Abstract
Borna disease virus (BDV) is a neurotropic virus that causes a persistent infection in the central nervous system (CNS) of many vertebrate species. Although a severe reactive gliosis is observed in experimentally BDV-infected rat brains, little is known about the glial reactions contributing to the viral persistence and immune modulation in the CNS. In this regard, we examined the expression of an astrocyte-derived factor, S100B, in the brains of Lewis rats persistently infected with BDV. S100B is a Ca2+-binding protein produced mainly by astrocytes. A prominent role of this protein appears to be the promotion of vascular inflammatory responses through interaction with the receptor for advanced glycation end products (RAGE). Here we show that the expression of S100B is significantly reduced in BDV-infected brains despite severe astrocytosis with increased glial fibrillary acidic protein immunoreactivity. Interestingly, no upregulation of the expression of S100B, or RAGE, was observed in the persistently infected brains even when incited with several inflammatory stimuli, including lipopolysaccharide. In addition, expression of the vascular cell adhesion molecule 1 (VCAM-1), as well as the infiltration of encephalitogenic T cells, was significantly reduced in persistently infected brains in which an experimental autoimmune encephalomyelitis was induced by immunization with myelin-basic protein. Furthermore, we demonstrated that the continuous activation of S100B in the brain may be necessary for the progression of vascular immune responses in neonatally infected rat brains. Our results suggested that BDV infection may impair astrocyte functions via a downregulation of S100B expression, leading to the maintenance of a persistent infection.
Astrocytes are activated in response to damage to the central nervous system (CNS) caused by ischemia, trauma, neurodegenerative disorders, autoimmunity, and infectious diseases (35). The process by which reactive astrocytes are recruited to the injured CNS remains rather obscure, but astrocytosis is postulated to play an important role in the maintenance of homeostasis in the CNS (5). Reactive astrocytes show higher levels of adhesion molecules and also increase the production of a variety of cytokines, chemokines, growth factors, and neuropeptides (35), consequently eliciting a brain inflammatory response. Although the modulation of CNS-based immune responses followed by astrocytosis seems to be engaged in negative effects on the injured brain, reactive astrocytes are nevertheless essential for the repair of damage to immune-privileged organs attacked by pathogens (5, 7, 35).
Infections by neurotropic viruses generally reactivate glial cells in infected brains. Borna disease virus (BDV) is a highly neurotropic virus that causes severe neurological disorders in many vertebrate species (18, 38). Like many pathogens targeting the CNS, BDV strongly induces glial reactivation in the brains of experimentally infected animals (16, 39, 50). BDV characteristically establishes a persistent infection without any cytopathic effects in brain cells (8, 12, 14, 39), and so studies of this virus provide a good understanding of the modulation of the brain immune response during the persistence of CNS pathologies. Numerous studies have demonstrated that persistent infections of BDV induce a chronic astrocytosis, as well as a stable upregulation of the expression of proinflammatory cytokines, such as interleukin 1-beta (IL-1β) and tumor necrosis factor alpha (TNF-α), in the brain (16, 31, 39). Despite such lasting inflammatory responses, this virus efficiently maintains the infection and has a life-long survival in the CNS. At present, modulation of the immune responses, such as Th1-specific T-cell tolerance, has been proposed as the mechanism for the maintenance of BDV persistence in mice (8, 12, 14). Although reactive astrocytes appear to be involved in the homeostatic preservation of infected brains, little is known about the glial reactions contributing to the persistence of CNS pathologies or to the regulation of the inflammatory responses in damaged brains.
In this study, we examined the expression of an astrocyte-derived factor, S100B, in rats persistently infected with BDV to understand the glial reactions occurring during a chronic viral infection. S100B is an EF-hand Ca2+-binding protein produced mainly by astrocytes that exerts autocrine and paracrine effects on neural cells, stimulating cellular survival, proliferation, and differentiation (1, 3, 6, 32, 40). A prominent role of this protein appears to be the promotion of inflammatory responses, as a cytokine, through binding to its cellular surface receptor, the receptor for advanced glycation end products (RAGE) (36, 37, 51). Here we demonstrate that the expression of S100B is significantly reduced in persistently infected brains despite severe astrocytosis with increased glial fibrillary acidic protein (GFAP) immunoreactivity. Interestingly, no upregulation of the expression of S100B, or RAGE, was found in the brains of persistently infected rats exposed to a bacterial lipopolysaccharide (LPS) and immunized with myelin-basic protein (MBP), suggesting the possibility of a constitutive downmodulation of S100B in the persistently infected brains. Furthermore, expression of the vascular cell adhesion molecule 1 (VCAM-1) in the vascular endothelium and the subsequent vasodilatation were downregulated in the infected rat brains sensitized with MBP. We also showed that S100B signaling may be necessary for the development of mononuclear cell infiltrates via the activation of vascular immune responses in neonatally infected rat brains. Our findings may provide a novel mechanism by which chronic viral infections abrogate vascular inflammatory responses through the downregulation of an astrocyte-derived cytokine, S100B, leading to a persistent infection.
MATERIALS AND METHODS
Preparation and inoculation of the virus.
A viral stock was prepared from the homogenate of rat brains infected with BDV strain huP2br (25), and the titer of the BDV source was measured as described previously (49). Briefly, semiconfluent monolayers of C6 cells were inoculated with serial 10-fold dilutions of the cell-free BDV stock. Three days after the inoculation, the cells were fixed with 4% paraformaldehyde and subjected to immunofluorescence staining. The viral titer was calculated as focus-forming units (FFU) per milliliter of cell-free BDV stock.
For viral infection, Lewis rats (SLC, Shizuoka, Japan) within 24 h after birth (neonate) and at 4 weeks old (adult) were intracranially inoculated in the left-brain hemisphere with 2,000 and 20,000 FFU of BDV stock per animal, respectively.
Administration of LPS.
BDV-infected and uninfected rats were injected intraperitoneally with 200 or 500 μg/kg of LPS (Escherichia coli strain O111:B4; Sigma-Aldrich, St. Louis, MO) at 5 weeks postinfection (p.i.). The rats were sacrificed at 24, 48, and 72 h after the injection. As age-matched controls, animals were injected with phosphate-buffered saline (PBS) and sacrificed at 72 h after the administration.
Induction and clinical evaluation of experimental autoimmune encephalomyelitis.
Experimental autoimmune encephalomyelitis (EAE) was induced in at least seven Lewis rats in each BDV-infected and uninfected group by subcutaneously injecting 100 μg of MBP (Sigma-Aldrich) in the lateral left upper leg. The injected emulsion contained 2 mg of MBP in 1 ml of PBS mixed with 1 ml of complete Freund's adjuvant (Wako Junyaku, Osaka, Japan). For immunohistochemical (IHC) analysis, animals were sacrificed at 14 days after the sensitization, at which time the clinical symptoms of EAE reached a peak in the uninfected controls. Animals were scored daily for clinical signs of disease on a severity scale ranging from 0 to 6 (where 0, normal; 1, limp tail; 2, hind limb weakness; 3, unilateral hind limb paralysis; 4, bilateral hind limb paralysis; 5, bilateral hind limb paralysis and incontinence; and 6, moribund). The results are presented as the mean daily clinical score of each experimental group.
Inoculation of recombinant BDV nucleoprotein antigen.
