStart DNA SYBR green I master mix (Roche), using importin α1, α3, and α5 isoform-specific primers as follows: forward α1, 5′-GCATAATAGAACCGTTGATG-3′; reverse α1, 5′-AGGAGCCCCATCCTGAAC-3′; forward α3, 5′-AGTGGCTTACCTTATCCAAC-3′, reverse α3, 5′-TGTTGGTACATTGGCAGATG-3′; forward α5, 5′-GTGATCTCCTCACGGTCATG-3′; and reverse α5, 5′-CATAGGAGCCTCACACTG-3′. Hypoxanthine phosphoribosyltransferase (HPRT) cDNA was amplified as a normalization control, using the primers forward HPRT (5′-GCCCTGGCGTCGTGATTAGT-3′) and reverse HPRT (5′-GCTCTACTAAGCAGATGGCC-3′).
Microinjection and imaging analysis.
GST and GFP fusion proteins (1 mg/ml) were injected into the cytoplasm of primary macrophages grown on a 35-mm glass-bottomed dish, using an InjectMan N12 microinjector (Eppendorf). Images were captured with a 40× long-distance objective lens on an Olympus IX70 inverted microscope equipped with a Yokogawa CSU10 confocal laser scanning system controlled by Metamorph software (Universal Imaging).
siRNA transfection.
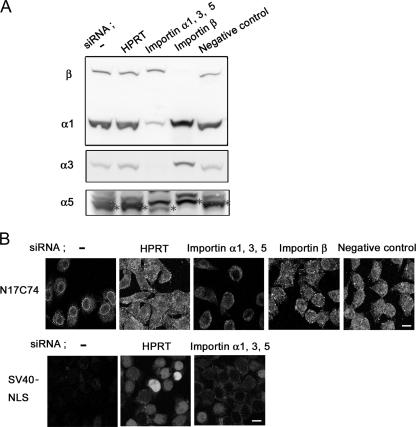
Small interfering RNAs (siRNAs) corresponding to importins α1, α3, α5, and β were designed with BLOCK-iT RNAi Designer (Invitrogen) and obtained. The siRNA sequences targeting importins α1, α3, α5, and β were CCAAGCUACUCAAGCUGCCAGGAAA for α1, CAGUGAUCGAAAUCCACCAAUUGAU for α3, CCGGAAUGCAGUAUGGGCUUUGUCU for α5, and CAGUCUGGCUGAAGCUGCUUAUGAA and CACAGCACUGCAGUCUGGAUUCCUU for β. HeLa cells were seeded 1 day before transfection with siRNAs. On day 0, transfection of importin α1-, α3-, α5-, and β-specific siRNAs, an HPRT-specific siRNA (HPRT-S1) as a positive control, or a nonspecific siRNA as a negative control was carried out with Lipofectamine RNAiMAX (Invitrogen) following the company protocol. After 2 days, second transfections were performed. After another 2 days, cells were harvested, and cytoplasmic extracts were prepared. Transfection efficiencies for siRNAs were determined by using Western blotting with MAbs against importins α1, α3, α5/7, and β.
HIV-1 infection of macrophages.
HIV-1 was introduced into COS cells by electroporation of macrophage-tropic pNF462 viruses encoding wild-type Vpr, a mutant form (αLA), and a deficient form. Viral supernatants were harvested 72 h after transfection. Virus stocks were titrated by measuring the amount of p24 antigen in the culture supernatants, using an enzyme-linked immunosorbent assay (ELISA). Differentiated primary macrophages seeded onto a 48-well tissue culture plate (2 × 105/well) were exposed to virus containing 5 ng of p24 antigen for 2 h at 37°C. They were then washed three times, and the infected cells were maintained in RPMI 1640 containing 10% fetal calf serum, 5% human serum, and M-CSF (20 ng/ml). Culture supernatants were harvested at 3- or 4-day intervals, and viral production was monitored by the sequential quantification of p24 antigen in cell-free supernatants with an HIV-1 p24gag ELISA kit (ZeptoMetrix Corp.).
Other assays.
In vitro nuclear transport and in vitro pull-down assays were performed as previously described (15, 19).
RESULTS
Nuclear import of Vpr is promoted by importin α without a requirement for importin β.
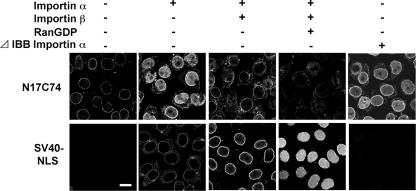
We previously reported that Vpr is localized at the nuclear envelope and that it then traverses the NPC in an importin α-dependent manner (19). To determine whether the human importin α-driven nuclear import of Vpr requires importin β, we reconstituted the candidate factors required for Vpr nuclear transport from digitonin-permeabilized, semi-intact HeLa cells. For this experiment, we used the region between residues 17 and 74 (N17C74) of Vpr because this is a functionally transportable region (18, 19). The chimeric protein comprised of N17C74 fused at the N terminus to GST and at the C terminus to GFP (∼63 kDa) was larger than the limitation for passive diffusion into the nucleus. As shown in Fig. 1, N17C74 localized predominantly to the perinuclear region in the absence of soluble factors. Interestingly, importin α1 alone had the highest activity for the nuclear import of N17C74, whereas the addition of importin β decreased the efficiency of N17C74 import, in the presence or absence of RanGDP (Fig. 1, upper panels). These results contrast with the classical nuclear import of SV40 NLS, which requires importin α1, importin β, and RanGDP (Fig. 1, lower panels), but are in good agreement with our recent report (19). Next, to exclude the possibility that residual endogenous importin β in the permeabilized cells contributed to the nuclear migration of Vpr, an importin α1 mutant (ΔIBB) lacking the IBB domain and unable to bind to importin β was used instead of full-length importin α1. ΔIBB importin α1 alone enhanced the nuclear import of N17C74, indicating that Vpr can enter the nucleus in an importin β-independent manner.
FIG. 1.
