Abstract
Abnormal activation of B lymphocytes is a feature commonly seen in human immunodeficiency virus type 1 (HIV-1)-infected persons. However, the mechanism(s) responsible for this dysfunction is still poorly understood. Having recently shown that CD40L, the ligand for CD40, is inserted within emerging HIV-1 particles, we hypothesized that the contact between virus-anchored host CD40L and CD40 on the surface of B lymphocytes might result in the activation of this cell type. We report here that CD40L-bearing viruses, but not isogenic virions lacking host-derived CD40L, can induce immunoglobulin G and interleukin-6 production. Furthermore, such viral entities were found to induce B-cell homotypic adhesion. These effects were paralleled at the intracellular level by the nuclear translocation of the ubiquitous transcription factor NF-κB. The presence of host-derived CD40L within virions resulted in an increased virus attachment to B cells and a more-efficient B-cell-mediated transfer of HIV-1 to autologous CD4+ T lymphocytes. All the above processes were independent of the virus-encoded envelope glycoproteins. Altogether, the data gathered from this series of investigations suggest that the incorporation of host-encoded CD40L in HIV-1 is likely to play a role in the B-cell abnormalities that are seen in infected individuals.
Over the past few years, studies of a rare immunodeficiency disease named X-linked hyper-immunoglubulin (Ig) syndrome revealed the importance of interactions between CD40L and CD40 in the normal immune response (reviewed in reference 37). The CD40L molecule is a type II membrane glycoprotein belonging to the tumor necrosis factor (TNF) superfamily that is expressed mainly in activated CD4+ T lymphocytes (4). It binds to and trimerizes CD40 (69), a type I membrane glycoprotein of the TNF receptor superfamily which is expressed on B lymphocytes from diverse sources, such as tonsils (5). The degree of multimerization of CD40 regulates the strength of the intracellular signal transduction events (29, 56, 70), resulting, notably, in the activation and differentiation of B cells (24). This activation leads to homotypic aggregation of these cells and the secretion of a number of cytokines, including interleukin-6 (IL-6), IL-10, IL-12, and TNF-α (reviewed in reference 9). The differentiation of B lymphocytes into Ig-secreting plasma cells is actually the final step of the isotype switching process (27). In the case of IgG, their synthesis normally occurs following the stimulation of CD40 and the IL-4 receptor, leading to NF-κB- and signal transducer and activator of transcription 6 (STAT-6)-dependent transcription of germ line genes and subsequent DNA recombination to Cγ genes (2, 62).
Although B lymphocytes are not considered to be a cellular reservoir for human immunodeficiency virus type 1 (HIV-1), this cell type is afflicted by a wide spectrum of abnormalities in the setting of HIV-1 infection. For example, it has been reported that B cells display a hyperactivation state in the presence of active viral replication which translates into hypergammaglobulinemia in vivo (reviewed in reference 58) and the spontaneous secretion, especially of the IgG subclass, of Ig in culture (33, 38, 46, 48). Other B-cell dysfunctions include an increased expression of cell surface activation markers (41), the appearance of B-cell subpopulations characteristic of cellular activation and terminal differentiation (19, 50), augmented B-cell turnover (18), and an increased incidence of B-cell malignancies (45). With respect to the appearance of hypergammaglobulinemia, this disorder is characterized by the production of low-affinity antibodies and antibodies directed against various self-antigens (19), which are both believed to be due to ongoing viral replication (63). Indeed, viremia has been found to be associated with abnormal levels of IgG in sera of HIV-1-infected patients (50, 51). In addition, in many patients, hypergammaglobulinemia appears as early as a few months following the primary infection (21, 52). Interestingly, this disorder is seen even before the appearance of quantitative and functional defects in CD4+ T cells (47, 66).