The recombinant BDV nucleoprotein (N) protein was produced as reported previously (48). Briefly, the cDNA encoding BDV N was inserted into the plasmid pGEX, and the recombinant protein was expressed in E. coli as a fusion construct with glutathione S-transferase (GST) and then purified by using glutathione Sepharose 4B. The purified protein was cleaved by factor Xa to remove the GST, and the endotoxin in the protein solution was removed by a polymyxin B-coupled matrix (Bio-Rad Laboratories Inc., Hercules, CA). The recombinant N (rN) was analyzed using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For sensitization, 50 μg of BDV rN was injected into rats at 3 weeks after the inoculation of BDV.
Inoculation with the soluble form of RAGE.
To block S100B signaling, a soluble form of RAGE (RAGE-Fc; R&D Systems Inc., Minneapolis, MN), which is a recombinant, truncated form of rat RAGE spanning the extracellular domain and serving as a decoy (34), was employed. Rats received 100 μg/kg of RAGE-Fc at 2-day intervals by intraperitoneal (i.p.) injection, starting from the day of immunization with the rN.
Immunoblotting analysis.
Brain extracts of Lewis rats were prepared by sonication in lysis buffer (1% NP-40, 50 mM Tris-HCl [pH 7.5], 1 mM EDTA, 100 mM NaCl) with a protease inhibitor cocktail (Nacalai Tesque, Inc., Kyoto, Japan). The aliquots were resolved in SDS-PAGE sample buffer. Equal amounts of total protein were subjected to 12% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked with 5% skimmed milk in PBS-0.1% Tween 20. The membranes were reacted with antibody against RAGE (1:100; Santa Cruz Biotechnology Inc., Santa Cruz, CA), HMGB1 (20) (1:1,000), tubulin (1:4,000; Sigma-Aldrich) at room temperature, or S100B (1:2,000; BD Bioscience, San Jose, CA) at 4°C. After being washed, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h at 37°C. Reacted proteins on the membrane were then visualized using an ECL system Western blotting kit (Amersham Pharmacia Biotech, Uppsala, Sweden). The intensity of each band was quantified using NIH image software.
Histological and immunohistochemical analyses.
Rat brains were fixed in 4% paraformaldehyde in PBS and embedded in paraffin. Deparaffinized sections (5 μm) were stained with hematoxylin and eosin (H&E). For IHC analysis, thin sections (4 μm) were incubated with a trypsin solution (0.1% trypsin, 0.1% CaCl2, and 0.05 M Tris-HCl [pH 7.6]) for 15 min at 37°C. Endogenous peroxide was quenched with 0.5% HIO4. After a blocking step, the sections were incubated with anti-GFAP mouse antibody (1:1,000; Lab Vision, Fremont, CA), anti-GFAP rabbit antibody (1:1,000; Chemicon, Temecula, CA), anti-rat CD68 (ED-1) (1:100; Serotec Ltd., Kidlington, Oxford, United Kingdom), anti-CD4 (1:100; Serotec Ltd.), anti-VCAM-1 (1:200; BD Bioscience), anti-RAGE (1:1,000) and/or anti-S100B (1:2,000; BD Bioscience) antibodies. After several washes, primary antibodies were detected by incubation with a biotinylated goat anti-biotin peroxidase complex (1:200; Vector, Burlingame, CA). Thereafter, the sections were incubated with the avidin-biotin peroxidase complex (1:125; Vector). Specific reactions were visualized with 3′,3′-diaminobenzidine-tetrahydrochloride (DAB). For immunofluorescence analysis, fluorescein-conjugated streptavidin (Vector) was used instead of an ABC kit. Alexa Fluor 555-conjugated secondary antibodies (Invitrogen, San Diego, CA) and 4′,6′-diamidino-2-phenylindole (DAPI) were used for counterstaining. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) was performed using an in situ cell death detection kit (Roche Molecular Diagnostics, Pleasanton, CA). For IHC analysis, polyclonal antibodies to human RAGE were generated by immunization of a rabbit with recombinant RAGE peptide (amino acids 102 to 346) expressed by E. coli.
Quantification of VCAM-1 expression in rat brains.
For a quantitative analysis of VCAM-1 levels in the brain, an image of the area of interest was captured using a Nikon E600 microscope and charge-coupled device camera (Hamamatsu Photonics Inc., Hamamatsu, Japan) under the same optical and lighting conditions. The average of the optical densities of positive signals was measured in four different fields of the cerebellum in each section for four animals by using Photoshop and NIH image software. Using the software, the pixel intensity of DAB staining was extracted above the threshold to determine the optical density.
Semiquantitative reverse transcription-PCR for chemokine expression.
Total RNA was extracted from rat brain homogenates by using the RNA isolation reagent TRIzol (Invitrogen). First-strand cDNAs were synthesized from aliquots of 2 μg of total RNA by a Thermoscript reverse transcription (RT)-PCR system (Invitrogen). The PCR primers used were as follows: macrophage-inflammatory protein 1β-sense (MIP-1β-sense) (5′-ATG AAG CTC TGC GTG TCT GCC TTC-3′); MIP-1β-antisense (5′-TCA GTT CAA CTC CAA GTC ATT CAC-3′); monocytes chemoattractant protein 1-sense (MCP-1-sense) (5′-ATG CAG GTC TCT GTC ACG CTT CTG GGC-3′); MCP-1-antisense (5′-CTA GTT CTC TGT CAT ACT GGT CAC-3′); glyceraldehyde-3-phosphate dehydrogenase sense (GAPDH-sense) (5′-ACC ACA GTC CAT GCC ATC AC-3′); and GAPDH-antisense (5′-TCC ACC ACC CTG TTG CTG TA-3′). PCR was performed in a total volume of 25 μl containing 1 μl of cDNA and 2 U of Taq polymerase (TaKaRa ExTaq; Takara Bio Inc., Shiga, Japan). The PCR was performed at 94°C for 30 s, at the annealing temperature for 30s, and at 72°C for 30s. The optimal number of amplification cycles and annealing temperature were changed for each primer.
Quantification of proinflammatory cytokines in rat brain.
Estimations of the expression levels of the proinflammatory cytokines IL-1β and TNF-α in BDV-infected rat brains were performed by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Inc.), following the manufacturer's directions. Plates were analyzed at 492 nm, and levels of IL-1β and TNF-α were determined by comparison to a standard curve. The data were normalized to the amount of total protein used for the analysis.
Statistical analysis.
Data were expressed as means ± standard error of the means. Statistical analyses were performed using an unpaired Student's t test or one-way analysis of variance followed by post hoc Dunnett's test compared with the control group. An n of 4 was used for each treatment group in statistical analyses, except for the indication.
RESULTS
Reduced expression of S100B, but not GFAP, in persistently infected rat brains.
To understand the environment in the CNS during a persistent infection of BDV, neonatal BDV-infected (NBI) or 4-week-old adult BDV-infected (ABI) Lewis rats were sacrificed at 5 weeks p.i. As shown in numerous studies (21, 39), no significant immune cell infiltration was observed in NBI rats, whereas ABI rats showed a slight encephalitis with perivascular mononuclear cell infiltrates. Furthermore, a marked astrocytosis, as well as microgliosis, was observed in both NBI and ABI rat brains at 5 weeks p.i. and was present during the persistent stage of BDV infection in the rats (data not shown).
