Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia (ATL). To develop a better animal model for the investigation of HTLV-1 infection, we established a transgenic (Tg) rat carrying the human CRM1 (hCRM1) gene, which encodes a viral RNA transporter that is a species-specific restriction factor. At first we found that CRM1 expression is elaborately regulated through a pathway involving protein kinase C during lymphocyte activation, initially by posttranscriptional and subsequently by transcriptional mechanisms. This fact led us to use an hCRM1-containing bacterial artificial chromosome clone, which would harbor the entire regulatory and coding regions of the CRM1 gene. The Tg rats expressed hCRM1 protein in a manner similar to expression of intrinsic rat CRM1 in various organs. HTLV-1-infected T-cell lines derived from these Tg rats produced 100- to 10,000-fold more HTLV-1 than did T cells from wild-type rats, and the absolute levels of HTLV-1 were similar to those produced by human T cells. We also observed enhancement of the dissemination of HTLV-1 to the thymus in the Tg rats after intraperitoneal inoculation, although the proviral loads were low in both wild-type and Tg rats. These results support the essential role of hCRM1 in proper HTLV-1 replication and suggest the importance of this Tg rat as an animal model for HTLV-1.
Human T-cell leukemia virus type I (HTLV-1) is etiologically associated with human adult T-cell leukemia (ATL), a chronic progressive neurological disorder termed HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (17, 27, 54, 55), and several other human diseases (23, 40, 42, 48). Examination of the viral nucleotide sequences associated with different disease groups has not revealed any specific determinants that distinguish a particular HTLV-1-associated disease (11, 35, 67). Thus, a primary determinant of HTLV-1-associated disease may be host related.
In order to investigate HTLV-1 infection and related disease development in detail, suitable animal models are required. HTLV-1 can immortalize simian, feline, rat, and rabbit lymphocytes in vitro (2, 29, 46). HTLV-1 can also infect experimental animals, such as rabbits, monkeys, and rats (2, 45, 53, 62). Using these susceptible animals, several models have been developed to study HTLV-1-associated diseases. The HAM/TSP-like disease model in strain WKA rats is well established and has been used to dissect the pathogenic mechanisms of the disease (31, 39). In contrast, only a few ATL model systems have been established using rabbits and rats, and their utility is limited. For instance, the rabbit ATL model shows reproducible development of an ATL-like disease in adult animals (58), but few immunological studies can be performed with this animal, primarily because of the difficulty of obtaining inbred strains of rabbits. In the rat models, the development of ATL-like disease was observed only in newborn animals, with a very short period of disease onset (64), making it difficult to perform oncological and immunological studies at the same time. Ohashi et al. have established a rat model of ATL-like disease in which they were able to examine the growth and spread of HTLV-1-infected cells, as well as to assess the effects of T cells on the development of the disease in T-cell-deficient nude rats (51). This model system has been used to assess DNA- or peptide-based vaccine development (25, 52) and to study the effects of Tax-directed small interfering RNA on HTLV-1-induced tumors (50). However, since the growth of HTLV-1 tumors could be monitored only in immune-deficient nude rats in this model system, better animal models are still necessary.
HTLV-1 replicates poorly in rats, which may be one of the reasons why previously established models could not completely reproduce the features of HTLV-1-related diseases. We have previously examined the differences in the pattern of viral gene expression between human and rat T cells infected with HTLV-1 (69). In rat cells, the levels of viral mRNAs encoding the Gag and Env proteins were much lower than those encoding the Tax and Rex proteins (36). Rex plays an important role in escorting unspliced and incompletely spliced viral mRNAs to the cytoplasm, resulting in enhanced synthesis of viral structural proteins (5, 34, 69). Human CRM1 (hCRM1) is a critical factor for Rex-dependent viral mRNA export to the cytoplasm, and rat CRM1 (rCRM1) cannot substitute for this function (19, 22, 69). Thus, it is reasonable to assume that transgenic (Tg) rats carrying the hCRM1 gene should provide a better environment for HTLV-1 replication and that such animals would provide a better animal model of HTLV-1 infection.
CRM1 is involved in numerous cellular activities, suggesting its essential function in viability, which is supported by the high conservation of CRM1 genes from yeast to humans (37) and by the demonstration that both yeast and mammalian cells defective in CRM1 are inviable (1, 15). In contrast, overexpression of CRM1 has been reported to inhibit early embryogenesis in Xenopus laevis (8). Therefore, proper expression of hCRM1 in rats will be essential to produce Tg rats. However, the regulation of CRM1 expression and synthesis has not yet been investigated in detail. Some immortalized cell lines have been reported to maintain CRM1 protein at constant levels throughout the cell cycle, which is compatible with an essential function (37), but other reports have indicated differences in the level of expression of CRM1 among different tissues (28, 37), implying that the expression is regulated. Therefore, we first investigated the expression profile of the CRM1 gene, especially during lymphocyte activation, to determine means for the proper expression of hCRM1 as a transgene. Our results indicate that expression of the CRM1 gene is elaborately regulated during the activation of lymphocytes, including CD4+ T cells, the major targets of HTLV-1. These data suggested that it would be necessary to use a construct harboring the entire regulatory and coding regions of CRM1 for Tg rat construction.
Using a bacterial artificial chromosome (BAC) clone containing the entire CRM1 gene, we have established hCRM1-Tg rats and examined the proliferation of HTLV-1 in vitro and in vivo. Our results demonstrate that T-cell lines isolated from hCRM1-Tg rats produced 100 to 10,000 times more HTLV-1 Gag antigen than T cells from wild-type (Wt) control rats and that Tg rats displayed more-extensive invasion of the thymus by HTLV-1 when infected intraperitoneally. These results indicate the essential role of hCRM1 in proper HTLV-1 replication and suggest the importance of this Tg rat model as a basis for the development of better HTLV-1 animal models.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors using Ficoll-Hypaque (Pharmacia) or Ficoll Paque Plus (Amersham Biotechnology) density centrifugation. CD4+ T lymphocytes were purified by negative selection using an immunomagnetic cell sorting apparatus, the MidiMACS cell separator (Miltenyi Biotec), and a cocktail of MACS MicroBeads coupled to hapten-conjugated monoclonal antibodies (MAbs) specific for CD8, CD11b, CD16, CD19, CD36, and CD56. The purity of CD4+ T cells was evaluated by flow cytometry (FACSCalibur; Becton Dickinson) to be approximately 95%.
For activation, cells were cultured with various combinations of 50 nM phorbol 12-myristate 13-acetate (PMA), 100 nM ionomycin, and 10 ng/ml interleukin-2 (IL-2).
The HTLV-1-producing rat and human T-cell lines, FPM1 and MT-2, have been described previously (36, 44). HTLV-1-immortalized cell lines from Wt or Tg rats were established by cocultivating thymocytes or splenocytes with MT-2 cells, which had been treated with mitomycin C (50 μg/ml) for 30 min at 37°C. These cells were maintained in a medium supplemented with 10 U/ml of IL-2 (PeproTech EC) at the beginning of coculture. Some cell lines were eventually freed from exogenous IL-2.
Western blotting.
Cells were lysed in ice-cold extraction buffer (10 mM Tris-HCl [pH 7.4], 1 mM MgCl2, 0.5% NP-40) containing a protease inhibitor cocktail (Complete Mini; Roche Diagnostics). The protein concentration of each sample was determined using a protein assay kit (QB Perbio; Pierce). The cell lysates were sonicated or, in some cases, treated with DNase 1 solution (Takara) and then dissolved in sample buffer. The same amounts (approximately 20 μg) of cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred to a nitrocellulose membrane and probed with anti-hCRM1 or anti-rCRM1 (34), anti-β-actin (AC40; Sigma), or anti-Rex (34) antibodies followed by secondary antibodies conjugated to alkaline phosphatase or horseradish peroxidase. Proteins were visualized by staining with 5-bromo-4-chloro-3-indolylphosphate-nitroblue tetrazolium or by ECL+ (Amersham Pharmacia Biotech) followed by the LAS-100 Plus system (Fujifilm) and were evaluated by Image Gauge (version 3.4) software (Fujifilm).
hCRM1 mRNA quantitative reverse transcription-PCR (RT-PCR).