Importin α mediates the nuclear import of Vpr without importin β. HeLa cells were permeabilized by treatment with 35 μg/ml digitonin. The cells were incubated with 1 μM GST- and GFP-tagged N17C74 or SV40 NLS in the presence (+) or absence (−) of soluble factors for 20 min at 30°C. After fixation, cells were analyzed by confocal laser scanning microscopy (Radiance 2100; Bio-Rad). Soluble factors were included at the following concentrations: importin α1, 1 μM; importin β, 1 μM; RanGDP, 2 μM; and ΔIBB importin α1, 1 μM. Bar = 20 μm.
These results support the notion that Vpr is targeted to the perinuclear region and then transported into the nucleus by importin α1 alone, without importin β. Thus, the mechanism of nuclear entry of Vpr is quite different from that mediated by the classical transport system.
Nuclear import of Vpr is promoted by all three major isoforms of importin α.
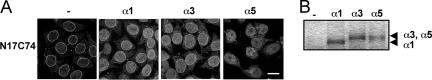
In human cells, at least six importin α isoforms have been identified, and they are believed to differ in efficiency with respect to classical substrate-specific import (31, 45). To determine whether the other two major importin α isoforms, α3 and α5, promote the nuclear import of Vpr similarly to α1, we performed an in vitro transport assay using recombinant human α1, α3, and α5. As shown in Fig. 2A, N17C74 was imported into the nucleus by either importin α3 or α5 as well as by α1, suggesting that Vpr is able to utilize all three major importin α isoforms for nuclear entry. The classical nuclear import of SV40 NLS, the positive control for the in vitro transport assay, occurred in the presence of any of the three importin α isoforms in combination with importin β and RanGDP (data not shown).
FIG. 2.
Importin α1, α3, and α5 isoforms interact with Vpr and mediate the nuclear import of Vpr. (A) Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 and 1 μM of each recombinant importin α isoform. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm. (B) Glutathione-Sepharose beads coupled with 100 pmol GST- and GFP-tagged N17C74 were incubated with 100 pmol recombinant GFP-tagged importin α1, α3, or α5. After incubation for 1 h at 4°C, the bound fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Coomassie staining. The positions of the three importin α isoforms are indicated.
To further examine whether N17C74 interacts directly with the three importin α isoforms, recombinant importin α1, α3, and α5 were incubated with GST- and GFP-tagged N17C74 that was immobilized on glutathione-Sepharose (Fig. 2B). Although the three isoforms share only approximately 50% overall amino acid sequence similarity, the tagged N17C74 protein was able to interact with all of them. These results suggest that the three importin α isoforms can directly interact with Vpr and support its nuclear entry.
Importin α in differentiated macrophages promotes the nuclear import of Vpr.
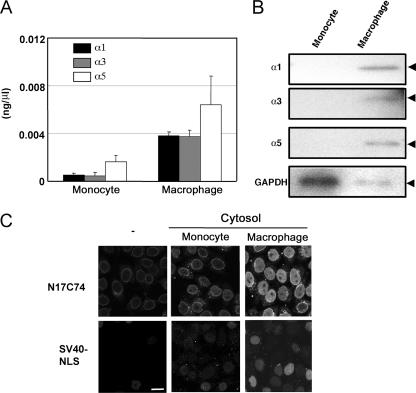
Vpr has been implicated as playing an important role in the nuclear import of proviral DNA in monocyte-derived macrophages (7, 11, 14). Neil et al. reported that although the HIV-1 PIC entered the nuclei of primary monocyte-derived macrophages, active nuclear import of PIC was inefficient in monocytes (34). These results suggested that the efficiency of HIV-1 PIC nuclear import in monocytes and macrophages might correlate with that of Vpr. However, it was unclear whether importin α was expressed in monocytes and macrophages, interacted with Vpr, and contributed to HIV-1 replication. Therefore, we first investigated the mRNA expression level of each importin α isoform in primary monocytes and differentiated macrophages. Primary monocytes were prepared from PBMC of healthy donors and allowed to differentiate into macrophages in vitro in the presence of M-CSF. Total cellular RNA was isolated from primary monocytes and macrophages, and real-time quantitative RT-PCR was conducted using specific primers for each isoform (Fig. 3A). Differentiated macrophages showed higher levels of expression of all three isoforms than did monocytes. Differences in the protein levels of each importin α isoform between monocytes and macrophages were confirmed by Western blotting with MAbs against importin α1, α3, and α5 (Fig. 3B). These results were consistent with the observed levels of mRNA expression. These data clearly show that the expression of the three major isoforms of importin α is low in primary monocytes but is markedly increased by inducing differentiation into macrophages.
FIG. 3.
Importin α in cytoplasmic extracts from primary macrophages promotes the nuclear import of Vpr. Monocytes were isolated from human PBMC. Macrophages were differentiated from monocytes by M-CSF. (A) Detection of importin α isoform mRNAs by real-time quantitative PCR. Total RNAs were extracted from monocytes and differentiated primary macrophages, and quantitative RT-PCR analysis was performed on a LightCycler system, using specific primers for each importin α isoform. Bars represent the mean values and standard errors for three experiments. (B) Detection by Western blotting of importin α proteins. Lysates containing 100 μg protein from monocytes and differentiated primary macrophages were subjected to Western blotting with MAbs against importins α1, α3, and α5 and against GAPDH as a control. The positions of the three importin α isoforms and GAPDH are indicated. (C) In vitro nuclear transport assay. Cytoplasmic extracts were prepared from monocytes and differentiated primary macrophages. Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 or GST- and GFP-tagged SV40 NLS and extracts containing 100 μg protein. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm.
Next, we performed an in vitro nuclear transport assay using cytoplasmic extracts from monocytes and differentiated macrophages (Fig. 3C). As expected from the above results, N17C74 was efficiently transported into the nucleus when a cytoplasmic extract from differentiated macrophages was added, whereas the nuclear import of N17C74 was inefficient when a monocyte extract was used. Under these conditions, the classical nuclear import of SV40 NLS was also inefficient with monocyte extracts and efficient with macrophage extracts. These results suggest that the efficiency of nuclear transport of Vpr and SV40 NLS depends strongly on the expression level of importin α in monocytes and macrophages, which are important targets for HIV-1 infection.