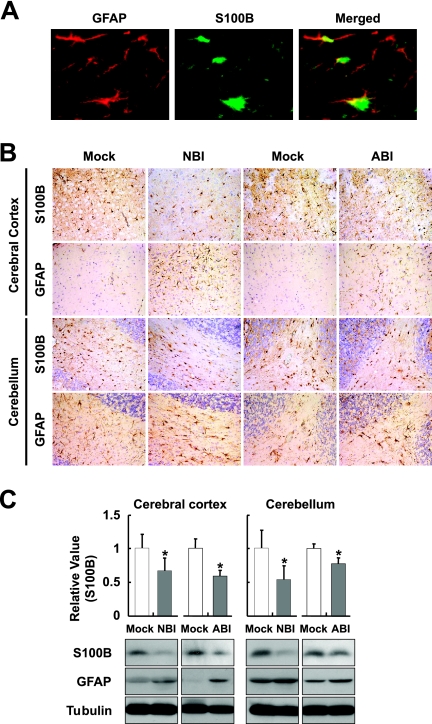
S100B is a Ca2+-binding protein produced mainly by astrocytes in the CNS (35, 37). The expression level of S100B is most likely to be correlated with brain injuries, as well as the degree of astrocytosis (37, 44, 51), suggesting that the expression level may be a biomarker for the activity of astrocytes in inflamed brains. We therefore monitored the expression of S100B in rat brains persistently infected with BDV, in addition to the expression of GFAP, which is also specific to astrocytes. The immunoreaction of S100B is found mainly in the GFAP-positive astrocytes in the rat brain (Fig. 1A). In both NBI and ABI rat brains, a marked increase of GFAP staining was demonstrated at 5 weeks p.i. (Fig. 1B). In contrast, immunoreactivity of S100B appeared to be weaker in the persistently infected brains than uninfected brains in both the cerebral cortex and cerebellum (Fig. 1B). We estimated GFAP and S100B levels by Western immunoblotting at 5 weeks p.i. As shown in Fig. 1C, although persistent infections induced an upregulation of GFAP expression in association with the astrocytosis, interestingly, the expression of S100B was significantly reduced at 5 weeks p.i. in both NBI and ABI rat brains.
FIG. 1.
Downregulation of S100B expression in BDV persistently infected brain. (A) S100B expression in BDV persistently infected Lewis rat brain. The cerebellum regions of NBI rats were stained with anti-GFAP (red) and anti-S100B (green) antibodies at 5 weeks p.i. The overlap in the distribution of GFAP and S100B is revealed in the merged image. The immunoreaction of S100B is found mainly in the GFAP-positive astrocytes. (B) Expressions of GFAP and S100B were detected in the cerebral cortex and cerebellum persistently infected with BDV. Brain sections from NBI and ABI rats at 5 weeks p.i. were immunostained with anti-GFAP and anti-S100B antibodies. Magnification, ×200. Mock, age-matched mock-infected rats. (C) Expression of S100B and GFAP proteins. The brain homogenates were obtained from the cerebral cortex and cerebellum regions of rat brains at 5 weeks p.i. and subjected to immunoblotting. The quantitative analysis of S100B expression is also shown. The band intensities were determined by NIH image. An n of 6 and n of 3 were used for the statistical analyses of NBI and ABI rats, respectively. Values were normalized to the tubulin level (*, P < 0.05 with mock-infected age-matched control rats).
No upregulation of S100B or RAGE expression in the brains of persistently infected rats administered LPS.
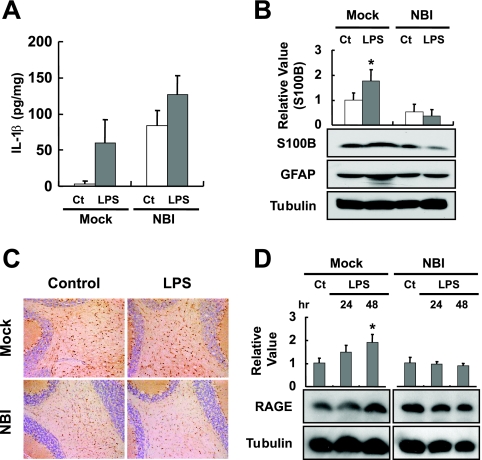
To understand whether the expression of S100B is constitutively reduced in the persistently infected brain, we intraperitoneally injected LPS into NBI rats at 5 weeks p.i., following previous studies demonstrating that the i.p. injection of LPS rapidly induces the release of proinflammatory cytokines, such as IL-1β and TNF-α, in the brain and subsequently upregulates the expression of S100B (19, 43). We used NBI rats in the subsequent experiments, since the expression of S100B represents an intense increase in the brains from the second postnatal week onward (45), suggesting that it could be difficult to detect the upregulated level of S100B by additional inflammatory stimuli in ABI rat brains. In addition, the inflammatory responses by the stimuli could be easy to detect in the NBI rat brains in which the background infiltrations developed by the initial infection are lacking. On the injection of LPS, the expression of IL-1β was induced in both infected and uninfected rat brains, although it was expressed more so in the NBI than in mock-infected rats (Fig. 2A). In contrast, the expression of S100B was slightly reduced in the cerebellum of NBI rats at 48 h after the injection of LPS, while it was significantly increased in the uninfected rats (Fig. 2B). To confirm the distinct reactivation of S100B in the brain, we performed an IHC analysis using antibodies against the glial antigen. Consistent with the immunoblotting results, no upregulation of S100B expression was observed in the cerebellum of LPS-injected NBI rats; rather, levels appeared to be slightly reduced in the infected brains (Fig. 2C).
FIG. 2.
LPS administration does not induce S100B expression in persistently infected brain. LPS was intraperitoneally administrated to BDV-infected rats at 5 weeks p.i., and the brains were collected 24 or 48 h after the injection. (A) Induction of IL-1β expression in LPS-injected bat brains. Amounts of IL-1β were measured with an ELISA kit. Ct, PBS-injected control rats; Mock, age-matched mock-infected rats. An n of 3 was used for the statistical analyses. (B) Expressions of S100B and GFAP were detected by immunoblotting. The brain homogenates were obtained from the cerebellum regions at 48 h after LPS injection. The quantitative analysis of S100B expression is also shown. The band intensities were determined by NIH image. Values were normalized to the tubulin level (*, P < 0.05 with PBS-injected control [Ct] rats). (C) Immunohistological analysis of S100B expression in the cerebellum regions of LPS- or PBS-treated (Control) rats. Magnification, ×200. Mock, age-matched mock-infected rats. (D) RAGE expression in LSP-injected animal brains. The quantitative analysis of RAGE expression is also shown. The band intensities were determined by NIH image. Values were normalized to the tubulin level. (*, P < 0.05 with PBS-injected control [Ct] rats).
That there was no upregulation of the expression of S100B in persistently infected brains suggested that the amount of this protein that is secreted may also be reduced in the CNS. To examine the secretion of S100B in the brain, we investigated the induction level of an S100B receptor, RAGE, in BDV-infected rat brains, because the expression of RAGE is upregulated by the direct interaction of S100B with RAGE (51). On the administration of LPS, the expression of RAGE was significantly upregulated in the cerebellum of uninfected rats by 48 h postinjection, whereas no expression of RAGE was induced in the persistently infected brains (Fig. 2D). It is worth noting that the expression of an alternative RAGE ligand, HMGB1, was upregulated in both infected and uninfected rat brains by LPS (data not shown), indicating that the unresponsiveness of RAGE expression is likely to be correlated with the decrease in the secretion of S100B in the infected brain.
Reduced stress resistance of brain cells in persistently infected NBI rats.