Total RNA was extracted using the RNeasy Mini kit (QIAGEN) and was treated with RNase-free DNase I (QIAGEN) to minimize contamination of chromosomal DNA. The RNA concentration was measured by absorbance at 260 nm, and purity was ascertained by the ratio of the optical density at 260 nm to that at 280 nm and by gel electrophoresis.
To quantify CRM1 mRNA, RNA samples (5 μg) were subjected to quantitative RT-PCR with the Platinum quantitative RT-PCR Thermoscript one-step system (Invitrogen) using the forward primer 5′-GCT GAA AAC TCA ACC GAG ATG G-3′, the reverse primer 5′-CTG TTG CTC TTG CTG ATG CTG TA-3′, and the probe 6-carboxyfluorescein-AAA ATG CCG CAG GCA TTT CGT TCA G-6-carboxytetramethylrhodamine. RT-PCR was performed by incubation for 2 min at 50°C, 30 min at 60°C, and 10 min at 95°C, followed by 50 cycles of 20 s at 95°C and 1 min at 62°C in an Applied Biosystems Prism 7700 sequence detector thermocycler with Sequence Detector software (Applied Biosystems). To make standard curves, the region from bp −943 to +38 of the CRM1 cDNA was amplified by PCR using Human Lung Marathon-Ready cDNA (Clontech) with adaptor primer 1 and 5′-GCTGCATGGTCTGCTAACATT-3′ and by nested PCR with adaptor primer 2 and 5′-CTGCATGGTCTGCTAACATTG-3′. The PCR product was cloned into the pCR 2.1 vector (Invitrogen), and a 981-base single-stranded RNA was synthesized in vitro with MegaScript T7 (Ambion).
Establishment of hCRM1-Tg rats.
pBeloBAC hCRM1, which harbors the entire hCRM1 genomic sequence including approximately 50 kb of 5′ upstream sequence and 10 kb of 3′ downstream sequence, was microinjected into 450 fertilized 1-cell eggs prepared from Fischer 344/Du Crj female rats by the YS Institute. Integration of the transgene was confirmed by PCR using genomic DNA, extracted with the PUREGENE tissue kit (Gentra) from the rat tail, as a template with the hCRM1-specific primer pairs 5′-TTATGTGGCTGCAGTGTGGA-3′ and 5′-ACATACCAGGGTTCTCTGGA-3′, and 5′-GTCACCTGATGTCGGGAGTT-3′ and 5′-GGATTACAGGTGTGAGCCA-3′. All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals, Institute for Genetic Medicine, Hokkaido University.
Detection of genomic copies of hCRM1 and G3PDH.
Genomic DNA was subjected to PCR with the following primer pairs: for hCRM1, forward primer 5′-TGA GGT CAG GAG TTC AGG AT-3′ and reverse primer 5′-CTC TGC CTC CTG GGT TCA A-3′; for glyceraldehyde-3-phosphate dehydrogenase (G3PDH), forward primer 5′-AGA GCT GAA CGG GAA G-3′ and reverse primer 5′-GGA AGA ATG GGA GTT GC-3′. PCR conditions were as follows: 5 min at 94°C; 10 cycles of 30 s at 94°C, 60 s at 69°C, with a decrease of 0.5°C/cycle, and 30 s at 72°C; 8 cycles of 30 s at 94°C, 60 s at 65°C, and 30 s at 72°C; and a final extension for 10 min at 72°C.
Quantification of HTLV-1 proviral load by LightCycler-based real-time PCR.
The HTLV-1 proviral loads of HTLV-1-infected cells were quantified by real-time PCR on a LightCycler PCR instrument (Roche Diagnostics). Briefly, 20 μl of a PCR mixture in a capillary tube containing each HTLV-1 pX-specific inner primer pair (pX1 and pX4) at 0.4 μM, 1× LightCycler-FastStart SYBR Green PCR master mix, and 30 ng of genomic DNA was subjected to 40 cycles of denaturation (95°C, 15 s), annealing (69°C, 10 s), and extension (72°C, 10 s) following an initial Taq polymerase activation step (95°C, 15 min). The copy numbers of HTLV-1 provirus in the samples were estimated from a standard regression curve using LightCycler software, version 3 (Roche Diagnostics). The standard curve for HTLV-1 provirus was obtained by PCR data using 1 × 102 to 1 × 108 copies of pCR-pX1-4 plasmids, which were constructed by inserting a PCR fragment amplified with pX1 (5′-CCC ACT TCC CAG GGT TTG GAC AGA GTC TTC-3′) and pX4 (5′-GGG GAA GGA GGG GAG TCG AGG GAT AAG GAA-3′) from the genomic DNA of MT-2 cells into pCR2.1. The copy numbers of HTLV-1 provirus were normalized by dividing by the copy numbers of the G3PDH gene in the same samples.
Detection of HTLV-1 p19.
Each cell line (105 cells/well) was cultured in 24-well flat-bottom plates for 4 days. The amount of HTLV-1 p19 protein in the culture supernatant or in rat plasma was quantified using an HTLV-1/2 p19 antigen enzyme-linked immunosorbent assay (ZeptoMetrix).
Detection of intracellular Tax and Gag proteins.
Cells (106) were fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS) containing 20 μg/ml of lysolecithin (Sigma) for 2 min at room temperature, centrifuged, and resuspended in cold methanol. The cells were then sorted at 4°C for 15 min, centrifuged, and incubated in 0.1% NP-40 in PBS at 4°C for 5 min. After centrifugation, the cells were stained with the mouse anti-Tax MAb LT-4 (63) or the mouse anti-Gag MAb GIN-7 (38), followed by a fluorescein isothiocyanate-conjugated goat antibody against mouse immunoglobulin G (IgG) plus IgM (Immunotech). Finally, the cells were washed and fixed with 1% formalin in PBS prior to analysis by cell sorting.
Inoculation of HTLV-1 into rats.
Various numbers of mitomycin C-treated or untreated MT-2 cells were intraperitoneally administered to 3- to 6-week-old Wt or hCRM1-Tg rats. Peripheral blood samples were collected from the rats every 2 or 4 weeks after inoculation, and the presence of HTLV-1 provirus in peripheral blood cells and levels of p19 in plasma were determined. In some experiments, rats were euthanized 1 week after inoculation and samples were collected to assess plasma p19 concentrations, proviral loads, and the presence of HTLV-1 provirus.
Detection of provirus in HTLV-1-infected rats.
To determine the rate of HTLV-1 provirus positivity in various organs, 200 μg of genomic DNA was subjected to PCR for the amplification of the pX region of HTLV-1 as described previously (51). The first-step PCR was performed with the primer pair pX1-pX4, followed by the second-step PCR with the primer pair consisting of pX2 (5′-CGGATACCCAGTCTACGTGTTTGGAGACTGT-3′) and pX3 (5′-GAGCCGATAACGCGTCCATCGATGGGGTCC-3′). PCR conditions were as follows: activation of Taq polymerase (94°C, 3 min); 35 cycles of denaturation (94°C, 30 s), annealing (60°C, 30 s), and extension (72°C, 30 s); and a final elongation of the product (72°C, 3 min). For nested PCR, an aliquot of the first PCR product was subjected to another 35 PCR cycles with the second set of primers.
RESULTS
Regulated expression of CRM1 in lymphocytes.