Importin α depletion prevents the nuclear import of Vpr in macrophages.
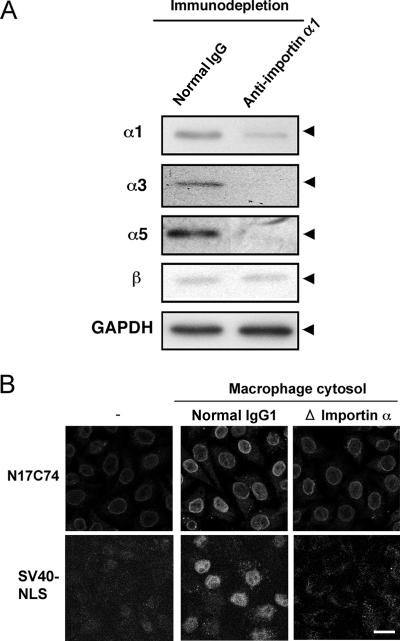
To further investigate the requirement for importin α in the nuclear import of Vpr in primary macrophages, cytoplasmic extracts were depleted, using a MAb against importin α1 and protein A-Sepharose, prior to the in vitro nuclear transport assay. Western blotting analysis of depleted lysates unexpectedly demonstrated that most importin α, including importins α3 and α5 as well as importin α1, was successfully removed, whereas importin β remained in the extracts (Fig. 4A). The addition of these extracts drastically decreased the nuclear import of N17C74 compared to that observed with cytoplasmic extracts incubated with preimmune normal mouse IgG (Fig. 4B). Similar results were obtained with SV40 NLS as a control for classical import. In addition, we successfully knocked down the three importin α isoforms α1, α3, and α5 in HeLa cells by isoform-specific siRNA transfection (Fig. 5A). The nuclear import of N17C74, which was enhanced by a cytoplasmic extract of HeLa cells, was greatly decreased by a cytoplasmic extract from importin α-specific siRNA-transfected cells but not by that from importin β-specific, HPRT-specific, or negative control siRNA-transfected cells (Fig. 5B).
FIG. 4.
Importin α depletion prevents the nuclear import of Vpr in macrophages. (A) Lysates of differentiated primary macrophages were treated with a MAb against importin α1 or with preimmune normal mouse IgG, followed by treatment with protein A-Sepharose. The cytoplasmic extracts were clarified by centrifugation, and equivalent amounts of the immunodepleted extracts were subjected to Western blotting with MAbs against importins α1, α3, and α5, importin β, or GAPDH. The positions of the three importin α isoforms, importin β, and GAPDH are indicated. (B) In vitro nuclear transport assay. Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 or SV40 NLS and 100 μg of control or immunodepleted cytoplasmic extract. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm.
FIG. 5.
siRNA-induced down regulation of importin α isoforms prevents the nuclear import of Vpr. (A) Duplicate siRNA transfections corresponding to importins α1, α3, α5, and β, HPRT-specific siRNA transfection as a positive control, and nonspecific siRNA transfection as a negative control were carried out with HeLa cells. Cytoplasmic extracts were prepared from HeLa cells transfected with siRNA. The transfection efficiencies of siRNAs were determined by Western blotting using importin α1-, α3-, α5/7-, and β-specific antibodies. The positions of importins α1, α3, α5 (*), and β are indicated. (B) In vitro nuclear import assay. Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 or GST- and GFP-tagged SV40 NLS and 100 μg proteins from a cytoplasmic extract prepared from HeLa cells transfected with siRNA. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm.
These results suggest that endogenously expressed importin α is essential for the efficient nuclear import of Vpr.
Interaction of importin α with the αΗ1 domain of Vpr is essential for its nuclear import in macrophages.
To understand the mechanism of the importin α-driven nuclear import of Vpr in detail, we examined the effects of mutations on the nuclear import of Vpr. Vpr consists of three α-helical domains (αH1, αH2, and αH3) (Fig. 6A). As we showed previously, N17C74 interacts directly with importin α1 through the αH1 and αH3 domains, and the interaction via αH1 is essential for nuclear entry (19). Therefore, in this study, we focused on the αH1 domain interaction. Three mutants were used in this study, including αLA/N17C74, in which the Leu residues at positions 20, 22, 23, and 26 within αH1 of Vpr were replaced by Ala residues; the αH1 domain alone; and its mutant, αLA/αH1 (Fig. 6A). In pull-down assays, we found that the αH1 chimeric protein interacted strongly with endogenous importin α in the cytoplasmic extract of HeLa cells, whereas the αLA/αH1 protein interacted very poorly. A similar result was obtained with recombinant importin α (Fig. 6Β). These results indicate that the Leu residues at positions 20, 22, 23, and 26 in the αH1 region of Vpr are crucial for binding to importin α.
FIG. 6.