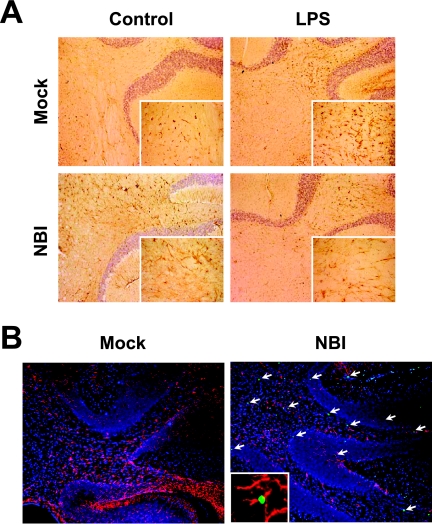
The downregulation of S100B and RAGE expression suggested that the functions of S100B may be impaired in the NBI brains. Thus, we examined the vulnerability of neural cells under stressful environment conditions because previous reports demonstrated that S100B expression prevents neural cell apoptosis at an appropriate concentration via interaction with RAGE (17). In this regard, we estimated apoptotic cell death in the LPS-injected NBI rat brains, which show a decrease in the level of both S100B and RAGE reactivities (Fig. 2). The administration of LPS is known to upregulate production of nitric oxide in the brain, showing that LPS contributes to the development of a stressful environment within the CNS (42). We observed the immunoreactivity of GFAP, as well as conducted TUNEL staining. As shown in Fig. 3A, the reaction level of GFAP seems to be slightly reduced by the injection of LPS in BDV-infected brains at 48 h after the injection, although uninfected rats exhibited significantly enhanced GFAP expression in the brain. The immunoblot data also verified the reduced expression of GFAP in the NBI cerebellum (see Fig. 2B), suggesting that the number of astrocytes may decrease in the infected brain on the injection of LPS. In addition, the largest numbers of TUNEL-positive cells were found in the LPS-injected NBI rats at 5 weeks p.i., whereas the uninfected rat brains rarely contained positive cells (Fig. 3B). The TUNEL-positive cell population in the NBI brains contained both GFAP-positive and -negative cells in the cerebellum (Fig. 3B). These results indicated that S100B signaling may be disturbed in the persistently infected NBI rat brains.
FIG. 3.
Induction of neural apoptosis in LPS-injected persistently infected rat brains. (A) Immunohistological analysis of GFAP expression in the cerebellum regions of LPS- or vehicle-treated (Control) rats. Brain sections were obtained at 48 h after the injection and stained with anti-GFAP antibody. Magnification, ×200 (insets magnification, ×1,000). (B) TUNEL staining of LPS-treated NBI and mock-infected rats. Cerebellar areas at 48 h postinjection are shown. Arrows indicate apoptotic cells (green). GFAP-positive cells are shown in red. Counterstaining was done with DAPI (blue) for nuclear staining. Magnification, ×100 (insets magnification, ×1,000). Mock, age-matched mock-infected rats. An apoptosis-induced GFAP-positive glial cell is shown in the inset.
Reduced vascular inflammatory responses in the brains of persistently infected rats immunized with MBP.
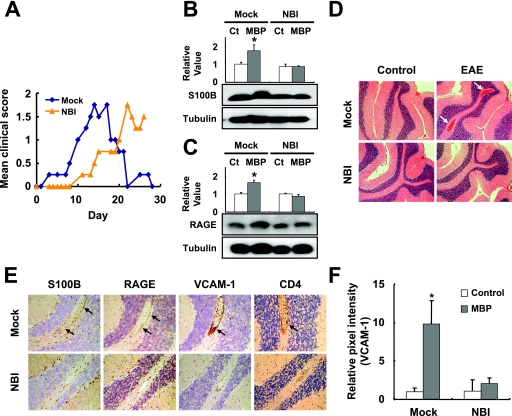
S100B signaling is also known to induce inflammatory reactions through the activation of adhesion molecules on vascular endothelial cells via binding to RAGE (4, 15, 46, 51). The inhibition of RAGE signaling is also known to suppress EAE via a selective blockage of encephalitogenic T-cell infiltration in the mouse CNS (51). These findings give rise to the possibility that brains persistently infected with BDV may have attenuated vascular inflammatory responses to inflammatory stimuli. To understand this, we examined the development of EAE in NBI rats immunized with MBP. We focused on the cerebellum region because this region showed relatively clear vascular inflammatory responses in control rat brains by MBP injection. Following immunization at 7 weeks p.i., NBI rats exhibited a delayed progression of EAE and appeared to induce a significantly less severe EAE than MBP-immunized, mock-infected rats over the observation period (Fig. 4A). We detected the expression of S100B, as well as RAGE, in the cerebellum at 14 days after the sensitization. As shown in Fig. 4B and C, no upregulation of both S100B and RAGE expressions was detected in the MBP-immunized, NBI rat cerebellum, while a significant increase in levels of the proteins was detected in the mock-infected rat brains. The H&E staining revealed that the EAE-developed, mock-infected rats had an apparently increased (in terms of number and diameter) cerebellar vascularity compared with that of the MBP-sensitized NBI rats (Fig. 4D, arrows). The IHC revealed that the immunized, mock-infected rats showed strong immunoreactivity to S100B, RAGE, and VCAM-1 in the perivascular area or vascular endothelium in comparison with that of BDV-positive rats (Fig. 4E, arrows, and F). Furthermore, a large number of CD4-positive cells were found around the neovessels in mock-infected animals (Fig. 4E, arrow). This observation suggested that the persistent infection may prevent the adhesion molecule from being expressed on vascular endothelial cells through the downregulation of S100B expression, resulting in reduced responses during vascular inflammation in the brain.
FIG. 4.
Vascular inflammatory responses in EAE-induced rat brains. (A) Clinical symptoms of EAE. The means of daily clinical scores are shown (see Materials and Methods). Expressions of S100B (B) and RAGE (C) in the cerebellum of the BDV-infected rats at 14 days after the MBP injection are shown. Quantitative analyses of the expressions are also shown. The band intensities were determined by NIH image. Values were normalized to the tubulin level. (*, P < 0.05 with PBS-injected age-matched control [Ct] rats). Mock, age-matched mock-infected rats. (D) Neuropathological analysis of MBP-injected rats. Brain sections were stained with H&E. Arrows indicate the regions of vasodilatation in the cerebellum of a mock-infected, EAE-induced rat. (E) IHC analysis of EAE-induced rat brains. Serial brain sections from the cerebellar regions were stained with anti-S100B, RAGE, VCAM-1, and CD4 antibody. Magnification, ×200. Arrows indicate positive signals in the perivascular regions. (F) Quantification of VCAM-1 expression in rat brains. For a quantitative analysis of VCAM-1 levels in the brain, the optical density of positive signals was measured as described in Materials and Methods. All optical density measurements were performed under the same optical and lighting conditions. The relative pixel intensities to PBS-injected control animals are shown. (*, P < 0.05).
S100B signaling is necessary for the development of mononuclear cell infiltrates in NBI rat brains.