Given that HIV-1 pathogenesis is associated with the appearance of numerous B-cell defects and considering that we recently demonstrated that CD40L constitutes one of the host cell-derived proteins incorporated into emerging HIV-1 particles (44), we tested the possibility that interactions between virus-associated host CD40L and CD40 on the surface of B lymphocytes might result in cellular activation. The activation status of B cells was assessed by monitoring NF-κB nuclear translocation, cytokine production, the secretion of IgG, and the induction of cell-to-cell adhesion. Moreover, because B lymphocytes continually percolate through peripheral lymphoid tissues, where they establish an intimate contact with activated CD4+ T lymphocytes, we also assessed whether the presence of host-derived CD40L within mature HIV-1 particles could result in a more-efficient B-cell-dependent spreading of the virus to CD4+ T cells.
(This work was performed by G. Martin in partial fulfillment of a Ph.D. degree from the Microbiology-Immunology Program, Faculty of Medicine, Laval University, Quebec, Canada.)
MATERIALS AND METHODS
Cells.
Human tonsils removed during routine tonsillectomies were received within a few hours of excision. The tonsillar tissue was mechanically disrupted in cold RPMI 1640 medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin G, 100 μg/ml streptomycin, and 50 μM 2-mercaptoethanol [RPMI 1640-10% FCS-2ME]. The resulting cellular suspension was filtered through a 30-μm mesh nylon filter and subjected to a Ficoll-Hypaque gradient for the isolation of mononuclear cells. Thereafter, autologous B cells and CD4+ T lymphocytes were negatively separated as described previously (57). B lymphocytes were purified using EasySep human B-cell enrichment cocktail (StemCell Technologies), resulting in a B-cell population almost free of CD4+ T lymphocytes, as assessed by flow cytometry (i.e., <1% CD4+ T cells), whereas CD4+ T lymphocytes were isolated with the EasySep human CD4+ T-cell enrichment cocktail (StemCell Technologies). Before the initiation of virus transmission assays, B and T lymphocytes were maintained separately in RPMI 1640-10% FCS-2ME medium. Human embryonic kidney 293T cells were cultured in complete Dulbecco's modified Eagle's medium (DMEM) (i.e., DMEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin G, and 100 μg/ml streptomycin).
Plasmids and antibodies.
pNL4-3 is a full-length infectious molecular clone of HIV-1 (X4-tropic) (1) that was obtained through the AIDS Repository Reagent Program (Germantown, MD). pNL4-3balenv was created by replacing the env gene of NL4-3 with that of the R5-tropic strain Bal (kindly provided by R. J. Pomerantz, Thomas Jefferson University) (20). The envelope (Env)-deficient plasmid pNL4-3 Env− was a generous gift of D. E. Ott (National Cancer Institute) (53). The vector coding for human CD40L (i.e., pcDNA3.1-CD40L) was provided by R. Kornbluth (University of California San Diego) (36). The anti-CD40 hybridoma G28.5 (IgG1) (15) and the anti-CD40L hybridoma 5c8 (IgG2a) (39) were generously provided by W. Mourad (Université de Montréal). The antibody against the NF-κB p50 subunit was purchased from Santa Cruz, while the antibody specific for the anti-NF-κB p65 subunit was kindly given by N. Rice (National Cancer Institute-Frederick Cancer Research and Development Center) (60). Antibodies from these hybridomas were purified with a HiTrap protein G affinity column by following the manufacturer's instructions (Amersham Biosciences AB). For purposes of the virus capture assay, biotinylation of the tested antibodies was performed using EZ-link Sulfo-NHS-LC-biotin (Pierce).
Virus production and capture assay.