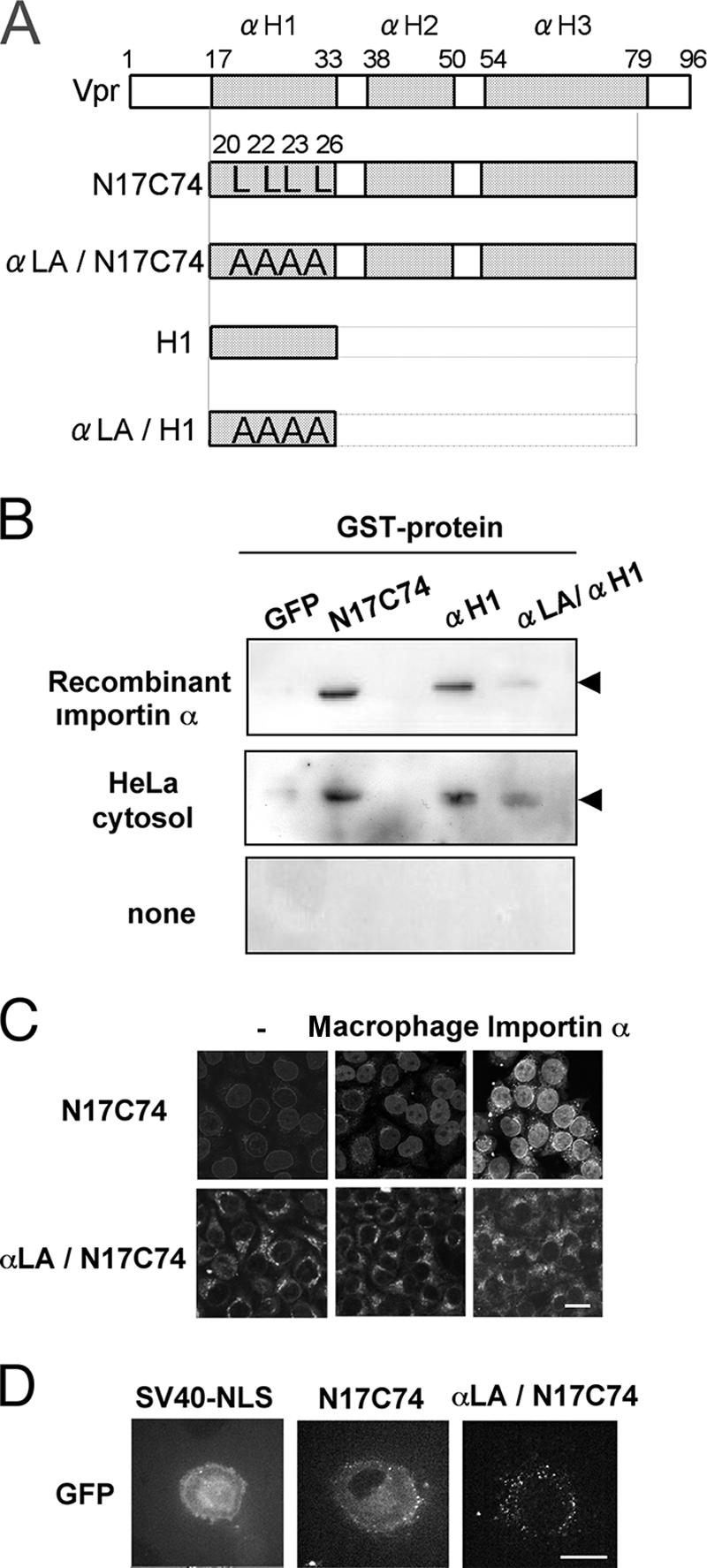
Vpr mutants that cannot bind importin α are not imported into the nucleus. (A) Construction of plasmids encoding GST- and GFP-tagged mutant forms of Vpr. The three α-helical domains (αH1, αH2, and αH3) are represented by shaded boxes. The Leu residues at positions 20, 22, 23, and 26 in the αH1 domain were replaced by Ala. (B) Glutathione-Sepharose beads were coupled with the recombinant proteins, namely, GST-tagged GFP, GST-N17C74-GFP, GST-αH1-GFP, and GST-αLA/αH1-GFP, and incubated with 100 pmol recombinant importin α (top), 100 μg cytosol of HeLa cells (middle), or nothing as a control (bottom). The proteins recovered from the beads were subjected to Western blotting with a MAb against importin α1. The position of importin α is indicated. (C) Digitonin-permeabilized HeLa cells were incubated with 1 μM GST- and GFP-tagged N17C74 or αLA/N17C74 in the presence of 100 μg cytoplasmic extract prepared from primary macrophages or 1 μM recombinant importin α. After fixation, cells were analyzed by confocal laser scanning microscopy. Bar = 20 μm. (D) GST- and GFP-tagged N17C74, αLA/N17C74 or SV40 NLS was injected into the cytoplasm of differentiated primary macrophages grown on a glass-bottomed dish. After 15 min, the transport reactions were captured by confocal laser scanning microscopy. Bar = 20 μm.
To further demonstrate the importance of this region for the nuclear transport of Vpr, we carried out an in vitro nuclear transport assay with the αLA/N17C74 chimeric protein, using digitonin-permeabilized HeLa cells. As shown in Fig. 6C, the αLA/N17C74 mutant had completely lost the ability to migrate into the nucleus in the presence of a cytoplasmic extract prepared from primary macrophages, even in the presence of recombinant importin α. The localization of N17C74 and of the αLA/N17C74 mutant protein was determined after cytoplasmic microinjection into primary macrophages. N17C74 clearly localized in the nucleus, whereas the mutant did not, suggesting that the mutant had lost nuclear import activity (Fig. 6D). These findings clearly indicate that the ability of Vpr to interact with importin α is indispensable for its nuclear import in macrophages.
Interaction of importin α through the αΗ1 domain of Vpr is crucial for the efficient replication of HIV-1 in macrophages.
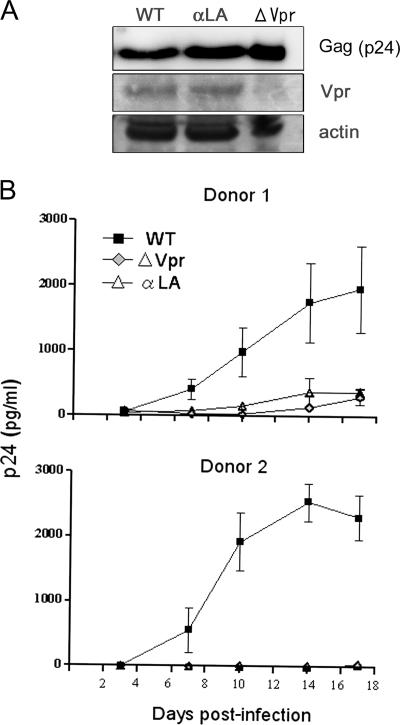
Finally, it was important to determine whether the nuclear entry of Vpr mediated by its interaction with importin α is crucial for macrophage-tropic HIV-1 replication in primary macrophages. We compared the replication in primary macrophages of HIV-1 carrying wild-type Vpr, a Vpr ATG mutant (ΔVpr), and a mutant form (αLA) that cannot interact with importin α and thus is defective in nuclear transport. The αLA mutant retained the ability to induce G2 arrest and apoptosis (data not shown). To examine whether the αLA mutant virus synthesized mutant Vpr protein, HeLa cells were transfected with pNF462 proviral DNA encoding wild-type Vpr or the Vpr mutant form (αLA), and the cell lysates were analyzed by Western blotting using anti-Vpr antibody and anti-Gag antibody. The wild-type and αLA mutant transfectants had similar amounts of Vpr and p24Gag (Fig. 7A). Typical kinetics for the replication of these wild-type and mutant viruses in the primary macrophages of two donors are shown in Fig. 7B. The virus carrying wild-type Vpr replicated well in both donors, with viral inputs of 5 ng p24 antigen, and replication reached a peak 14 to 17 days after infection. In contrast, ΔVpr HIV-1 displayed a markedly decreased level of replication compared to the wild-type virus, suggesting the importance of Vpr in HIV-1 replication in primary macrophages. Interestingly, replication of the αLA mutant virus was also reduced to a level equivalent to that of the ΔVpr virus. Similar results were obtained with macrophages isolated from four additional donors (data not shown).