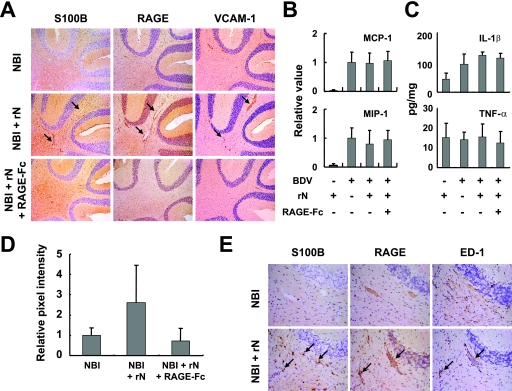
The results above suggested that the downregulation of S100B expression may be responsible for the elimination of the vascular inflammatory responses, as well as the infiltration of encephalitogenic T cells, in persistently infected NBI brains. Therefore, we sought to determine whether the expression level of endogenous S100B is associated with the development of the mononuclear cell infiltrates in the NBI brain. Previous studies have demonstrated that immunization of the viral antigen into BDV-infected animals maintains inflammatory responses in the brains (9, 12). Thus, we immunized NBI rats with rN antigen at 3 weeks p.i. to continuously stimulate the activation of astrocytes, as well as the expression of S100B, in the brain. The immunization at 3 weeks p.i successfully revealed enhanced immunoreactivity of S100B in the perivascular CNS areas even at 5 weeks p.i. compared with that of nonimmunized NBI rats (Fig. 5A and E, arrows). On the other hand, the expression levels of several chemokines and proinflammatory cytokines were quite similar to those of the rN-immunized and nonimmunized, BDV-infected rat brains at 5 weeks p.i. (Fig. 5B and C). In the immunized rat brains, however, an apparently extensive vasodilatation along with intense immunoreactivity of RAGE and VCAM-1 in the vascular endothelium was found (Fig. 5A, arrows, and D). In addition, the immunoreactivity of ED-1 was found in the rN-injected animals, both in the perivascular tissue and the parenchyma (Fig. 5E, arrows). To understand the role of S100B signaling in the development of the vascular responses, we blocked the S100B-RAGE interaction by injecting RAGE-Fc at 2-day intervals from the day of immunization with rN. Previous studies have demonstrated that a low level of endogenous soluble RAGE exists in normal plasma, and the injection of rat RAGE-Fc does not induce any immune responses (34, 51, 52). Interestingly, interference of S100B-RAGE interaction by the repeated i.p. injection of RAGE-Fc drastically repressed the vascular responses, as well as the vasodilatation, in the rN-inoculated rats (Fig. 5A and D), indicating that the S100B signaling may be important for the progression of vascular inflammation in NBI rat brain.
FIG. 5.
Expression of S100B is involved in vascular inflammatory responses in BDV-infected neonatal rat brains. (A) IHC analysis of rN-immunized rat brains. The immunized NBI rats were treated with or without RAGE-Fc and sacrificed at 14 days after the immunization (5 weeks p.i.). Serial brain sections from the cerebellum regions were stained with anti-S100B, RAGE, and VCAM-1 antibody. Magnification, ×100. Arrows indicate positive perivascular regions. Chemokine (B) and cytokine (C) expressions in rN-immunized rat brains. Expressions of MIP-1β and MCP-1 were monitored in the cerebellum by semiquantitative RT-PCR at 14 days after the rN immunization. The band intensities were determined by NIH image, and values were normalized to the GAPDH mRNA level. (C) Levels of IL-1β and TNF-α expression in the cerebellum were estimated with ELISA kits at 14 days after the rN immunization. (D) Quantification of VCAM-1 expression in rat brains. The relative pixel intensities of NBI animals at 5 weeks p.i. are shown. (E) Induction of mononuclear cell infiltration by rN-immunized rat cerebellum. Infiltrated mononuclear cells positive for ED-1 are found in perivascular areas (arrows). IHC results of S100B and RAGE are also shown.
DISCUSSION
In the present study, we demonstrated that the expression of an astrocyte-derived factor, S100B, is reduced in both the cerebral cortex and cerebellum of Lewis rat brains persistently infected with BDV. Although the level of S100B secreted within the rat CNS could not be determined in this experiment, the immunoreactivity level appears to correlate with the amount of this protein secreted in the brain (30). The downregulation of S100B expression is likely to affect the expression of RAGE in the persistently infected brains, suggesting that the secretion of S100B could also be disrupted in the CNS. On the other hand, a significant upregulation of GFAP expression, as well as the chronic production of proinflammatory cytokines and chemokines, was demonstrated in the persistently infected brains, indicating that the downregulation of S100B expression is not due to the deletion of astrocytes in the CNS.
A decreased level of S100B is observed in both NBI and ABI brains, suggesting that the downregulation may be associated with the duration of CNS inflammatory responses in the CNS, such as a chronic expression of inflammatory mediators. A recent study found that treatment with an inflammatory cytokine, gamma interferon (IFN-γ), which has been shown to activate astrocytes to acquire immune functions, downregulates S100B gene expression in primary mouse astrocytes, by using a microarray (11). This observation suggested that a negative regulatory effect of cytokines to immune responsive cells contributes to the downregulation of S100B in the brains. In addition, it has been reported that reactive astrocytes are regulated by the signal transducer and activator of transcription 3 (STAT3) and the protein suppressor of cytokine signaling 3 (SOCS3) (27). Considering that the expression of S100B is also controlled by the STAT3-SOCS3 signaling in the CNS (13), the downregulation of S100B may be caused by the aberration of the signaling via sustained activation of astrocytes. It has also been reported that continual release of S100B during chronic brain stresses directly causes S100B deprivation within astrocytes (10), suggesting that depletion of S100B protein may occur in astrocytes of persistently infected brains. Further study will be needed to elucidate the regulatory mechanism underlying S100B downregulation in the persistently infected brain.
Our analysis using immunoblotting demonstrated that the reduction of the S100B level in persistently infected brains is statistically significant, but the overall difference seems to be modest. The NBI rats showed about 30% reduction of S100B in both the cerebral cortex and cerebellum. A previous study revealed that prenatal stresses induced a 25% reduction in hippocampal S100B content in rats, leading to abnormal postnatal brain development (47). In addition, a significant suppression of S100B (a 24% decrease) by arundic acid (ONO-2506) in the brains of transgenic mice, which overproduce a mutant form of amyloid precursor protein, was markedly ameliorated in β-amyloid plaque burden and amyloid-β peptide levels (24). In these experiments, although expression levels of many other genes associated with neurodevelopment or brain damage could also be changed in the brains, some correlations were observed between the expression level of S100B and specific neurological effects in the animals (24, 26, 47). These observations suggested that even at a moderate level, altered expression of S100B could affect astrocyte activities, resulting in specific biological effects in the brains. On the other hand, in this study we examined the effects of S100B in the brains of NBI rats exposed to LPS or MBP. Interestingly, no upregulation of S100B expression was found in the NBI rat brains by the injection of the immune stimuli, while the protein expression increases about 75% in mock-infected brains (Fig. 2B and Fig. 4B). Along with the modest reduction of S100B at the basal level, the unresponsiveness of S100B expression indicated that the level of this protein is considerably lower in the brains of stimulated NBI rats than control rats, suggesting that the reduced level of S100B has potentially important implications for biological significances found in the NBI brains.