Progeny viruses were produced by calcium phosphate transfection of 293T cells as previously described (23). It should be noted that 293T cells do not constitutively express CD40L (data not shown). Briefly, 293T cells were cotransfected with a plasmid coding for the human CD40L molecule (i.e., pcDNA3.1-CD40L) and one of the following molecular clones of HIV-1: a plasmid coding for wild-type NL4-3 particles (i.e., pNL4-3) (virus stock called NL4-3/CD40L), a vector coding for R5-tropic Bal Env glycoproteins inserted within the NL4-3 backbone (i.e., pNL4-3balenv/CD40L) (virus stock called NL4-3balenv/CD40L), or a plasmid coding for Env-deficient NL4-3 viruses (i.e., pNL4-3 Env−) (virus stock called NL4-3 Env−/CD40L). Viruses lacking host CD40L were made by transfection of 293T cells with only one of the listed NL4-3 constructs: wild-type NL4-3 (virus stock called NL4-3/null) and NL4-3balenv (virus stock called NL4-3balenv/null). Controls consisted of 293T cells transfected with pcDNA3.1-CD40L alone (called Mock/CD40L). Supernatants from such transfected 293T cells were filtered through a 0.45-μm cellulose acetate membrane (Millipore Corporation). To eliminate free p24, each supernatant was ultrafiltrated in CentriconPlus-20 Biomax-100 filter devices (Millipore Corporation). An enzyme-linked immunosorbent assay (ELISA) developed in our laboratory was used to estimate the amount of p24 in all virus stocks (12). For all virus stocks, the presence or absence of the CD40L molecule was confirmed by performing our virus capture assay using an initial virus input of 1 ng of p24 as we described previously (44).
IgG production assay.
Purified B lymphocytes (1 × 106) were cultured in 1 ml of RPMI 1640-10% FCS-2ME containing 400 U/ml of human recombinant IL-4 (R&D Systems) (25) and 50 ng/ml of human recombinant IL-10 (PeproTech Inc.) (14, 16). Thereafter, such B cells were incubated at 37°C either with medium alone, Mock/CD40L preparation (always added in the same volume as the less-concentrated viral stock), anti-CD40 (clone G28.5) (1 μg/ml), or one of the studied virus stocks (standardized in terms of p24 contents/200 ng of p24). In some conditions, wedelolactone (BIOMOL International LP), an inhibitor of the IκB kinase complex, was added at 10 μM along with the virus stock. Supernatants were harvested at 5 and 12 days following initiation of the culture, frozen, and kept at −20°C until assayed for their IgG content by ELISA (Bethyl Inc.).
Cellular adhesion and cytokine production.
Purified B lymphocytes (1 × 106) were cultured using conditions similar to those for IgG production, either with medium alone, Mock/CD40L, anti-CD40 (clone G28.5) (1 μg/ml), or one of the tested virus preparations (200 ng of p24). Photographs of cultures were taken after 72 h, and supernatants were harvested and then frozen and kept at −20°C until assayed for their cytokine content. The simultaneous evaluation of multiple cytokines/chemokines was achieved through the use of the Luminex 100 instrument and the Beadlyte human multicytokine detection system 4 (Upstate), which is a multiplex assay kit used for measuring 12 different cytokines (i.e., IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, TNF-α, gamma interferon, MCP-1, and RANTES). Quantitative measurement of IL-6 was also carried out with a regular ELISA test (eBioscience).
Preparation of nuclear extracts and EMSA.