FIG. 7.
Interaction between Vpr and importin α is crucial for the efficient replication of macrophage-tropic HIV-1 in macrophages. (A) Cell lysates derived from HeLa cells transfected with pNF462 proviral DNA encoding wild-type Vpr (WT), the Vpr mutant form (αLA), or a deficient form of Vpr (ΔVpr) were analyzed by Western blotting with anti-Vpr and anti-Gag antibodies. (B) Differentiated primary macrophages from two independent donors were infected with macrophage-tropic viruses (5 ng/well) that encoded wild-type Vpr (▪), the mutant αLA form (▵), or a deficient form of Vpr ( ), and the kinetics of virus production were analyzed. Cells were maintained for 3 weeks, and the levels of virus production in culture supernatants were measured by p24 antigen ELISA. All samples were tested in triplicate, and the data presented are the mean levels of p24 antigen. The bars indicate the standard errors of the measurements.
), and the kinetics of virus production were analyzed. Cells were maintained for 3 weeks, and the levels of virus production in culture supernatants were measured by p24 antigen ELISA. All samples were tested in triplicate, and the data presented are the mean levels of p24 antigen. The bars indicate the standard errors of the measurements.
Our results strongly support the notions that the binding of Vpr to importin α is essential for the nuclear import of Vpr and that this nuclear import is crucial for viral replication in macrophages. In addition, our results indicate that expression of importin α is essential for efficient viral replication in macrophages.
DISCUSSION
Novel nuclear import mechanism for Vpr promoted by importin α.
We previously showed that Vpr traverses the NPC in an importin α-dependent manner (19). Our present study clearly demonstrates that importin α promotes nuclear import of Vpr in digitonin-permeabilized cells, without the need for importin β or other soluble proteins. The importin α derivative ΔIBB, which cannot bind to importin β, also promotes nuclear import of Vpr. In addition, depletion of three importin α isoforms from HeLa cells by use of siRNAs markedly decreased the nuclear import of Vpr in an in vitro nuclear transport assay using cytoplasmic extracts but not in importin β-depleted extracts. These results strongly suggest that Vpr is transported into the nucleus by importin α alone, without utilizing the classical importin β-dependent transport pathway. To our knowledge, Vpr is the first retroviral protein that has been shown to use a nuclear import mechanism involving importin α alone, without any other soluble factors. Moreover, as previously reported (19), Vpr directly localizes to the perinuclear region, without a requirement for any soluble factors, before it is transported into the nucleus by importin α. This perinuclear localization distinguishes the nuclear import of Vpr from that of other NLS-bearing proteins, suggesting that the karyophilic properties of Vpr rely on a novel mechanism.
Our present and previous results enable us to characterize the nuclear import of Vpr as follows. (i) Vpr is targeted to the perinuclear region in the absence of soluble factors, as shown by in vitro transport assays (Fig. 1) (19). (ii) The transport of Vpr is mediated by importin α alone, without the intervention of importin β, transportin, RanGDP, or NTF2 (Fig. 1) (19). Moreover, the ΔIBB derivative of importin α can also promote nuclear import of Vpr, confirming the transport of Vpr by importin α without the aid of importin β (Fig. 1). (iii) Vpr interacts with importin α through two α-helical domains, αH1 and αH3, which play distinct roles in nuclear import (18, 19). (iv) Vpr interacts with the C-terminal region of importin α, and this interaction is required for nuclear entry (19). (v) The nuclear import of Vpr is promoted by the three major isoforms of importin α (Fig. 2). It was recently reported that importin α alone can carry CaMKIV into the nucleus without utilizing importin β (23). Similar to the case for Vpr, the nuclear transport of CaMKIV requires the C-terminal region of importin α and is promoted by all three major isoforms of importin α. We have found that Vpr and CaMKIV share a highly conserved amino acid region that is rich in Leu and Gln residues. In our study, the αLA mutant, in which all Leu residues are replaced with Ala in the αH1 domain, is incapable of nuclear import due to its reduced interaction with importin α. In addition, this mutant appears to retain not only its G2 arrest and apoptosis-promoting activities, at half the levels for wild-type Vpr (data not shown), but also the same secondary profile as wild-type Vpr, as calculated by a secondary structure prediction program (27). Therefore, this Leu-rich region may be important for the atypical nuclear import promoted by importin α alone. Additional analysis will be required to completely elucidate this novel import mechanism.
We have previously demonstrated that the perinuclear localization of Vpr is necessary for its nuclear transport (19). In the present study, Vpr appeared to be targeted to the perinuclear region, without the assistance of soluble factors, prior to entering the nucleus in an importin α-dependent manner, which is notably distinct from the mechanism of CaMKIV nuclear import mediated by importin α. Earlier studies have shown that Vpr binds to several nucleoporins, including human p54 and p58 Nups, rodent POM121, Saccharomyces cerevisiae NUP1P, and human CG1 (10, 25, 42, 48). It has been suggested that Vpr itself may directly dock on the NPC before entry into the nucleus and that this is the focal point of the new nuclear import mechanism. Therefore, further study is required to define the role of Vpr docking to the nuclear envelope for nuclear entry.