S100B is an EF-hand type Ca2+-binding multifunctional protein in the CNS. This protein exerts autoparacrine and paracrine effects on neurons and glia and is involved in a variety of cellular responses, such as protein phosphorylation, cell proliferation and differentiation, the structural organization of membranes, cytoskeleton modifications, intracellular Ca2+ homeostasis, and the promotion of cell survival (1, 37, 40, 41). In this experiment, we found that the administration of LPS at 5 weeks p.i. does not enhance the expression level of S100B in NBI rat brains and causes significant cell death of both neurons and glia in the cerebellum. The administration of LPS is known to induce CNS stress responses via upregulation of the production of proinflammatory cytokines and nitric oxide (19, 42). The studies using S100B transgenic and knockout mice demonstrated that S100B plays a role in astrocyte proliferation under stressful conditions (3, 6, 32). Considering the neurotrophic effects of reactive astrocytes in inflamed CNS (5, 27, 35), the downregulation of S100B may induce neural cell death as a consequence of impairing astrocyte proliferation in NBI rat brains. In addition, the level of S100B is also known to be directly linked with cellular survival by preventing apoptosis (1, 17, 37, 41). In either mechanism, the brains with downregulated S100B expression might succumb to the stressful environment and cause the apoptosis of neural cells. Previous studies have demonstrated that in BDV-infected neonatal rat brains, reactivation of glial cells may be associated with specific neuronal cell apoptosis rather than viral tropism and virus-specific immune responses (29, 50). These studies support our hypothesis that sustained activation of astrocytes in the persistently infected brains may exhaust the glial functions concerned with cellular survival. Elucidation of how the downregulation of S100B expression is involved in the functional exhaustion of astrocytes may be important to understanding the pathology of neurodegenerative disorders.
A prominent role of this protein seems to be the promotion of inflammation through binding to RAGE as an inflammatory cytokine (36, 37, 46, 51). Expression of S100B is associated with the activation of astrocytes followed by damage to the CNS, during which production of proinflammatory cytokines, such as IL-1β, is increased (6, 22). Stimulation of RAGE signaling through the binding of S100B can lead to the activation of MAP kinase and increased NF-κB activity (17, 22). The RAGE-S100B interaction was confirmed to induce MCP-1 expression, which is often associated with localized inflammation (2, 46). Furthermore, the signaling also enhances the expression of cell adhesion molecules, including VCAM-1, on vascular endothelial cells at inflammatory sites, which is recognized by the integrin VLA-4 expressed on lymphocytes and monocytes, resulting in infiltration by the cells (15, 46, 51). Thus, there is a large body of evidence suggesting that S100B is involved in the development and/or amplification of CNS-based inflammatory responses via modification of the expression of the molecules on the vascular endothelium (46, 51), indicating that the regulation of S100B expression in the injured CNS plays a key role in determining the severity of inflammation in the brain. We found that the downregulation prevents severe EAE in MBP-injected NBI rats. In the rat brain, no significant immunoreactivity of S100B or RAGE was observed in the perivascular areas and vascular endothelium, respectively. The lack of vascular inflammatory reactions may result in a reduction in vascularity and mononuclear cell infiltration in the NBI rat brain. This observation strongly suggests that the activation of S100B is required for the progression of the vascular inflammatory responses, followed by encephalitogenic T-cell infiltration in MBP-immunized rat brains.
In contrast with the MBP immunization, the rN-injected NBI rat brains developed severe vasodilatation and mononuclear cell infiltration in the cerebellum, with the expression of RAGE and VCAM-1 on the vascular endothelium. The role of S100B in the inflammatory reactions in the rN-injected rats was confirmed by the experiment in which the binding of S100B to RAGE was interfered with following repeated administration of RAGE-Fc. These results also implied an important role for S100B signaling in the progression of the vascular inflammatory reaction in the NBI rat brain. Intriguingly, the rN injection into NBI rats successfully upheld the strong immunoreactivity of S100B in the perivascular areas of the cerebellum at 5 weeks p.i. This may be because upon the injection of rN at 3 weeks p.i., astrocytes can continue to retain a phenotype characteristic of astrocytosis with the reactivity of S100B in the rat brain. On the other hand, the MBP immunization at 7 weeks p.i. could not reactivate the astrocytes and S100B expression in the NBI brains. These results may indicate that continuous stimulation with initial antigens is necessary to maintain intact astrocyte activation in the brains. This hypothesis is under investigation using NBI rats at 3 weeks p.i. and immunization with several different antigens.
Our results strongly suggested that downregulation of S100B in NBI rat brains is considerably responsible for the neural cell death and reduced vascular inflammatory responses found in the brains. It should be noted, however, that a large number of different genes could also be altered in BDV-infected rat brains. These altered factors could make it difficult to assess the effects related only to the expression level of S100B in the brains. On the other hand, in this study we focused on NBI instead of ABI brains to observe the effects of S100B downregulation. This is because vascular inflammatory responses by additional stimuli could be easy to detect in NBI brains, in which background infiltration and vascular immune response are totally lacking. As described above, however, it is also certain that NBI rat brain shows a complex environment, with alteration of many different factors associated with abnormal neurodevelopment (16, 53). A comprehensive analysis using a proteomics technique and/or a comparative experiment between NBI and ABI rat brains would be helpful for further evaluation of biological importance of S100B downregulation in persistently infected brains. Such studies will be challenged in the future.
In conclusion, we demonstrated that the expression of an astrocyte-derived inflammatory cytokine, S100B, was downregulated in rat brains persistently infected with BDV. At present, we can only speculate as to the impact of this downregulation, owing to the multiple functions of this protein in the CNS. However, our results are consistent with observations from previous reports that the expression of S100B is involved in the induction of vascular immune responses and the infiltration of encephalitogenic T cells in the CNS of experimental animals (46, 51), implying that BDV may evade CNS-based immune responses through the regulation of astrocyte functions by downregulating the expression of S100B in the brain, resulting in a persistent infection. Given that the downregulation of S100B expression is established as a common course of chronic activation of astrocytes in virus-infected brain, the phenomenon may also be involved in the mechanism of persistent infection of other CNS viruses, such as lymphocytic choriomeningitis virus (28). Interestingly, in patients with human immunodeficiency virus and human T-cell leukemia virus type 1 infections, both of which show severe degenerative effects on brain functions, an increased level of S100B release from activated astrocytes is observed (33, 44). These findings may represent a depletion of S100B protein in the reactive astrocytes of patient brains. The analysis of the regulation of S100B expression in astrocytes would be important to understand the role of this protein in the neuropathogenesis of persistent virus infections. Recently, a remarkable therapeutic approach, referred to as immunocytotherapy, has been discussed as a treatment to eliminate persistent viral infection from the CNS (23). Although the direct role of S100B in the regulation of T-cell responses remains to be elucidated, it might be possible that the abnormal level of S100B influences antiviral functions of transferred T lymphocytes in the CNS immunocytotherapy. Further experiments would be necessary to understand the involvement of the downregulation of S100B in the persistent mechanism of CNS viruses, as well as the therapeutic approach for persistent viral infection, in the brain.
Acknowledgments
This research was partially supported by the Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan; by Grants-in-Aid for Scientific Research (B) 15390148 and 18390139; a Grant-in-Aid from the Zoonosis Control Project of the Ministry of Agriculture, Forestry and Fisheries of Japan; and a research grant for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare of Japan.
Footnotes
Published ahead of print on 21 March 2007.