Purified B lymphocytes were incubated in RPMI 1640-10% FCS-2ME for 30 or 60 min at 37°C either with medium alone, Mock/CD40L, phorbol myristate acetate (20 ng/ml) and ionomycin (1 μM) (both from Sigma), or some of the studied virus stocks (i.e., NL4-3/null or NL4-3/CD40L at a final concentration of 10 or 30 ng of p24 per 1 × 105 B cells). The treatment of the B cells was terminated by the addition of cold phosphate-buffered saline (PBS), and nuclear extracts were prepared as previously described (64). Electrophoretic mobility shift assays (EMSAs) were performed by incubating 10 μg of nuclear proteins in 20 μl of binding buffer (10 mM HEPES [pH 7.9], 4% glycerol, 1% Ficoll, 25 mM KCl, 1 mM dithiothreitol, 0.5 mM EDTA, 25 mM NaCl) containing 2 μg of poly(dI-dC), 10 μg of nuclease-free bovine serum albumin fraction V, and 0.8 ng of a γ-32P-labeled double-stranded DNA oligonucleotide for 20 min at room temperature. The following double-stranded DNA oligonucleotides were used as probes and/or competitors: the consensus NF-κB binding site (5′-ATGTGAGGGGACTTTCCCAGGC-3′), the consensus SP1 binding site (5′-ATTCGATCGGGGCGGGGCGAG-3′), and the consensus binding site for Oct-2A (5′-GGAGTATCCAGCTCCGTAGCATGCAAATCCTCTGG-3′) (for nonspecific competition). Cold competition assays were carried out by adding a 100-fold molar excess of an unlabeled double-stranded DNA oligonucleotide simultaneously with the labeled probe. Supershift assays were performed by preincubating nuclear extracts with 1 μg of an antibody against NF-κB p50 or NF-κB p65 for 30 min on ice prior to the addition of the binding buffer containing the labeled probe. DNA-protein complexes were resolved from unbound labeled DNA by electrophoresis in native 4% (wt/vol) polyacrylamide gels. Then, the gels were dried, exposed, and autoradiographed.
Virus binding assay.
Purified B lymphocytes (3.5 × 106) were incubated at 37°C for 1 h in 1 ml of RPMI 1640-10% FCS-2ME containing NL4-3/null, NL4-3/CD40L, NL4-3balenv/null, or NL4-3balenv/CD40L (350 ng of p24). In some cases, cells were also incubated with NL4-3/CD40L or NL4-3balenv/CD40L in combination with 10 μg/ml of a trimeric and soluble form of CD40L (sCD40L) (CEDARLANE Laboratories Ltd). The cells were extensively washed with PBS and resuspended in 350 μl of a lysis buffer (PBS-0.05% Tween-2.5% Triton X-100 -2.5% trypan blue). The p24 content was determined by ELISA.
Virus entry assay.
Purified B lymphocytes (3.5 × 106) were incubated at 37°C for 1 h in 1 ml of RPMI 1640-10% FCS-2ME containing NL4-3/null or NL4-3/CD40L (350 ng of p24). Next, the cells were washed with PBS and exposed to trypsin for 5 min to remove uninternalized viruses. Next, RPMI 1640-10% FCS-2ME was added, and then cells were washed in PBS and resuspended in 350 μl of a lysis buffer (PBS-0.05% Tween-2.5% Triton X-100-2.5% trypan blue). The amount of internalized viruses was estimated by measuring the p24 content.
Virus transmission assay.
Purified B lymphocytes (1.5 × 105) were initially pulsed with NL4-3/null, NL4-3/CD40L, NL4-3balenv/null, or NL4-3balenv/CD40L (10 ng of p24) for 1 h at 37°C in 100 μl of RPMI 1640-10% FCS-2ME. In some experiments, cells were also incubated with NL4-3/CD40L or NL4-3balenv/CD40L used in combination with 10 μg/ml of sCD40L (CEDARLANE Laboratories Ltd). Next, the cells were extensively washed with PBS and then cocultured with phytohemagglutinin (PHA)/IL-2-stimulated autologous CD4+ T lymphocytes (1 × 105). Supernatants were harvested at days 4, 6, and 8 postinfection to quantify the p24 content.
Statistical analysis.
The means of variables of matched pairs were compared using Student's t test with Microsoft Excel. P values of less than 0.05 were considered statistically significant.
RESULTS
Incorporation of host-derived CD40L in HIV-1 is independent of Env glycoproteins.