We first examined the level of expression of CRM1 mRNA in human tissues by PCR using cDNA derived from the tissues. Expression of CRM1 mRNA was variable in different tissues. Notably, CRM1 mRNA was expressed at very low levels in PBMCs (data not shown). This result was unexpected, because PBMCs include CD4+ T cells, which are the targets of human immunodeficiency virus (HIV) and HTLV-1 (14). Lymphocytes in the PBMC population are mainly in a resting state, leading us to hypothesize that the production of CRM1 is stimulated during lymphocyte activation. Consequently, activated hematopoietic cells should contain CRM1 protein at levels similar to those observed in lymphocyte-derived cell lines. We prepared CD4+ T helper cells, macrophages, and dendritic cells from PBMCs, cultured them in the presence of appropriate cytokines, and compared the amount of CRM1 present in these cells with amounts found in Jurkat cells, a transformed cell line that constitutively expresses CRM1. Western blotting indicated that all activated lymphocyte subsets and monocyte lineage cells expressed CRM1 at levels similar to those in Jurkat cells (data not shown). These results indicate that lymphocyte activation induces high levels of CRM1 expression.
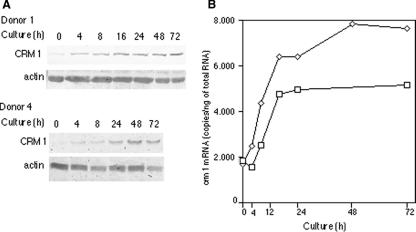
To demonstrate that CRM1 is induced during lymphocyte activation, we stimulated freshly isolated PBMCs with calcium ionophore, PMA, and IL-2, and we examined CRM1 levels at several times by Western blotting (Fig. 1A). The level of CRM1 in resting PBMCs was very low. The CRM1 level clearly increased 4 h after stimulation and then gradually increased further up to 72 h, although some differences were observed between donors 1 and 2. Little change in the level of CRM1 was observed in the absence of stimulation. Actin was used as a loading control, because its level remained relatively constant. These results indicate that the CRM1 gene belongs to the class of early response genes that are induced during lymphocyte activation.
FIG. 1.
Expression of CRM1 during activation of PBMCs. (A) PBMCs isolated from donor 1 and donor 2 were activated with ionophore, PMA, and IL-2 and were analyzed by Western blotting. (B) PBMCs isolated from donor 1 (□) and donor 3 (⋄) were activated with ionophore, PMA, and IL-2 and were analyzed by quantitative RT-PCR. Each value is the average of duplicate measurements. (C) PBMCs isolated from donor 2 were activated with various combinations of ionophore, PMA, and IL-2 and were analyzed by Western blotting. (D) PBMCs isolated from donor 2 were activated in the presence of various inhibitors and analyzed by Western blotting.
We next measured the levels of CRM1 mRNA by quantitative RT-PCR to determine how the expression of CRM1 is stimulated in PBMCs (Fig. 1B). The amount of CRM1 transcript did increase, but the expression profile differed among individuals. For example, the level of CRM1 mRNA observed in donor 3 was relatively constant up to 24 h after stimulation and then started to increase, while the level of CRM1 mRNA in donor 1 increased gradually over the course of activation. Nevertheless, we consistently found in four experiments that the increase in CRM1 mRNA levels occurred after the increase in CRM1 protein levels. Specifically, up to 4 h after stimulation, marked increases in the level of CRM1 protein were detected, in contrast to nearly constant levels of CRM1 mRNA. Therefore, these results suggest that during lymphocyte activation, CRM1 production is initially stimulated posttranscriptionally and then is further enhanced by upregulating transcription.
In order to identify the signaling pathway responsible for the induction of CRM1 transcription, we activated PBMCs in the presence of various combinations of IL-2, calcium ionophore, and PMA. As shown in Fig. 1C, IL-2 and PMA fully induced CRM1, whereas IL-2 and calcium ionophore did not. Next, we examined whether PMA alone is sufficient to induce CRM1. PMA alone enhanced CRM1 production as efficiently as IL-2 plus PMA. Since PMA is an activator of protein kinase C (PKC) (49), these data suggest that induction of CRM1 is PKC dependent.
To confirm the results described above, we examined the effects of various inhibitors, including staurosporine (a PKC inhibitor) (60) and cyclosporine (a Ca2+ cascade inhibitor) (66). As shown in Fig. 1D, staurosporine, but not cyclosporine, inhibited the induction of CRM1, consistent with the results shown in Fig. 1C. We further examined the effects of pyrrolidine dichiocarmate (PDTC) (an NF-κB inhibitor) (43) and PD98059 (a mitogen-activated protein kinase kinase inhibitor) (3) and found that PDTC inhibited CRM1 induction at the highest dose but PD98059 had only a minor effect.
Regulated expression of CRM1 in CD4+ T lymphocytes.
To examine CRM1 regulation in CD4+ T lymphocytes, resting CD4+ T lymphocytes were purified by negative selection and activated by treatment with a combination of IL-2, ionophore, and PMA. CRM1 levels were estimated by Western blotting (Fig. 2A). CRM1 expression was induced by the same stimuli as in PBMCs, although the kinetics of induction were somewhat different among donors. In contrast to CRM1, the level of actin was constant during T-cell activation. Staurosporine inhibited the enhanced production of CRM1 (data not shown), indicating the involvement of PKC in the induction of CRM1 in CD4+ T cells.
FIG. 2.
Time course of CRM1 induction during activation of CD4+ T cells. (A) CD4+ T cells isolated from donor 1 and donor 4 were activated with ionophore, PMA, and IL-2 and were analyzed by Western blotting. (B) Time course of CRM1 mRNA induction during activation of CD4+ T cells. CD4+ T cells isolated from donor 1 (□) and donor 4 (⋄) were activated with ionophore, PMA, and IL-2 and were analyzed by quantitative RT-PCR. Each value is the average of duplicate measurements.
To examine the mechanism underlying the stimulation of CRM1 in CD4+ T cells, we measured the amount of CRM1 mRNA by quantitative RT-PCR (Fig. 2B). As with PBMCs, the amount of CRM1 mRNA also increased during CD4+ T-cell activation. Although the levels of CRM1 mRNA during T-cell activation differed to some extent among donors, similar profiles of induction were observed; after a lag of approximately 4 h, the level of CRM1 mRNA started to increase and continued to do so up to 24 h after stimulation. These results suggest that the increase in CRM1 mRNA is delayed compared to the increase in CRM1 protein, as seen in PBMCs. The level of CRM1 mRNA was constant at times later than 24 h poststimulation, but purified CD4+ T cells appeared unhealthy 2 and 3 days after stimulation in these cultures, as judged by microscopic observation. Therefore, further examination is required to definitively determine the levels of CRM1 protein and mRNA in CD4+ T cells at later times after stimulation.
Expression of hCRM1 in the Tg rat.
The results described above indicate that regulation of CRM1 expression during the activation of lymphocytes is complex. Considering the lack of characterization of CRM1 regulatory elements, we used a BAC clone, which is likely to harbor the entire regulatory and coding regions of the CRM1 gene, to establish an hCRM1-Tg rat. One rat strain carrying the hCRM1 transgene was obtained from microinjection of the hCRM1-containing BAC clone into 450 fertilized 1-cell eggs from Fischer 344/Du Crj female rats. We assessed the expression of hCRM1 protein in each tissue by immunoblotting using an hCRM1-specific antibody (22). As shown in Fig. 3A, hCRM1 expression was detected in all organs tested. The expression level of this protein was especially high in the ovary and thymus compared to other organs. In addition, expression levels of hCRM1 in the organs were similar to those of endogenous rCRM1 (Fig. 3B). hCRM1 expression was not detected in any organs prepared from Wt rats (data not shown). These data indicate that the Tg rats express hCRM1 in a physiologically relevant manner.
FIG. 3.