REFERENCES
- 1.Baudier, J., C. Delphin, D. Grunwald, S. Khochbin, and J. J. Lawrence. 1992. Characterization of the tumor suppressor protein p53 as a protein kinase C substrate and a S100b-binding protein. Proc. Natl. Acad. Sci. USA 89:11627-11631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucciarelli, L. G., T. Wendt, W. Qu, Y. Lu, E. Lalla, L. L. Rong, M. T. Goova, B. Moser, T. Kislinger, D. C. Lee, Y. Kashyap, D. M. Stern, and A. M. Schmidt. 2002. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation 106:2827-2835. [DOI] [PubMed] [Google Scholar]
- 3.Chang, M. S., L. M. Ariah, A. Marks, and E. C. Azmitia. 2005. Chronic gliosis induced by loss of S-100B: knockout mice have enhanced GFAP-immunoreactivity but blunted response to a serotonin challenge. Brain Res. 1031:1-9. [DOI] [PubMed] [Google Scholar]
- 4.Chavakis, T., A. Bierhaus, N. Al-Fakhri, D. Schneider, S. Witte, T. Linn, M. Nagashima, J. Morser, B. Arnold, K. T. Preissner, and P. P. Nawroth. 2003. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 198:1507-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y., and R. A. Swanson. 2003. Astrocytes and brain injury. J. Cereb. Blood Flow Metab. 23:137-149. [DOI] [PubMed] [Google Scholar]
- 6.Craft, J. M., D. M. Watterson, A. Marks, and L. J. Van Eldik. 2005. Enhanced susceptibility of S-100B transgenic mice to neuroinflammation and neuronal dysfunction induced by intracerebroventricular infusion of human beta-amyloid. Glia 51:209-216. [DOI] [PubMed] [Google Scholar]
- 7.Dong, Y., and E. N. Benveniste. 2001. Immune function of astrocytes. Glia 36:180-190. [DOI] [PubMed] [Google Scholar]
- 8.Engelhardt, K. R., K. Richter, K. Baur, P. Staeheli, and J. Hausmann. 2004. The functional avidity of virus-specific CD8+ T cells is down-modulated in Borna disease virus-induced immunopathology of the central nervous system. Eur. J. Immunol. 35:487-497. [DOI] [PubMed] [Google Scholar]
- 9.Fassnacht, U., A. Ackermann, P. Staeheli, and J. Hausmann. 2004. Immunization with dendritic cells can break immunological ignorance toward a persisting virus in the central nervous system and induce partial protection against intracerebral viral challenge. J. Gen. Virol. 85:2379-2387. [DOI] [PubMed] [Google Scholar]
- 10.Gerlach, R., G. Demel, H.-G. König, U. Gross, J. H. M. Prehn, A. Raabe, V. Seifert, and D. Kögel. 2006. Active secretion of S100B from astrocytes during metabolic stress. Neuroscience 141:1697-1701. [DOI] [PubMed] [Google Scholar]
- 11.Halonen, S. K., T. Woods, K. McInnerney, and L. M. Weiss. 2006. Microarray analysis of IFN-γ response genes in astrocytes. J. Neuroimmunol. 175:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausmann, J., W. Hallensleben, J. C. de la Torre, A. Pagenstecher, C. Zimmermann, H. Pircher, and P. Staeheli. 1999. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 96:9769-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, F., W. Ge, K. Martinowich, S. Becker-Catania, V. Coskun, W. Zhu, H. Wu, D. Castro, F. Guillemot, G. Fan, J. de Vellis, and Y. E. Sun. 2005. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat. Neurosci. 8:616-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer, M., J. Hausmann, P. Staeheli, and A. Pagenstecher. 2004. Cerebral expression of interleukin-12 induces neurological disease via differential pathways and recruits antigen-specific T cells in virus-infected mice. Am. J. Pathol. 165:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann, M. A., S. Drury, C. Fu, W. Qu, A. Taguchi, Y. Lu, C. Avila, N. Kambham, A. Bierhaus, P. Nawroth, M. F. Neurath, T. Slattery, D. Beach, J. McClary, M. Nagashima, J. Morser, D. Stern, and A. M. Schmidt. 1999. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97:889-901. [DOI] [PubMed] [Google Scholar]
- 16.Hornig, M., H. Weissenbock, N. Horscroft, and W. I. Lipkin. 1999. An infection-based model of neurodevelopmental damage. Proc. Natl. Acad. Sci. USA 96:12102-12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huttunen, H. J., J. Kuja-Panula, G. Sorci, A. L. Agneletti, R. Donato, and H. Rauvala. 2000. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J. Biol. Chem. 275:40096-40105. [DOI] [PubMed] [Google Scholar]
- 18.Ikuta, K., K. Hagiwara, H. Taniyama, and N. Nowotny. 2002. Epidemiology and infection of natural animal hosts, p. 87-123. In K. M. Carbone (ed.), Borna disease virus and its role in neurobehavioral disease. ASM Press, Washington, DC.
- 19.Iravani, M. M., C. C. Leung, M. Sadeghian, C. O. Haddon, S. Rose, and P. Jenner. 2005. The acute and the long-term effects of nigral lipopolysaccharide administration on dopaminergic dysfunction and glial cell activation. Eur. J. Neurosci. 22:317-330. [DOI] [PubMed] [Google Scholar]
- 20.Kamitani, W., Y. Shoya, T. Kobayashi, M. Watanabe, B.-J. Lee, G. Zhang, K. Tomonaga, and K. Ikuta. 2001. Borna disease virus phosphoprotein binds a neurite outgrowth factor, amphoterin/HMG-1. J. Virol. 75:8742-8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis, A. J., J. L. Whitton, C. G. Hatalski, H. Weissenbock, and W. I. Lipkin. 1999. Effect of immune priming on Borna disease. J. Virol. 73:2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, L., Y. Li, L. J. Van Eldik, W. S. Griffin, and S. W. Barger. 2005. S100B-induced microglial and neuronal IL-1 expression is mediated by cell type-specific transcription factors. J. Neurochem. 92:546-553. [DOI] [PubMed] [Google Scholar]
- 23.McGavern, D. B. 2006. Immunotherapeutic relief from persistent infections and amyloid disorders. Neurology 66:S59-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori, T., T. Town, J. Tan, N. Yada, Y. Horikoshi, J. Yamamoto, T. Shimoda, Y. Kamanaka, N. Tateishi, and T. Asano. 2006. Arundic acid ameliorates cerebral amyloidosis and gliosis in Alzheimer transgenic mice. J. Pharmacol. Exp. Ther. 318:571-578. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura, Y., H. Takahashi, Y. Shoya, T. Nakaya, M. Watanabe, K. Tomonaga, K. Iwahashi, K. Ameno, N. Momiyama, H. Taniyama, T. Sata, T. Kurata, J. C. de la Torre, and K. Ikuta. 2000. Isolation of Borna disease virus from human brain tissue. J. Virol. 74:4601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohtani, R., H. Tomimoto, H. Wakita, H. Kitaguchi, K. Nakaji, and R. Takahashi. 2007. Expression of S100 protein and protective effect of arundic acid on the rat brain in chronic cerebral hypoperfusion. Brain Res. 1135:195-200. [DOI] [PubMed] [Google Scholar]
- 27.Okada, S., M. Nakamura, H. Katoh, T. Miyao, T. Shimazaki, K. Ishii, J. Yamane, A. Yoshimura, Y. Iwamoto, Y. Toyama, and H. Okano. 2006. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat. Med. 12:829-834. [DOI] [PubMed] [Google Scholar]