Although it is clear that CD40L is acquired by R5- and X4-tropic variants of HIV-1 once expanded in natural cellular reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo (44), there is no information regarding the molecular mechanism responsible for the insertion of this cell surface molecule within viruses or on the potential role of this host-derived protein in HIV-1 biology. It was previously reported that the incorporation of host HLA-DR glycoproteins within HIV-1 is independent of virus-encoded Env glycoproteins (43). Therefore, we initially investigated whether the acquisition of CD40L by emerging virions was independent of Env as well. To this end, virions were produced using a transient transfection and expression system based on cotransfection of 293T cells with an expression vector coding for human CD40L and an infectious molecular clone of HIV-1 (i.e., wild-type NL4-3 or Env-deficient NL4-3). The physical presence of host-derived CD40L within mature HIV-1 particles was assessed by a virus capture assay using beads coated with anti-CD40L antibodies. It should be noted that 293T cells were transfected with an amount of the CD40L expression vector resulting in a surface expression level of CD40L comparable to what is seen in primary human CD4+ T lymphocytes following stimulation through the T-cell receptor and CD28 costimulatory molecule (data not shown). Data from the capture test performed on virus preparations indicate that CD40L is efficiently acquired by HIV-1 and that this process is independent of viral Env glycoproteins (Fig. 1). The estimated recovery rates for CD40L-bearing virions either bearing or lacking Env glycoproteins varied between 14 and 18%. Similar observations were made when using virus stocks obtained from two independent transfection protocols (data not shown).
FIG. 1.
Env glycoproteins are dispensable for the incorporation of CD40L within HIV-1. NL4-3/CD40L and NL4-3 Env−/CD40L virus stocks were incubated with streptavidin-coated magnetic beads coated with anti-CD40L antibodies (i.e., 5c8) or isotype-matched irrelevant antibodies (i.e., IgG2a). The amounts of precipitated viruses were estimated by a p24 test. The data shown are the means ± standard deviations of triplicate samples and are representative of three independent transfections.
Virus-anchored host CD40L promotes IgG production.
We next studied the putative biological function(s) of such virus-associated CD40L molecules. Since the majority of antibodies detected in HIV-1-associated B-cell abnormalities are IgG, we studied the modulatory effect of virus-anchored host CD40L on the production of this class of Ig by B cells purified from human tonsils. We found that incubation of purified B lymphocytes from three different donors with CD40L-bearing NL4-3 particles resulted in a statistically significant induction of IgG synthesis, a phenomenon that was not seen when using isogenic virions devoid of CD40L (Fig. 2A). Similar findings were made when using R5-tropic viruses (i.e., NL4-3balenv) (Fig. 2B). It is important to emphasize that the levels of IgG mediated by CD40L-bearing virions were sometimes higher than the values obtained with a monoclonal anti-CD40 antibody (i.e., G28.5) which was used as a positive control. The production of IgG by NL4-3/CD40L and NL4-3balenv/CD40L was completely abolished upon the treatment of B cells with wedelolactone, an inhibitor of the IκB kinase complex (data not shown). This suggests that the virus-mediated induction of IgG synthesis was relying on NF-κB activation (see below). Experiments performed with Env-deficient viruses bearing host-derived CD40L confirmed that Env glycoproteins are not required to permit the acquisition of this cell membrane constituent by HIV-1 (Fig. 2C). They also demonstrated that CD40L is still functional on virions devoid of Env.
FIG. 2.
CD40L-bearing virions, but not viruses lacking host CD40L, induce IgG production. B lymphocytes purified from human tonsils were treated with IL-4 and IL-10 and incubated at 37°C either in medium alone or with G28.5 (used as a positive control) or one of the following: (A) Mock/CD40L, NL4-3/null, or NL4-3/CD40L, (B) Mock/CD40L, NL4-3balenv/null, or NL4-3balenv/CD40L, or (C) NL4-3/CD40L or NL4-3 Env−/CD40L. After 5 (filled bars) and 12 (empty bars) days in culture, supernatants were harvested and the IgG content was measured using a commercial ELISA. In panels A and B, the data shown represent the means ± standard deviations of triplicate samples for three independent experiments, whereas in panel C, the data shown represent the means ± standard deviations of triplicate samples and are representative of three independent experiments. The asterisks indicate statistically significant differences (i.e., P < 0.05) between the following matched pairs: NL4-3/CD40L versus NL4-3/null (A), NL4-3balenv/CD40L versus NL4-3balenv/null (B), NL4-3/CD40L versus medium alone (C) and NL4-3 Env−/CD40L versus medium alone (C).