It is known that various importin α isoforms can recognize the same target proteins, although each isoform differs in its efficiency for importing NLS-bearing proteins. Our study shows that all three major importin α isoforms, i.e., α1, α3, and α5, can promote nuclear import of Vpr and that the efficiency of import depends on the expression level of importin α in monocytes and macrophages. This result implies that Vpr enters the nucleus by a mechanism that is common to importin α isoforms. Although the N-terminal IBB domain and the C-terminal domain of importin α isoforms exhibit approximately 50 to 85% amino acid identity, all isoforms interact with importin β and the cellular apoptosis susceptibility gene product (CAS) via their IBB and C-terminal domains (22). In contrast, Vpr can bind these two domains of importin α, and binding with the C-terminal region is essential for import (19). Likewise, the nuclear import of CaMKIV requires the C terminus of importin α. The C-terminal region of importin α presumably contributes to the nonclassical import that utilizes importin α alone.
Importin α is essential not only for the nuclear import of Vpr but also for viral replication in macrophages.
The levels of RT are similar in monocytes and differentiated macrophages after infection with the HIV-based vector, whereas there are no detectable two-long-terminal-repeat vector circles in monocytes, suggesting that the nuclear entry of this vector is blocked in monocytes (34). Based on the present study, we propose a possible mechanism to explain the inhibition of PIC nuclear entry in monocytes. Firstly, we have shown that the three major importin α isoforms are abundantly expressed in differentiated macrophages but poorly expressed in monocytes. Secondly, in vitro transport assays and in vivo microinjection experiments have demonstrated that the nuclear import of Vpr correlates well with the expression level of importin α. Thirdly, the requirement for importin α for Vpr nuclear import in macrophages has been confirmed by the observation that depletion of importin α from cytoplasmic extracts of macrophages prevents import. In addition, in vitro nuclear transport assays demonstrated that siRNA depletion of importin α from cytoplasmic extracts of HeLa cells markedly decreases Vpr nuclear import. Thus, we can conclude that importin α is required for the nuclear import of Vpr. Finally, experimental infection with a virus encoding a Vpr mutant protein that cannot bind importin α showed that the expression of importin α is essential for viral replication in macrophages. Although the low level of importin α in monocytes may also be the cause of the inefficient nuclear import of MA and IN, which utilize the classical importin α/β-dependent nuclear import pathway (11), the reduced replication of vpr-deficient HIV-1 indicates the importance of Vpr in PIC nuclear import in primary macrophages (Fig. 7) (7, 14). Taken together, these results show that there is a good possibility that Vpr nuclear import is inefficient in monocytes, which do not express importin α, and that therefore the PIC cannot enter the nuclei of these cells.
Importin α isoforms have been shown to differ in their cell- and tissue-specific expression patterns and to depend on the state of cellular metabolism and differentiation (20, 30, 47). Indeed, it has been reported that importins α1 and α5 are inducible and differentially expressed in the human Jurkat and Raji lymphocyte lines (33) and, in addition, that importin α isoforms are expressed in the human leukemia HL60 cell line during proliferation and differentiation into macrophages or neutrophils (21). However, the expression of importin α isoforms has not been investigated previously in human primary PBMC. In the present study, we demonstrated that importin α isoforms α1, α3, and α5 are more abundantly expressed in primary differentiated macrophages prepared from healthy donors than in undifferentiated monocytes, indicating that primary monocytes undergo a marked increase in the expression of these three isoforms upon differentiation. In addition, we have found that all three major importin α isoforms are more strongly expressed in activated CD4+ T cells than in resting CD4+ T cells (unpublished data). This result may provide significant information about the cell-specific expression of importin α. Further study is required to clarify whether or not other importin α isoforms, such as α4, α6, and α7, expressed in monocytes, macrophages, resting CD4+ T cells, and activated CD4+ T cells are targets of HIV-1 infection.
Importin 7, one of the importin β family members, is another host cellular protein that is relevant to the nuclear import of the HIV-1 PIC (9). However, recent experiments with RNA interference technology have shown that importin 7 deficiency does not alter the efficiency of HIV-1 and simian immunodeficiency virus cDNA synthesis, nuclear translocation, or infection, suggesting that importin 7 is dispensable for HIV-1 infection (49). This result clearly demonstrates that importin 7 is not important for HIV-1 nuclear import in macrophages and that HIV-1 and simian immunodeficiency virus nuclear transport may involve other karyopherins or another unconventional mechanism. In this regard, the atypical importin α-dependent nuclear import of Vpr may be considered a candidate mechanism for transport of the HIV-1 PIC.
Binding of Vpr to importin α is a promising target for blocking HIV-1 replication.
We demonstrated that after entering the host cell, the mutant αLA virus and L67P virus (15) may be restricted at the nuclear import step. We have speculated that Vpr nuclear import, mediated by its interaction with importin α, could play an important role in efficient HIV-1 infection, not only in monocyte-derived macrophages but also in activated CD4+ T cells (15). A more complete understanding of this novel nuclear import mechanism will require a detailed investigation of the interaction between the αH1 domain of Vpr and the C-terminal region of importin α that leads to nuclear import. The interaction between Vpr and importin α may be a potential target for an antiviral agent that inhibits the nuclear entry step.
Acknowledgments
We thank Akio Adachi for kindly providing HIV-1 pNL432 and pNF462. We thank Atushi Miyawaki for critical comments on imaging analysis.
This work was supported, in part, by a Health Sciences Research Grant from the Ministry of Health, Labor and Welfare of Japan (Research on HIV/AIDS 16150301), by Grants-in-Aid for Scientific Research on Priority Areas (1402113, 15019115, and 16017304), by a Grant-in-Aid for Exploratory Research (18659135) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan, by a President's Special Research Grant from RIKEN, and by the Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO) of Japan.
Footnotes
Published ahead of print on 7 March 2007.