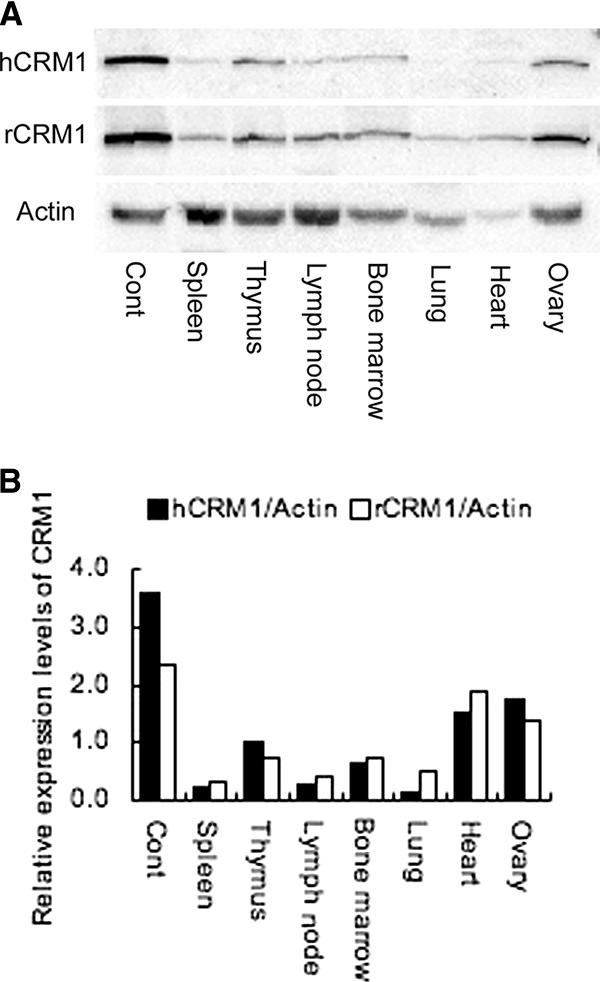
Tissue distribution of hCRM1 and rCRM1 in hCRM1 Tg rats. (A) Immunoblot assays showing the relative levels of hCRM1 and rCRM1 in rat tissues. Each protein level was determined on immunoblots containing 10 μg of total protein per lane. An FCMT18 cell extract was used as a positive control (Cont). (B) Relative levels of hCRM1 and rCRM1 expression by different organs are shown. Protein expression was quantified by ImageGauge software, and relative values are normalized to the amount of actin.
Enhanced production of p19gag in cell lines derived from Tg rats.
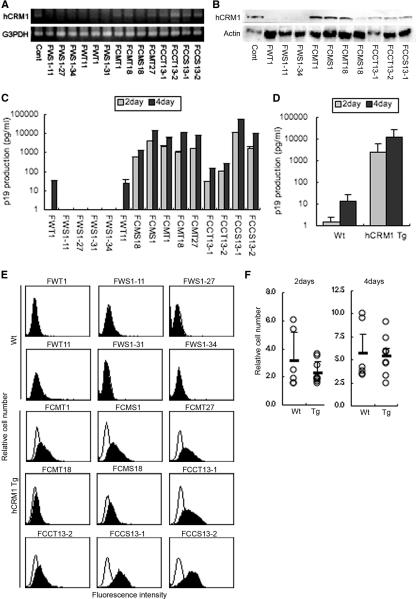
To assess the replication of HTLV-1 in T cells of hCRM1-Tg rats, we established several T-cell lines from both Wt and Tg rats by infecting with HTLV-1. Thymocytes and splenocytes isolated from Wt or hCRM1-Tg rats were cocultured with the HTLV-1-infected human T-cell line MT-2, which had been treated with mitomycin C and then maintained in a culture medium containing 10 U/ml of IL-2. After 2 months of cultivation, we obtained 6 lines from Wt rats and 9 lines from Tg rats (Table 1). As shown in Fig. 4, all of the Tg rat-derived cell lines were confirmed to have the hCRM1 gene (Fig. 4A) and to express hCRM1 (Fig. 4B), whereas none of the Wt rat-derived lines contained the gene or the protein. The expression levels of hCRM1 differed among the cell lines.
TABLE 1.
Constructed cell lines and surface markers
| Cell line | Presence or absencea of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| hCRM1 | Surface marker:
|
|||||||
| CD3 | CD4 | CD5 | CD8 | CD25 | MHC-I | MHC-II | ||
| FWT1 | − | + | + | + | − | + | + | + |
| FWS1-11 | − | − | − | + | − | + | + | + |
| FWS1-27 | − | − | − | + | − | + | + | + |
| FWS1-31 | − | − | − | + | (+) | + | + | − |
| FWS1-34 | − | − | − | + | − | + | + | − |
| FWT11 | − | + | − | + | − | + | + | + |
| FCMS1 | + | + | + | + | − | + | + | (+) |
| FCMT1 | + | + | + | + | − | + | + | + |
| FCMT18 | + | + | + | − | − | + | + | − |
| FCMS18 | + | + | + | (+) | − | + | + | (+) |
| FCMT27 | + | + | + | (+) | − | + | + | (+) |
| FCCT13-1 | + | − | − | (+) | − | + | + | + |
| FCCT13-2 | + | + | − | (+) | (+) | + | + | + |
| FCCS13-1 | + | − | − | − | − | + | + | + |
| FCCS13-2 | + | − | (+) | (+) | − | + | + | − |
+, positive; (+), weakly positive; −, negative.
FIG. 4.
Expression of HTLV-1 Gag and hCRM1 in cell lines immortalized with HTLV-1. (A) Detection of the hCRM1 transgene in cell lines by PCR. DNA extracted from each cell line (100 ng) was subjected to PCR with primers for hCRM1 and with primers for G3PDH as an internal control. (B) Protein expression of hCRM1 was detected by immunoblotting. Samples (10 μg of total protein per lane) were subjected to SDS-PAGE. A HeLa cell extract was used as a positive control (Cont). (C) HTLV-1 Gag protein levels in the supernatants of 2-day and 4-day cultures were quantified by an HTLV-1 p19 enzyme-linked immunosorbent assay. Results are means from three independent experiments. (D) Based on the data shown in panel C, the average p19gag production of Tg and Wt cell lines was calculated. (E) The amount of intracellular Gag in each cell line was analyzed by flow cytometry. Open histograms, cells stained with MAbs against p19gag and p55gag; solid histograms, cells stained with control mouse IgG. (F) The growth rates of Wt and Tg cell lines were measured. In parallel with the experiments described in the legend to panel C, the growth rate was monitored by the cell-counting Kit-8 (Dojinndo Laboratories). The relative numbers of cells in 2- or 4-day cultures versus day zero cultures are shown.
We next examined the expression of cell surface markers, including CD3, CD4, CD5, CD8, CD25, major histocompatibility complex class I (MHC-I), and MHC-II, in these cell lines (Table 1). All the cell lines expressed rat CD25 and MHC-I, indicating that they were derived from rat cells, not from the human MT-2 cells. Most of the cell lines also expressed rat CD5 and MHC-II, with the exception of two Wt rat-derived and three Tg rat-derived lines. Expression of rat CD3 was confirmed in six of nine Tg lines, whereas only two of six Wt lines were positive. Rat CD4 expression was detected in one Wt and six Tg cell lines. Rat CD8 was detected in one Wt rat-derived and one Tg rat-derived line. As judged by the expression of CD3, we established a total of eight T-cell lines, two from Wt and six from Tg rats.
We next examined the production of the p19gag protein in the cell lines to assess the effect of hCRM1 on HTLV-1 replication. Our results demonstrated that the Tg rat-derived cell lines produced much greater levels of p19 in the culture supernatant than the Wt rat-derived cells (Fig. 4C). After 2 and 4 days in culture, the mean p19 production by nine Tg rat-derived cell lines was 1,000 ± 10 and 10,000 ± 100 times higher, respectively, than the mean production of the six Wt rat-derived lines (Fig. 4D). The amounts (1 to 60 ng/ml) of p19 released from the Tg rat-derived cell lines are equivalent to those from human HTLV-1-producing T-cell lines, such as MT-2 and MT-4 (data not shown). These results clearly demonstrate the enhanced production of the HTLV-1 Gag protein in the cells expressing hCRM1.
To further examine the increased p19 production in each cell line expressing hCRM1, we conducted a fluorescence-activated cell sorter analysis to detect the intracellular Gag protein. As shown in Fig. 4E, we were able to detect p19 and the precursor p55gag protein in all cell lines derived from Tg rats. In contrast, no Wt rat-derived cell lines produced detectable amounts of Gag. These results further support the role of hCRM1 in the enhancement of HTLV-1 Gag production.