CD40L-bearing virions trigger IL-6 secretion and homotypic cell-to-cell adhesion.
The proinflammatory cytokines IL-6 and TNF-α are known to be produced by B cells isolated from HIV-1-infected persons (32). Thus, we evaluated the secretion of several soluble factors following the exposure of B lymphocytes to viruses either lacking or bearing host-derived CD40L. We used the Luminex 100 technology to screen for the production of 12 different cytokines/chemokines, including those mentioned above, in cell-free supernatants from B cells incubated with isogenic NL4-3-based particles. High levels of IL-4 and IL-10 were detected in the tested samples, and no increase in the production of these two cytokines could be observed following treatment with the tested virus preparations (i.e., NL4-3/null, NL4-3/CD40L, NL4-3balenv/null, and NL4-3balenv/CD40L) or anti-CD40 antibody (Fig. 3A). Although a basal level of IL-6 was measured in B cells exposed to supernatants from 293T cells transiently transfected with the CD40L expression vector (i.e., Mock/CD40L), a weak but statistically significant increase in IL-6 production was detected with CD40L-bearing virions (both NL4-3/CD40L and NL4-3balenv/CD40L) but not with isogenic viruses lacking host-derived CD40L (i.e., NL4-3/null and NL4-3balenv/null). The higher secretion of IL-6 upon exposure to CD40L-bearing NL4-3 particles was confirmed when a commercial human IL-6 ELISA kit was used (Fig. 3B). A comparable virus-mediated induction of IL-6 production was seen when using CD40L-bearing NL4-3balenv (data not shown). Env-deficient viruses that bear host-derived CD40L were also effective at mediating the production of IL-6 from purified B cells (Fig. 3C). Again, the monoclonal anti-CD40 antibody G28.5 was used as a positive control in this series of investigations.
FIG. 3.
CD40L-bearing HIV-1 particles, but not viruses lacking host CD40L, induce Il-6 production. Purified B lymphocytes from human tonsils were treated with IL-4 and IL-10 and incubated for 72 h at 37°C with (A) either Mock/CD40L, the indicated virus preparations, or G28.5, (B) either medium alone, Mock/CD40L, the indicated virus stocks, or G28.5, or (C) either medium alone, the listed virus preparations, or G28.5. The amounts of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12(p70), IL-13, TNF-α, IFN-γ, MCP-1, and RANTES were measured in cell-free supernatants with the Luminex technology (A). In some studies, the levels of IL-6 were determined with a commercial ELISA test (B and C). In panel A, the data shown represent the means ± standard deviations of triplicate samples for two independent experiments, whereas in panels B and C, the data shown represent the means ± standard deviations of triplicate samples and are representative of three different experiments. The asterisks indicate statistically significant differences (i.e., P < 0.05) between the following matched pairs: NL4-3/CD40L versus NL4-3/null and NL4-3balenv/CD40L versus NL4-3balenv/null (A), NL4-3/CD40L versus medium alone (B and C), and NL4-3 Env−/CD40L versus medium alone (C).
The activation of B lymphocytes can also be monitored by assessing homotypic aggegation (6, 26, 31). Microphotographs of purified B cells that were exposed to progeny virus either lacking or bearing host CD40L were taken. Large cell clusters were found only in B cells incubated with CD40L-bearing R5 virions and not with viruses devoid of host-derived CD40L (Fig. 4A). Comparable findings were obtained when B cells were exposed to CD40L-bearing X4 viruses as well as to Env-deficient particles that carry host CD40L on their surface (Fig. 4B).
FIG. 4.