REFERENCES
- 1.Agostini, I., S. Popov, J. Li, L. Dubrovsky, T. Hao, and M. Bukrinsky. 2000. Heat-shock protein 70 can replace viral protein R of HIV-1 during nuclear import of the viral preintegration complex. Exp. Cell Res. 259:398-403. [DOI] [PubMed] [Google Scholar]
- 2.Ayyavoo, V., A. Mahboubi, S. Mahalingam, R. Ramalingam, S. Kudchodkar, W. V. Williams, D. R. Green, and D. B. Weiner. 1997. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 3:1117-1123. [DOI] [PubMed] [Google Scholar]
- 3.Azuma, A., A. Matsuo, T. Suzuki, T. Kurosawa, X. Zhang, and Y. Aida. 2006. Human immunodeficiency virus type 1 Vpr induces cell cycle arrest at the G(1) phase and apoptosis via disruption of mitochondrial function in rodent cells. Microbes Infect. 8:670-679. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., N. Sharova, M. P. Dempsey, T. L. Stanwick, A. G. Bukrinskaya, S. Haggerty, and M. Stevenson. 1992. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc. Natl. Acad. Sci. USA 89:6580-6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukrinsky, M. I., N. Sharova, T. L. McDonald, T. Pushkarskaya, W. G. Tarpley, and M. Stevenson. 1993. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 90:6125-6129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 7.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 8.Cuomo, C. A., S. A. Kirch, J. Gyuris, R. Brent, and M. A. Oettinger. 1994. Rch1, a protein that specifically interacts with the RAG-1 recombination-activating protein. Proc. Natl. Acad. Sci. USA 91:6156-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassati, A., D. Gorlich, I. Harrison, L. Zaytseva, and J. M. Mingot. 2003. Nuclear import of HIV-1 intracellular reverse transcription complexes is mediated by importin 7. EMBO J. 22:3675-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fouchier, R. A., B. E. Meyer, J. H. Simon, U. Fischer, A. V. Albright, F. Gonzalez-Scarano, and M. H. Malim. 1998. Interaction of the human immunodeficiency virus type 1 Vpr protein with the nuclear pore complex. J. Virol. 72:6004-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallay, P., V. Stitt, C. Mundy, M. Oettinger, and D. Trono. 1996. Role of the karyopherin pathway in human immunodeficiency virus type 1 nuclear import. J. Virol. 70:1027-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghorpade, A., M. Q. Xia, B. T. Hyman, Y. Persidsky, A. Nukuna, P. Bock, M. Che, J. Limoges, H. E. Gendelman, and C. R. Mackay. 1998. Role of the beta-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J. Virol. 72:3351-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorlich, D., and I. W. Mattaj. 1996. Nucleocytoplasmic transport. Science 271:1513-1518. [DOI] [PubMed] [Google Scholar]
- 14.Heinzinger, N. K., M. I. Bukinsky, S. A. Haggerty, A. M. Ragland, V. Kewalramani, M. A. Lee, H. E. Gendelman, L. Ratner, M. Stevenson, and M. Emerman. 1994. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc. Natl. Acad. Sci. USA 91:7311-7315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iijima, S., Y. Nitahara-Kasahara, K. Kimata, W. Zhong Zhuang, M. Kamata, M. Isogai, M. Miwa, Y. Tsunetsugu-Yokota, and Y. Aida. 2004. Nuclear localization of Vpr is crucial for the efficient replication of HIV-1 in primary CD4+ T cells. Virology 327:249-261. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins, Y., M. McEntee, K. Weis, and W. C. Greene. 1998. Characterization of HIV-1 vpr nuclear import: analysis of signals and pathways. J. Cell Biol. 143:875-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2+M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamata, M., and Y. Aida. 2000. Two putative alpha-helical domains of human immunodeficiency virus type 1 Vpr mediate nuclear localization by at least two mechanisms. J. Virol. 74:7179-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamata, M., Y. Nitahara-Kasahara, Y. Miyamoto, Y. Yoneda, and Y. Aida. 2005. Importin-alpha promotes passage through the nuclear pore complex of human immunodeficiency virus type 1 Vpr. J. Virol. 79:3557-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamei, Y., S. Yuba, T. Nakayama, and Y. Yoneda. 1999. Three distinct classes of the alpha-subunit of the nuclear pore-targeting complex (importin-alpha) are differentially expressed in adult mouse tissues. J. Histochem. Cytochem. 47:363-372. [DOI] [PubMed] [Google Scholar]
- 21.Kohler, M., A. Fiebeler, M. Hartwig, S. Thiel, S. Prehn, R. Kettritz, F. C. Luft, and E. Hartmann. 2002. Differential expression of classical nuclear transport factors during cellular proliferation and differentiation. Cell. Physiol. Biochem. 12:335-344. [DOI] [PubMed] [Google Scholar]
- 22.Kohler, M., C. Speck, M. Christiansen, F. R. Bischoff, S. Prehn, H. Haller, D. Gorlich, and E. Hartmann. 1999. Evidence for distinct substrate specificities of importin alpha family members in nuclear protein import. Mol. Cell. Biol. 19:7782-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotera, I., T. Sekimoto, Y. Miyamoto, T. Saiwaki, E. Nagoshi, H. Sakagami, H. Kondo, and Y. Yoneda. 2005. Importin alpha transports CaMKIV to the nucleus without utilizing importin beta. EMBO J. 24:942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuramitsu, M., C. Hashizume, N. Yamamoto, A. Azuma, M. Kamata, N. Yamamoto, Y. Tanaka, and Y. Aida. 2005. A novel role for Vpr of human immunodeficiency virus type 1 as a regulator of the splicing of cellular pre-mRNA. Microbes Infect. 7:1150-1160. [DOI] [PubMed] [Google Scholar]
- 25.Le Rouzic, E., A. Mousnier, C. Rustum, F. Stutz, E. Hallberg, C. Dargemont, and S. Benichou. 2002. Docking of HIV-1 Vpr to the nuclear envelope is mediated by the interaction with the nucleoporin hCG1. J. Biol. Chem. 277:45091-45098. [DOI] [PubMed] [Google Scholar]