We also assessed the proliferation of each cell line to exclude the possibility that the enhanced production was not due to increased production by individual cells but was the result of increases in the number of cells in the Tg rat-derived lines. As shown in Fig. 4F, we confirmed that there was no difference in the proliferation rate between Wt rat-derived and Tg rat-derived cell lines after 2 or 4 days in culture. In addition, there was no correlation between the rate of cell growth and the amount of p19 in the culture in any cell line.
The state of HTLV-1 infection is not correlated with levels of p19 production.
We also assessed the proviral load of each cell line to rule out the possibility that enhanced production of Gag was due to increased provirus copy numbers in Tg cell lines. Real-time PCR analysis using a pair of primers for the Tax gene was performed to quantify the number of integrated provirus copies. As a relative standard, we used genomic DNA from FPM1 cells, which contain 3 copies of HTLV-1 provirus per cell (36). As shown in Fig. 5A, all five Wt cell lines contained more than 2 copies of the provirus, whereas most of the Tg lines appeared to have only 1 provirus copy per cell, with the exception of FCCT13-1 cells, which possessed 4 copies. Thus, there was no correlation between the provirus copy number and p19 production, indicating that differences in the amount of provirus were not responsible for the enhanced Gag production in Tg rat-derived cells.
FIG. 5.
Viral loads and expression in HTLV-1-transformed T cells derived from Tg and Wt rats. (A) The proviral load of each cell line was measured by quantitative real-time PCR. The copy number of HTLV-1 provirus was normalized by dividing by the G3PDH copy number in the same sample. (B) The production of intracellular Tax in each cell line was analyzed by flow cytometry. Solid histograms, cells stained with an anti-Tax MAb; open histograms, cells stained with control mouse IgG. (C) The Rex expression of each cell line was detected by immunoblotting. Ten micrograms of total protein per lane was subjected to SDS-PAGE. Lower bands in FCMS1 and FCMS18 samples represent p21rex.
Altered expression of Tax and Rex could also be associated with enhanced expression of Gag in Tg rat-derived cells. Thus, we investigated the expression of Tax in the cell lines. As shown in Fig. 5B, fluorescence-activated cell sorter analysis revealed that all of the cell lines tested expressed detectable levels of Tax proteins. Although we observed differences in the levels of Tax expression among the cell lines, there was no significant difference in Tax expression between Wt rat- and Tg rat-derived lines.
We next examined Rex expression by immunoblotting. As shown in Fig. 5C, the Rex protein was expressed in all cell lines tested. Again, there was no statistical difference in Rex protein expression between Wt and Tg cells. Two Tg cell lines, FCMS1 and FCMS18, expressed p21 protein as well as p27rex. This expression was not associated with elevated expression of Gag, since the amounts of p19gag produced by these two cell lines were similar to those for the other Tg rat-derived cell lines (Fig. 4C and D). These results indicate that the number of integrated provirus copies and the expression levels of Tax and Rex are not correlated with the enhanced expression of Gag observed in cell lines derived from hCRM1-Tg rats.
Enhanced dissemination of HTLV-1 in hCRM1-Tg rats.
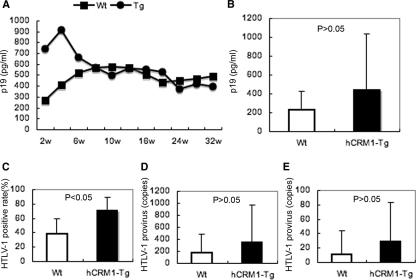
We next examined the proliferation of HTLV-1 in Tg rats by inoculating animals with the HTLV-1-producing human T-cell line MT-2 as a virus source. Analysis of plasma p19 concentrations in the infected rats over time did not show significant differences between Tg and Wt rats, although p19 concentrations in Tg rats tended to be higher during the first 6 weeks after infection (Fig. 6A). Figure 6B shows the mean plasma p19 concentration in rats after 1 week of infection and again demonstrates higher, but not significantly different, levels of the viral protein in Tg rat-derived samples. To evaluate the dissemination of the virus in vivo, we determined the presence of HTLV-1 provirus DNA in various organs 1 week after intraperitoneal infection by a nested PCR that specifically amplifies a part of the px region. We calculated the percentage of rats that sustained the px gene in five independent experiments and found that the rate at which the virus disseminated to the thymus in Tg rats was significantly higher than that for Wt rats (Fig. 6C). However, we have not detected notable differences between the two groups in HTLV-1 proviral loads in various organs, including peripheral blood cells and the thymus (Fig. 6D and E; also data not shown). These results indicate the limited effects of hCRM1 on the proliferation of HTLV-1 in vivo, in dramatic contrast to the significant enhancement of HTLV-1 production in Tg rat-derived cells in vitro.
FIG. 6.
Dissemination of HTLV-1 in hCRM1-Tg rats. (A) Mean plasma p19 concentration in Wt (n = 9) or hCRM1-Tg (n = 7) rats after intraperitoneal inoculation of mitomycin C-treated MT-2 cells (1 × 107 per animal). (B) Mean plasma p19 concentration in Wt (n = 16) or hCRM1-Tg (n = 17) rats 1 week after intraperitoneal inoculation of MT-2 cells (5 × 106 per animal). (C) Detection of the HTLV-1 provirus in thymuses derived from rats used for the experiment for which results are shown in panel B. The presence of the HTLV-1 provirus was analyzed by nested PCR. Results are mean percentages of HTLV-1 provirus-positive rats from five independent experiments. (D and E) HTLV-1 proviral loads of rats used in the experiment for which results are shown in panel B. HTLV-1 proviral loads in peripheral blood cells (D) or thymuses (E) were quantified by real-time PCR. The relative copy numbers of HTLV-1 provirus per 2 × 107 copies of G3PDH are shown. Results are expressed as means + standard deviations. The statistical significance of differences, shown in panels B to E, was determined by Student's t test, using Microsoft Excel 2004 software for Mac.
DISCUSSION
Unlike hCRM1, rCRM1 does not support Rex function, due to its inability to induce Rex-Rex dimerization, which is required for RNA export from the nucleus to the cytoplasm (22). This may be one reason why HTLV-1 replicates poorly in rats compared to humans. This observation suggests that the hCRM1-Tg rats would be novel animal models, since they would support better replication of HTLV-1.
The essential role of CRM1 in cell viability suggested that proper expression of the transgene would be key for the successful construction of Tg rats. Therefore, we examined the expression pattern of CRM1 and found that CRM1 is expressed in a manner similar to that of the early response genes induced during the activation of lymphocytes, including CD4+ T cells. Our results suggest that expression of CRM1 is stimulated in two steps: in the first phase, lasting approximately 4 h, induction is regulated primarily in a posttranscriptional manner, and in the second phase, transcriptional augmentation takes place. Alternatively, CRM1 protein in PBMCs may be rapidly turned over and then protected from degradation upon stimulation, giving rise to the early increase in protein levels. The profile of CRM1 expression further suggests that the initial induction occurs in the G1 phase of the cell cycle, a hypothesis also supported by the observation that mimosine, which blocks the cell cycle in late G1 (65), does not prevent the induction (data not shown).
The elaborate regulation of CRM1 expression led us to use a BAC clone harboring the entire hCRM1 gene for Tg rat construction. An initial unsuccessful trial using the mouse H2 promoter to express hCRM1 cDNA supports the necessity of using the hCRM1 BAC. Our results indicate that the hCRM1 BAC Tg rats express hCRM1 in various organs, including the thymus and spleen, in a manner similar to expression of endogenous rCRM1 in rats. Moreover, the distribution of hCRM1 in the Tg rats is similar to that observed in humans (28, 37). Therefore, use of the hCRM1 BAC construct may have resulted in physiological expression of the protein in Tg rats. We also demonstrated hCRM1 expression in all Tg rat-derived cell lines, which will be useful for the functional analysis of hCRM1 in HTLV-1-infected cells.