CD40L-bearing virions, but not viruses lacking host CD40L, mediate homotypic B-cell adhesion. B lymphocytes purified from human tonsils were treated with IL-4 and IL-10 and were incubated for 72 h at 37°C with either (A) medium alone, Mock/CD40L, NL4-3balenv/null, NL4-3balenv/CD40L, or G28.5, or (B) medium alone, NL4-3/CD40L, NL4-3 Env−/CD40L, or G28.5. The images were observed at ×100 magnification, and each is representative of three independent experiments.
CD40L-bearing viruses drive nuclear translocation of NF-κB p50 homodimer and p50/p65 heterodimer.
In the experiments described below, we attempted to characterize the intracellular events occurring after CD40 ligation on B lymphocytes by virus-associated CD40L. To this end, we performed mobility shift assays and found that NL4-3 particles bearing host-derived CD40L, but not viruses lacking CD40L, induced the nuclear translocation of NF-κB in B lymphocytes (Fig. 5A). As expected, a basal level of nuclear NF-κB was found in these cells (7). Competition with a 100-fold excess of a specific (i.e., cold NF-κB) or nonspecific (i.e., cold Oct-2A) oligonucleotide confirmed the specificity of the NF-κB binding complex. Moreover, an SP1-labeled probe was used to test the nonspecific binding, and no signal was detected (data not shown). Supershift experiments carried out with specific antibodies revealed that the NF-κB binding complex induced by the attachment of CD40L-bearing HIV-1 particles to the surface of purified B lymphocytes was composed of p50/p50 homodimers and p50/p65 heterodimers (Fig. 5B). Interestingly, a dose-dependent increase in the activation of NF-κB was seen when using increasing doses of CD40L-bearing virions (Fig. 5C).
FIG. 5.

CD40L-bearing virions, but not viruses lacking host CD40L, induce a dose-dependent nuclear translocation of NF-κB. (A) B lymphocytes purified from human tonsils were either left untreated or treated for 30 min at 37°C with medium alone, the listed virus stocks (30 ng of p24 per 1 × 105 B cells), Mock/CD40L, or phorbol myristate acetate-ionomycin (used as a positive control). The nuclear extracts were incubated with a labeled NF-κB probe, and the complexes were resolved on a native 4% polyacrylamide gel. Competitions were performed with a 100-fold molar excess of either specific (i.e., NF-κB) (S) or nonspecific (Oct 2A) (NS) oligonucleotides. (B) For the supershift assays, the reactions were also conducted in the absence or presence of antibodies specific for p50 and p65. The arrows on the right indicate the positions of the specific DNA-protein complexes. (C) In some experiments, B lymphocytes purified from human tonsils were either left untreated or treated for 60 min at 37°C with medium alone, the listed virus stocks (at 10 or 30 ng of p24 per 1 × 105 B cells), Mock/CD40L, or phorbol myristate acetate-ionomycin. Competition assays were performed with a 100-fold molar excess of either specific (S) (i.e., NF-κB) or nonspecific (NS) (i.e., Oct 2A) oligonucleotides. The signal band intensities are shown at the bottom of the graph and were quantified by laser densitometry scanning. The data shown are representative of three independent experiments.
CD40L enhances HIV-1 binding to B lymphocytes and augments infection of autologous CD4+ T cells in trans.