- 26.Lu, Y. L., P. Spearman, and L. Ratner. 1993. Human immunodeficiency virus type 1 viral protein R localization in infected cells and virions. J. Virol. 67:6542-6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahalingam, S., V. Ayyavoo, M. Patel, T. Kieber-Emmon, and D. B. Weiner. 1997. Nuclear import, virion incorporation, and cell cycle arrest/differentiation are mediated by distinct functional domains of human immunodeficiency virus type 1 Vpr. J. Virol. 71:6339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchior, F., T. Guan, N. Yokoyama, T. Nishimoto, and L. Gerace. 1995. GTP hydrolysis by Ran occurs at the nuclear pore complex in an early step of protein import. J. Cell Biol. 131:571-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyamoto, Y., M. Hieda, M. T. Harreman, M. Fukumoto, T. Saiwaki, A. E. Hodel, A. H. Corbett, and Y. Yoneda. 2002. Importin alpha can migrate into the nucleus in an importin beta- and Ran-independent manner. EMBO J. 21:5833-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyamoto, Y., N. Imamoto, T. Sekimoto, T. Tachibana, T. Seki, S. Tada, T. Enomoto, and Y. Yoneda. 1997. Differential modes of nuclear localization signal (NLS) recognition by three distinct classes of NLS receptors. J. Biol. Chem. 272:26375-26381. [DOI] [PubMed] [Google Scholar]
- 31.Miyamoto, Y., and Y. Yoneda. 1999. Molecular mechanisms of nucleocytoplasmic transport of proteins. Tanpakushitsu Kakusan Koso 44:1860-1868. [PubMed] [Google Scholar]
- 32.Moore, M. S., and G. Blobel. 1993. The GTP-binding protein Ran/TC4 is required for protein import into the nucleus. Nature 365:661-663. [DOI] [PubMed] [Google Scholar]
- 33.Nadler, S. G., D. Tritschler, O. K. Haffar, J. Blake, A. G. Bruce, and J. S. Cleaveland. 1997. Differential expression and sequence-specific interaction of karyopherin alpha with nuclear localization sequences. J. Biol. Chem. 272:4310-4315. [DOI] [PubMed] [Google Scholar]
- 34.Neil, S., F. Martin, Y. Ikeda, and M. Collins. 2001. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 75:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishizawa, M., M. Kamata, R. Katsumata, and Y. Aida. 2000. A carboxy-terminally truncated form of the human immunodeficiency virus type 1 Vpr protein induces apoptosis via G1 cell cycle arrest. J. Virol. 74:6058-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishizawa, M., M. Kamata, T. Mojin, Y. Nakai, and Y. Aida. 2000. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G(2) arrest of the cell cycle. Virology 276:16-26. [DOI] [PubMed] [Google Scholar]
- 37.Nishizawa, M., T. Myojin, Y. Nishino, Y. Nakai, M. Kamata, and Y. Aida. 1999. A carboxy-terminally truncated form of the Vpr protein of human immunodeficiency virus type 1 retards cell proliferation independently of G(2) arrest of the cell cycle. Virology 263:313-322. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill, R. E., and P. Palese. 1995. NPI-1, the human homolog of SRP-1, interacts with influenza virus nucleoprotein. Virology 206:116-125. [DOI] [PubMed] [Google Scholar]
- 39.Orenstein, J. M., M. Feinberg, C. Yoder, L. Schrager, J. M. Mican, D. J. Schwartzentruber, R. T. Davey, Jr., R. E. Walker, J. Falloon, J. A. Kovacs, K. D. Miller, C. Fox, J. A. Metcalf, H. Masur, and M. A. Polis. 1999. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS 13:2219-2229. [DOI] [PubMed] [Google Scholar]
- 40.Orenstein, J. M., C. Fox, and S. M. Wahl. 1997. Macrophages as a source of HIV during opportunistic infections. Science 276:1857-1861. [DOI] [PubMed] [Google Scholar]
- 41.Pemberton, L. F., G. Blobel, and J. S. Rosenblum. 1998. Transport routes through the nuclear pore complex. Curr. Opin. Cell Biol. 10:392-399. [DOI] [PubMed] [Google Scholar]
- 42.Popov, S., M. Rexach, L. Ratner, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates docking of the HIV-1 preintegration complex to the nuclear pore complex. J. Biol. Chem. 273:13347-13352. [DOI] [PubMed] [Google Scholar]
- 43.Popov, S., M. Rexach, G. Zybarth, N. Reiling, M. A. Lee, L. Ratner, C. M. Lane, M. S. Moore, G. Blobel, and M. Bukrinsky. 1998. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 17:909-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seki, T., S. Tada, T. Katada, and T. Enomoto. 1997. Cloning of a cDNA encoding a novel importin-alpha homologue, Qip1: discrimination of Qip1 and Rch1 from hSrp1 by their ability to interact with DNA helicase Q1/RecQL. Biochem. Biophys. Res. Commun. 234:48-53. [DOI] [PubMed] [Google Scholar]
- 45.Sekimoto, T., N. Imamoto, K. Nakajima, T. Hirano, and Y. Yoneda. 1997. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 16:7067-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takizawa, C. G., K. Weis, and D. O. Morgan. 1999. Ran-independent nuclear import of cyclin B1-Cdc2 by importin beta. Proc. Natl. Acad. Sci. USA 96:7938-7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuji, L., T. Takumi, N. Imamoto, and Y. Yoneda. 1997. Identification of novel homologues of mouse importin alpha, the alpha subunit of the nuclear pore-targeting complex, and their tissue-specific expression. FEBS Lett. 416:30-34. [DOI] [PubMed] [Google Scholar]
- 48.Vodicka, M. A., D. M. Koepp, P. A. Silver, and M. Emerman. 1998. HIV-1 Vpr interacts with the nuclear transport pathway to promote macrophage infection. Genes Dev. 12:175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zielske, S. P., and M. Stevenson. 2005. Importin 7 may be dispensable for human immunodeficiency virus type 1 and simian immunodeficiency virus infection of primary macrophages. J. Virol. 79:11541-11546. [DOI] [PMC free article] [PubMed] [Google Scholar]