We have previously reported that expression of hCRM1 induced an increase in HTLV-1 Gag production in both rat epithelial and T cells (21, 69). Our present study also showed that T-cell lines established from hCRM1-Tg rats produced significantly greater amounts of p19 than cell lines established from Wt rats, further indicating the positive effect of hCRM1 on viral protein synthesis. This effect was not due to the effects of Tax or Rex proteins, which enhance the transcription of total viral mRNAs and the nuclear export of unspliced and incompletely spliced mRNAs, respectively (12, 26, 30, 68), since the expression levels of these proteins were not significantly different in the Tg and Wt cell lines. Additionally, these results indicate that induction of hCRM1 expression does not affect the expression of HTLV-1 regulatory proteins in virus-infected rat cells. We also observed differences in the levels of p19 production among the cell lines derived from hCRM1-Tg rats. Since the amount of p19 did not correlate with the expression level of hCRM1, Tax, or Rex, the reason for the differences is not clear. Some other factors, including RanGTP and RanBP3, which play important roles in the nuclear export of CRM1-substrate complexes (14, 41, 47, 59), may affect the levels of p19 production in the rat cell lines. It is also possible that the integration sites of the provirus influence virus production. Further studies are required to identify the factors that result in different p19 production among Tg rat-derived cell lines.
Differences in the expression of cell surface proteins were also observed among the cell lines established (Table 1). It is especially interesting that most of the Wt rat-derived cells do not express CD3 or CD4, whereas the majority of the Tg rat-derived lines possess both of these molecules. Since we and others have established a number of CD4-positive cell lines from various strains of Wt rats (31, 36), the present results may be due to experimental disparities. However, it is possible that enhanced HTLV-1 production by the hCRM1-expressing cells and subsequent dissemination of the virus in the culture may influence the phenotypes of the transformed cells. Thus, additional studies are required to determine the significance and cause of the difference.
The Tg rats showed minimal effects on HTLV-1 replication in vivo. Since dramatic enhancement of HTLV-1 production was observed in all hCRM1-expressing cells in vitro, it is possible that the number of HTLV-1-infected cells in vivo was too low to detect differences in virus production between Wt and Tg rats. From this point of view, alteration of experimental conditions to improve the initial HTLV-1 infection rate may lead to enhanced viral replication in Tg rats. Repression of viral protein expression in vivo may also reduce the effects of hCRM1, masking the enhanced viral replication in Tg rats. Such responses have been well documented for HTLV-1-infected individuals (32, 33). It is also possible that HTLV-1-specific immune responses could affect the replication of HTLV-1 in the Tg rats. Indeed. Our preliminary experiments indicated that induction of HTLV-1-specific cytotoxic T-lymphocyte responses occurred as early as 1 week after virus infection. Alternatively, some other host factors may govern and modulate efficient HTLV-1 replication in vivo. Thus, further studies on both virological and immunological aspects are required to verify the importance of the Tg rats as an in vivo model of HTLV-1 infection.
The HTLV-1 Rex protein is able to functionally replace the Rev protein of HIV type 1 (HIV-1) (57). CRM1 is a nuclear export factor for HIV-1 Rev, and a truncated Rev mutant with weakened binding affinity to CRM1 results in reduced levels of HIV-1 Gag production (20). These results raise the possibility that rat cells expressing hCRM1 protein can produce enhanced levels of HIV-1 structural proteins. Indeed, our preliminary results demonstrate that hCRM1 promotes HIV-1 p24gag production in rat cells (unpublished data). Thus, the hCRM1-Tg rats generated in this study may also be useful as a small-animal model of HIV-1 infection, when HIV-1 receptors are simultaneously expressed in these rats.
HIV latently infects reservoirs of resting T cells (7, 9, 10, 13, 61), which are thought to be in the G0 state, and the virus is then reactivated during T-cell activation. Alternatively, HIV has also been reported to propagate efficiently in nonreplicating lymphatic T cells (18), which lack certain markers specific for activation. Since cytokine levels are high in lymphatic tissues, the progression of T cells from G0 to G1 may support HIV replication. Although release from the cell cycle block has been extensively investigated at the transcriptional level, a recent study has shown that the synthesis of unspliced HIV Gag RNA increases rapidly during the HIV reactivation process, to a much greater extent than the synthesis of multiply spliced RNAs (7). Our results demonstrating a rapid increase in CRM1 expression during lymphocyte activation provide a clue to the underlying mechanism, the efficient action of the HIV Rev protein, which leads to robust synthesis of unspliced RNA. We suggest that HIV gene expression is regulated in lymphocytes at both the transcriptional and RNA export levels.
Independently of viral replication, the first phase of enhancement of CRM1 expression is also coincident with the induction of cytokines, such as IL-2 (4). CRM1 interacts with the AU-rich element (ARE) located in the 3′ untranslated region of c-fos mRNA (via HuR and its ligands) and mediates export of this mRNA from the nucleus to the cytoplasm (6, 16). Therefore, CRM1 may transport cytokine mRNAs belonging to the early response genes, since many cytokine mRNAs harbor ARE sequences (24, 56). Collectively, these observations suggest that enhancement of mRNA export via the induction of CRM1 expression, in addition to regulation at the transcriptional and translational levels, may play an important role in coordinating gene expression during lymphocyte activation. The existence of a posttranscriptional mechanism leading to a rapid increase in CRM1 protein levels is consistent with this hypothesis.
In conclusion, we have established a novel Tg rat carrying the hCRM1 gene via examination of the expression of this gene, and we have isolated several HTLV-1-infected T-cell lines expressing hCRM1. Our results demonstrate that T cells from hCRM1-Tg rats produced enhanced levels of the HTLV-1 Gag protein compared to T cells from Wt control rats. These results indicate the essential role of hCRM1 in proper HTLV-1 replication and suggest the importance of this Tg rat in the development of HTLV-1 animal models. These animals may also contribute to the development of models for other human retroviruses, such as HIV-1.
Acknowledgments
Buffy coats for the isolation of lymphocytes were the kind gift of the Hokkaido Red Cross Blood Center (Sapporo, Japan). We thank A. Hirano, N. Mizuno, K. Nakajima, and J. Hioki for excellent technical assistance.
This study was supported by grants from the Ministry of Sports and Culture (Japan) and the Ministry of Health and Welfare (Japan).
Footnotes
Published ahead of print on 14 March 2007.