- 28.Oldstone, M. B. 2006. Viral persistence: parameters, mechanisms and future predictions. Virology 344:111-118. [DOI] [PubMed] [Google Scholar]
- 29.Ovanesov, M. V., C. Sauder, S. A. Rubin, J. Richt, A. Nath, K. M. Carbone, and M. V. Pletnikov. 2006. Activation of microglia by Borna disease virus infection: in vitro study. J. Virol. 80:12141-12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petzold, A., M. J. Eikelenboom, D. Gveric, G. Keir, M. Chapman, R. H. C. Lazeron, M. L. Cuzner, C. H. Polman, B. M. J. Uitdehaag, E. J. Thompson, and G. Giovannoni. 2002. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain 125:1462-1473. [DOI] [PubMed] [Google Scholar]
- 31.Plata-Salaman, C. R., S. E. Ilyin, D. Gayle, A. Romanovitch, and K. M. Carbone. 1999. Persistent Borna disease virus infection of neonatal rats causes brain regional changes of mRNAs for cytokines, cytokine receptor components and neuropeptides. Brain Res. Bull. 49:441-451. [DOI] [PubMed] [Google Scholar]
- 32.Reeves, R. H., J. Yao, M. R. Crowley, S. Buck, X. Zhang, P. Yarowsky, J. D. Gearhart, and D. C. Hilt. 1994. Astrocytosis and axonal proliferation in the hippocampus of Sl00b transgenic mice. Proc. Natl. Acad. Sci. USA 91:5359-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regner, A., O. Bianchini, C. Jardim, and M. Menna-Barreto. 2002. HTLV-I-associated myelopathy: are ferritin, S100β protein, or guanine nucleotides CSF markers of disease? J. Neurovirol. 8:64-67. [DOI] [PubMed] [Google Scholar]
- 34.Renard, C., O. Chappey, M. P. Wautier, M. Nagashima, E. Lundh, J. Morser, L. Zhao, A. M. Schmidt, J. M. Scherrmann, and J. L. Wautier. 1997. Recombinant advanced glycation end product receptor pharmacokinetics in normal and diabetic rats. Mol. Pharmacol. 52:54-62. [DOI] [PubMed] [Google Scholar]
- 35.Ridet, J. L., S. K. Malhotra, A. Privat, and F. H. Gage. 1997. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 20:570-577. [DOI] [PubMed] [Google Scholar]
- 36.Rong, L. L., S. F. Yan, T. Wendt, D. Hans, S. Pachydaki, L. G. Bucciarelli, A. Adebayo, W. Qu, Y. Lu, K. Kostov, E. Lalla, S. D. Yan, C. Gooch, M. Szabolcs, W. Trojaborg, A. P. Hays, and A. M. Schmidt. 2004. RAGE modulates peripheral nerve regeneration via recruitment of both inflammatory and axonal outgrowth pathways. FASEB J. 18:1818-1825. [DOI] [PubMed] [Google Scholar]
- 37.Rothermundt, M., M. Peters, J. H. Prehn, and V. Arolt. 2003. S100B in brain damage and neurodegeneration. Microsc. Res. Tech. 60:614-632. [DOI] [PubMed] [Google Scholar]
- 38.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 39.Sauder, C., and J. C. de la Torre. 1999. Cytokine expression in the rat central nervous system following perinatal Borna disease virus infection. J. Neuroimmunol. 96:29-45. [DOI] [PubMed] [Google Scholar]
- 40.Scotto, C., J. C. Deloulme, D. Rousseau, E. Chambaz, and J. Baudier. 1998. Calcium and S100B regulation of p53-dependent cell growth arrest and apoptosis. Mol. Cell. Biol. 18:4272-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selinfreund, R. H., S. W. Barger, W. J. Pledger, and L. J. Van Eldik. 1991. Neurotrophic protein S100 beta stimulates glial cell proliferation. Proc. Natl. Acad. Sci. USA 88:3554-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semmler, A., T. Okulla, M. Sastre, L. Dumitrescu-Ozimek, and M. T. Heneka. 2005. Systemic inflammation induces apoptosis with variable vulnerability of different brain regions. J. Chem. Neuroanat. 30:144-157. [DOI] [PubMed] [Google Scholar]
- 43.Sheng, J. G., K. Ito, R. D. Skinner, R. E. Mrak, C. R. Rovnaghi, L. J. Van Eldik, and W. S. T. Griffin. 1996. In vivo and in vitro evidence supporting a role for the inflammatory cytokine interleukin-1 as a driving force in Alzheimer pathogenesis. Neurobiol. Aging 17:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley, L. C., R. E. Mrak, R. C. Woody, L. J. Perrot, S. Zhang, D. R. Marshak, S. J. Nelson, and W. S. Griffin. 1994. Glial cytokines as neuropathogenic factors in HIV infection: pathogenic similarities to Alzheimer's disease. J. Neuropathol. Exp. Neurol. 53:231-238. [DOI] [PubMed] [Google Scholar]
- 45.Tramontina, F., S. Conte, D. Gonçalves, C. Gottfried, L. V. Portela, L. Vinade, C. Salbego, and C. A. Gonçalves. 2002. Developmental changes in S100B content in brain tissue, cerebrospinal fluid, and astrocyte cultures of rats. Cell. Mol. Neurobiol. 22:373-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valencia, J. V., M. Mone, J. Zhang, M. Weetall, F. P. Buxton, and T. E. Hughes. 2004. Divergent pathways of gene expression are activated by the RAGE ligands S100b and AGE-BSA. Diabetes 53:743-751. [DOI] [PubMed] [Google Scholar]
- 47.Van den Hove, D. L., H. W. Steinbusch, M. Bruschettini, D. Gazzolo, R. Frulio, A. Scheepens, J. Prickaerts, and C. E. Blanco. 2006. Prenatal stress reduces S100B in the neonatal rat hippocampus. Neuroreport 17:1077-1080. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe, M., Q. Zhong, T. Kobayashi, W. Kamitani, K. Tomonaga, and K. Ikuta. 2000. Molecular ratio between Borna disease viral-p40 and -p24 proteins in infected cells determined by quantitative antigen capture ELISA. Microbiol. Immunol. 44:765-772. [DOI] [PubMed] [Google Scholar]
- 49.Watanabe, M., B. J. Lee, W. Kamitani, T. Kobayashi, H. Taniyama, K. Tomonaga, and K. Ikuta. 2001. Neurological diseases and viral dynamics in the brains of neonatally Borna disease virus-infected gerbils. Virology 282:65-76. [DOI] [PubMed] [Google Scholar]
- 50.Weissenbock, H., M. Hornig, W. F. Hickey, and W. I. Lipkin. 2000. Microglial activation and neuronal apoptosis in Bornavirus infected neonatal Lewis rats. Brain Pathol. 10:260-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan, S. S., Z. Y. Wu, H. P. Zhang, G. Furtado, X. Chen, S. F. Yan, A. M. Schmidt, C. Brown, A. Stern, J. LaFaille, L. Chess, D. M. Stern, and H. Jiang. 2003. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat. Med. 9:287-293. [DOI] [PubMed] [Google Scholar]
- 52.Yonekura, H., Y. Yamamoto, S. Sakurai, R. G. Petrova, M. J. Abedin, H. Li, K. Yasui, M. Takeuchi, Z. Makita, S. Takasawa, H. Okamoto, T. Watanabe, and H. Yamamoto. 2003. Novel splice variants of the receptor for advanced glycation end products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 370:1097-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zocher, M., S. Czub, J. Schulte-Monting, J. C. de La Torre, and C. Sauder. 2000. Alterations in neurotrophin and neurotrophin receptor gene expression patterns in the rat central nervous system following perinatal Borna disease virus infection. J. Neurovirol. 6:462-477. [DOI] [PubMed] [Google Scholar]