REFERENCES
- 1.Adachi, Y., and M. Yanagida. 1989. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene CRM1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. J. Cell Biol. 108:1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi, T., I. Takeda, T. Oka, Y. Ohtsuki, S. Yano, and I. Miyoshi. 1985. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn. J. Cancer Res. 76:86-94. [PubMed] [Google Scholar]
- 3.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 4.Ashwell, J. D., and R. D. Klusner. 1990. Genetic and mutational analysis of the T-cell antigen receptor. Annu. Rev. Immunol. 8:139-167. [DOI] [PubMed] [Google Scholar]
- 5.Bogerd, H. P., R. A. Fridell, R. E. Benson, J. Hua, and B. R. Cullen. 1996. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol. Cell. Biol. 16:4207-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan, C. M., I. E. Gallouzi, and J. A. Steitz. 2000. Protein ligands to HuR modulate its interaction with target mRNAs in vivo. J. Cell Biol. 151:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brooks, D. G., S. G. Kitchen, C. M. Kitchen, D. D. Scripture-Adams, and J. A. Zack. 2001. Generation of HIV latency during thymopoiesis. Nat. Med. 7:459-464. [DOI] [PubMed] [Google Scholar]
- 8.Callanan, M., N. Kudo, S. Gout, M. Brocard, M. Yoshida, S. Dimitrov, and S. Khochbin. 2000. Developmentally regulated activity of CRM1/XPO1 during early Xenopus embryogenesis. J. Cell Sci. 113:451-459. [DOI] [PubMed] [Google Scholar]
- 9.Chun, T. W., D. Engel, M. M. Berrey, T. Shea, L. Corey, and A. S. Fauci. 1998. Early establishment of a pool of latently infected, resting CD4+ T cells during primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 95:8869-8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daenke, S., S. Nightingale, J. K. Cruickshank, and C. R. Bangham. 1990. Sequence variants of human T-cell lymphotropic virus type I from patients with tropical spastic paraparesis and adult T-cell leukemia do not distinguish neurological from leukemic isolates. J. Virol. 64:1278-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang, J., S. Kushida, R. Feng, M. Tanaka, T. Kawamura, H. Abe, N. Maeda, M. Onobori, M. Hori, K. Uchida, and M. Miwa. 1998. Transmission of human T-cell leukemia virus type 1 to mice. J. Virol. 72:3952-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 14.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 15.Fornerod, M., J. van Deursen, S. van Baal, A. Reynolds, D. Davis, K. G. Murti, J. Fransen, and G. Grosveld. 1997. The human homologue of yeast CRM1 is in a dynamic subcomplex with CAN/Nup214 and a novel nuclear pore component Nup88. EMBO J. 16:807-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallouzi, I. E., and J. A. Steitz. 2001. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science 294:1895-1901. [DOI] [PubMed] [Google Scholar]
- 17.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 18.Haase, A. T., K. Henry, M. Zupancic, G. Sedgewick, R. A. Faust, H. Melroe, W. Cavert, K. Gebhard, K. Staskus, Z. Q. Zhang, P. J. Dailey, H. H. Balfour, Jr., A. Erice, and A. S. Perelson. 1996. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science 274:985-989. [DOI] [PubMed] [Google Scholar]
- 19.Hakata, Y., T. Umemoto, S. Matsushita, and H. Shida. 1998. Involvement of human CRM1 (exportin 1) in the export and multimerization of the Rex protein of human T-cell leukemia virus type 1. J. Virol. 72:6602-6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hakata, Y., M. Yamada, N. Mabuchi, and H. Shida. 2002. The carboxy-terminal region of the human immunodeficiency virus type 1 protein Rev has multiple roles in mediating CRM1-related Rev functions. J. Virol. 76:8079-8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hakata, Y., M. Yamada, and H. Shida. 2003. A multifunctional domain in human CRM1 (exportin 1) mediates RanBP3 binding and multimerization of human T-cell leukemia virus type 1 Rex protein. Mol. Cell. Biol. 23:8751-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hakata, Y., M. Yamada, and H. Shida. 2001. Rat CRM1 is responsible for the poor activity of human T-cell leukemia virus type 1 Rex protein in rat cells. J. Virol. 75:11515-11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall, W. W., C. R. Liu, O. Schneewind, H. Takahashi, M. H. Kaplan, G. Roupe, and A. Vahlne. 1991. Deleted HTLV-I provirus in blood and cutaneous lesions of patients with mycosis fungoides. Science 253:317-320. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton, T. A., Y. Ohmori, and J. Tebo. 2002. Regulation of chemokine expression by antiinflammatory cytokines. Immunol. Res. 25:229-245. [DOI] [PubMed] [Google Scholar]
- 25.Hanabuchi, S., T. Ohashi, Y. Koya, H. Kato, A. Hasegawa, F. Takemura, T. Masuda, and M. Kannagi. 2001. Regression of human T-cell leukemia virus type I (HTLV-I)-associated lymphomas in a rat model: peptide-induced T-cell immunity. J. Natl. Cancer Inst. 93:1775-1783. [DOI] [PubMed] [Google Scholar]
- 26.Hidaka, M., J. Inoue, M. Yoshida, and M. Seiki. 1988. Post-transcriptional regulator (rex) of HTLV-1 initiates expression of viral structural proteins but suppresses expression of regulatory proteins. EMBO J. 7:519-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hinuma, Y., K. Nagata, M. Hanaoka, M. Nakai, T. Matsumoto, K. I. Kinoshita, S. Shirakawa, and I. Miyoshi. 1981. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc. Natl. Acad. Sci. USA 78:6476-6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holaska, J. M., and B. M. Paschal. 1998. A cytosolic activity distinct from CRM1 mediates nuclear export of protein kinase inhibitor in permeabilized cells. Proc. Natl. Acad. Sci. USA 95:14739-14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshino, H., H. Tanaka, K. Shimotohno, M. Miwa, M. Nagai, M. Shimoyama, and T. Sugimura. 1984. Immortalization of peripheral blood lymphocytes of cats by human T-cell leukemia virus. Int. J. Cancer 34:513-517. [DOI] [PubMed] [Google Scholar]
- 30.Inoue, J., M. Yoshida, and M. Seiki. 1987. Transcriptional (p40x) and post-transcriptional (p27x-III) regulators are required for the expression and replication of human T-cell leukemia virus type I genes. Proc. Natl. Acad. Sci. USA 84:3653-3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishiguro, N., M. Abe, K. Seto, H. Sakurai, H. Ikeda, A. Wakisaka, T. Togashi, M. Tateno, and T. Yoshiki. 1992. A rat model of human T lymphocyte virus type I (HTLV-I) infection. 1. Humoral antibody response, provirus integration, and HTLV-I-associated myelopathy/tropical spastic paraparesis-like myelopathy in seronegative HTLV-I carrier rats. J. Exp. Med. 176:981-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannagi, M., S. Matsushita, and S. Harada. 1993. Expression of the target antigen for cytotoxic T lymphocytes on adult T-cell-leukemia cells. Int. J. Cancer 54:582-588. [DOI] [PubMed] [Google Scholar]
- 33.Kannagi, M., K. Sugamura, K. Kinoshita, H. Uchino, and Y. Hinuma. 1984. Specific cytolysis of fresh tumor cells by an autologous killer T cell line derived from an adult T cell leukemia/lymphoma patient. J. Immunol. 133:1037-1041. [PubMed] [Google Scholar]
- 34.Kim, F. J., A. A. Beeche, J. J. Hunter, D. J. Chin, and T. J. Hope. 1996. Characterization of the nuclear export signal of human T-cell lymphotropic virus type 1 Rex reveals that nuclear export is mediated by position-variable hydrophobic interactions. Mol. Cell. Biol. 16:5147-5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kinoshita, T., A. Tsujimoto, and K. Shimotohno. 1991. Sequence variations in LTR and env regions of HTLV-I do not discriminate between the virus from patients with HTLV-I-associated myelopathy and adult T-cell leukemia. Int. J. Cancer 47:491-495. [DOI] [PubMed] [Google Scholar]
- 36.Koya, Y., T. Ohashi, H. Kato, S. Hanabuchi, T. Tsukahara, F. Takemura, K. Etoh, M. Matsuoka, M. Fujii, and M. Kannagi. 1999. Establishment of a seronegative human T-cell leukemia virus type 1 (HTLV-1) carrier state in rats inoculated with a syngeneic HTLV-1-immortalized T-cell line preferentially expressing Tax. J. Virol. 73:6436-6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kudo, N., S. Khochbin, K. Nishi, K. Kitano, M. Yanagida, M. Yoshida, and S. Horinouchi. 1997. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J. Biol. Chem. 272:29742-29751. [DOI] [PubMed] [Google Scholar]
- 38.Kurihara, K., N. Harashima, S. Hanabuchi, M. Masuda, A. Utsunomiya, R. Tanosaki, M. Tomonaga, T. Ohashi, A. Hasegawa, T. Masuda, J. Okamura, Y. Tanaka, and M. Kannagi. 2005. Potential immunogenicity of adult T cell leukemia cells in vivo. Int. J. Cancer 114:257-267. [DOI] [PubMed] [Google Scholar]
- 39.Kushida, S., H. Mizusawa, M. Matsumura, H. Tanaka, Y. Ami, M. Hori, K. Yagami, T. Kameyama, Y. Tanaka, A. Yoshida, H. Nyunoya, K. Shimotohno, Y. Iwasaki, K. Uchida, and M. Miwa. 1994. High incidence of HAM/TSP-like symptoms in WKA rats after administration of human T-cell leukemia virus type 1-producing cells. J. Virol. 68:7221-7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaGrenade, L., B. Hanchard, V. Fletcher, B. Cranston, and W. Blattner. 1990. Infective dermatitis of Jamaican children: a marker for HTLV-I infection. Lancet 336:1345-1347. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay, M. E., J. M. Holaska, K. Welch, B. M. Paschal, and I. G. Macara. 2001. Ran-binding protein 3 is a cofactor for CRM1-mediated nuclear protein export. J. Cell Biol. 153:1391-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann, D. L., P. DeSantis, G. Mark, A. Pfeifer, M. Newman, N. Gibbs, M. Popovic, M. G. Sarngadharan, R. C. Gallo, J. Clark, and W. Blattner. 1987. HTLV-I-associated B-cell CLL: indirect role for retrovirus in leukemogenesis. Science 236:1103-1106. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Martinez, S., P. Gomez del Arco, A. L. Armesilla, J. Aramburu, C. Luo, A. Rao, and J. M. Redondo. 1997. Blockade of T-cell activation by dithiocarbamates involves novel mechanisms of inhibition of nuclear factor of activated T cells. Mol. Cell. Biol. 17:6437-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura, H., M. Hayami, Y. Ohta, K. Ishikawa, H. Tsujimoto, T. Kiyokawa, M. Yoshida, A. Sasagawa, and S. Honjo. 1987. Protection of cynomolgus monkeys against infection by human T-cell leukemia virus type-I by immunization with viral env gene products produced in Escherichia coli. Int. J. Cancer 40:403-407. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura, H., Y. Tanaka, A. Komuro-Tsujimoto, K. Ishikawa, K. Takadaya, H. Tozawa, H. Tsujimoto, S. Honjo, and M. Hayami. 1986. Experimental inoculation of monkeys with autologous lymphoid cell lines immortalized by and producing human T-cell leukemia virus type-I. Int. J. Cancer 38:867-875. [DOI] [PubMed] [Google Scholar]
- 47.Nemergut, M. E., M. E. Lindsay, A. M. Brownawell, and I. G. Macara. 2002. Ran-binding protein 3 links CRM1 to the Ran guanine nucleotide exchange factor. J. Biol. Chem. 277:17385-17388. [DOI] [PubMed] [Google Scholar]
- 48.Nishioka, K., I. Maruyama, K. Sato, I. Kitajima, Y. Nakajima, and M. Osame. 1989. Chronic inflammatory arthropathy associated with HTLV-I. Lancet i:441. [DOI] [PubMed] [Google Scholar]
- 49.Nishizuka, Y. 1984. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693-698. [DOI] [PubMed] [Google Scholar]
- 50.Nomura, M., T. Ohashi, K. Nishikawa, H. Nishitsuji, K. Kurihara, A. Hasegawa, R. A. Furuta, J. Fujisawa, Y. Tanaka, S. Hanabuchi, N. Harashima, T. Masuda, and M. Kannagi. 2004. Repression of Tax expression is associated both with resistance of human T-cell leukemia virus type 1-infected T cells to killing by Tax-specific cytotoxic T lymphocytes and with impaired tumorigenicity in a rat model. J. Virol. 78:3827-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi, T., S. Hanabuchi, H. Kato, Y. Koya, F. Takemura, K. Hirokawa, T. Yoshiki, Y. Tanaka, M. Fujii, and M. Kannagi. 1999. Induction of adult T-cell leukemia-like lymphoproliferative disease and its inhibition by adoptive immunotherapy in T-cell-deficient nude rats inoculated with syngeneic human T-cell leukemia virus type 1-immortalized cells. J. Virol. 73:6031-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohashi, T., S. Hanabuchi, H. Kato, H. Tateno, F. Takemura, T. Tsukahara, Y. Koya, A. Hasegawa, T. Masuda, and M. Kannagi. 2000. Prevention of adult T-cell leukemia-like lymphoproliferative disease in rats by adoptively transferred T cells from a donor immunized with human T-cell leukemia virus type 1 Tax-coding DNA vaccine. J. Virol. 74:9610-9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oka, T., H. Sonobe, J. Iwata, I. Kubonishi, H. Satoh, M. Takata, Y. Tanaka, M. Tateno, H. Tozawa, S. Mori, T. Yoshiki, and Y. Ohtsuki. 1992. Phenotypic progression of a rat lymphoid cell line immortalized by human T-lymphotropic virus type I to induce lymphoma/leukemia-like disease in rats. J. Virol. 66:6686-6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 55.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raghavan, A., R. L. Robison, J. McNabb, C. R. Miller, D. A. Williams, and P. R. Bohjanen. 2001. HuA and tristetraprolin are induced following T cell activation and display distinct but overlapping RNA binding specificities. J. Biol. Chem. 276:47958-47965. [DOI] [PubMed] [Google Scholar]
- 57.Rimsky, L., J. Hauber, M. Dukovich, M. H. Malim, A. Langlois, B. R. Cullen, and W. C. Greene. 1988. Functional replacement of the HIV-1 rev protein by the HTLV-1 rex protein. Nature 335:738-740. [DOI] [PubMed] [Google Scholar]
- 58.Simpson, R. M., T. M. Zhao, B. S. Hubbard, S. Sawasdikosol, and T. J. Kindt. 1996. Experimental acute adult T cell leukemia-lymphoma is associated with thymic atrophy in human T cell leukemia virus type I infection. Lab. Investig. 74:696-710. [PubMed] [Google Scholar]
- 59.Stade, K., C. S. Ford, C. Guthrie, and K. Weis. 1997. Exportin 1 (CRM1p) is an essential nuclear export factor. Cell 90:1041-1050. [DOI] [PubMed] [Google Scholar]
- 60.Stein, G. M., U. Pfuller, M. Schietzel, and A. Bussing. 2000. Expression of interleukin-4 in apoptotic cells: stimulation of the type-2 cytokine by different toxins in human peripheral blood mononuclear and tumor cells. Cytometry 41:261-270. [DOI] [PubMed] [Google Scholar]
- 61.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taguchi, H., T. Sawada, A. Fukushima, J. Iwata, Y. Ohtsuki, H. Ueno, and I. Miyoshi. 1993. Bilateral uveitis in a rabbit experimentally infected with human T-lymphotropic virus type I. Lab. Investig. 69:336-339. [PubMed] [Google Scholar]
- 63.Tanaka, Y., A. Yoshida, H. Tozawa, H. Shida, H. Nyunoya, and K. Shimotohno. 1991. Production of a recombinant human T-cell leukemia virus type-I trans-activator (tax1) antigen and its utilization for generation of monoclonal antibodies against various epitopes on the tax1 antigen. Int. J. Cancer 48:623-630. [DOI] [PubMed] [Google Scholar]
- 64.Tateno, M., N. Kondo, T. Itoh, T. Chubachi, T. Togashi, and T. Yoshiki. 1984. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J. Exp. Med. 159:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, G., R. Miskimins, and W. K. Miskimins. 2000. Mimosine arrests cells in G1 by enhancing the levels of p27 (Kip1). Exp. Cell Res. 254:64-71. [DOI] [PubMed] [Google Scholar]
- 66.Werlen, G., E. Jacinto, Y. Xia, and M. Karin. 1998. Calcineurin preferentially synergizes with PKC-θ to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 17:3101-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshida, M., M. Osame, K. Usuku, M. Matsumoto, and A. Igata. 1987. Viruses detected in HTLV-I-associated myelopathy and adult T-cell leukaemia are identical on DNA blotting. Lancet i:1085-1086. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida, M., T. Suzuki, J. Fujisawa, and H. Hirai. 1995. HTLV-1 oncoprotein tax and cellular transcription factors. Curr. Top. Microbiol. Immunol. 193:79-89. [DOI] [PubMed] [Google Scholar]
- 69.Zhang, X., Y. Hakata, Y. Tanaka, and H. Shida. 2006. CRM1, an RNA transporter, is a major species-specific restriction factor of human T cell leukemia virus type 1 (HTLV-1) in rat cells. Microbes Infect. 8:851-859. [DOI] [PubMed] [Google Scholar]