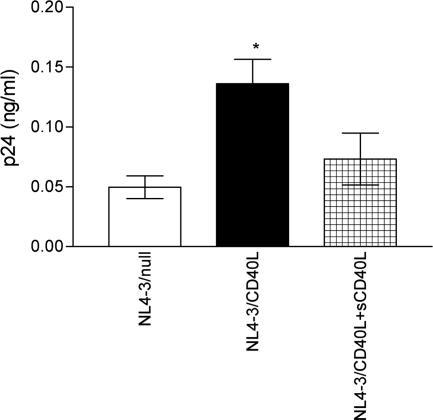
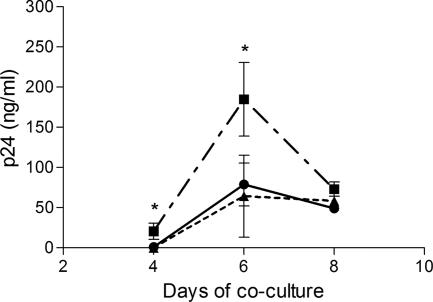
Finally, we tested whether the additional CD40L/CD40 interaction could translate into a more-efficient capture and transfer by B cells of HIV-1 to autologous CD4+ T lymphocytes. As illustrated in Fig. 6, the attachment of CD40L-bearing NL4-3 particles onto B lymphocytes was increased compared to the attachment of isogenic virions devoid of this cell surface constituent and this enhanced virus adsorption was abrogated upon treatment with sCD40L. Moreover, a more-significant HIV-1 transfer was seen when using CD40L-bearing virions than with progeny viruses lacking host-derived CD40L (Fig. 7). The more-efficient transmission of CD40L-bearing virions was abrogated upon the addition of sCD40L.
FIG. 6.
HIV-1 attachment to B lymphocytes is increased by interactions between virus-anchored host CD40L and cell surface CD40. B lymphocytes purified from human tonsils were incubated for 1 h at 37°C with NL4-3/null or NL4-3/CD40L virions in either the absence or presence of sCD40L. Next, the virus-cell mixture was extensively washed, lysed, and tested for the p24 content. The data shown represent the means ± standard deviations of triplicate samples and are representative of three independent experiments. The asterisk indicates statistically significant differences (P < 0.05) between the following matched pairs: NL4-3/CD40L versus NL4-3/null and NL4-3/CD40L versus NL4-3/CD40L+sCD40L.
FIG. 7.
B-cell mediated transmission of HIV-1 to autologous CD4+ T lymphocytes is increased with CD40L-bearing virions. B lymphocytes purified from human tonsils were incubated for 1 h at 37°C with NL4-3/null (triangles), NL4-3/CD40L (squares), or NL4-3/CD40L used in combination with sCD40L (10 μg/ml) (circles). Thereafter, the virus-cell mixtures were extensively washed and cocultured with PHA/IL-2-treated autologous CD4+ T cells. The supernatants were harvested to estimate the p24 content by ELISA at 4, 6, and 8 days following the initiation of the coculture. The data shown represent the means ± standard deviations of triplicate samples and are representative of three independent experiments. The asterisks indicate statistically significant differences (i.e., P < 0.05) between the following matched pair: NL4-3/CD40L versus NL4-3/null.
Virus entry assays performed with purified B lymphocytes that were first exposed to the tested virus preparations and next treated with trypsin to remove uninternalized virions indicated that virions do not enter such cells and remain on the cell surface (data not shown). In addition, p24 levels measured until 14 days following acute infection of purified B cells with viruses either lacking or bearing host-derived CD40L remained below the detection limit of our p24 test (data not shown). This indicates that virus replication in the B-cell-T-cell cocultures was not a result of direct, cis infection of B cells by HIV-1. Comparable findings were made when transfer studies were carried out with the R5-tropic NL4-3balenv variant, although the levels of HIV-1 p24 were lower than with the X4-tropic NL4-3 strain (data not shown). Taken together, our data indicate that the physical presence of host-derived CD40L on the exterior of virions resulted in a more-efficient trans infection of autologous CD4+ T lymphocytes when using B cells as transmitters.
DISCUSSION
While working on the mechanistic aspects of the process of incorporation of host molecules within emerging HIV-1 particles, we recently provided evidence that HIV-1 acquires HLA-DR independently of Env (43). Here, we provide evidence that the virus-encoded Env glycoproteins are also not required for the incorporation of host-derived CD40L within HIV-1 particles. Although the exact process by which CD40L is acquired by HIV-1 remains to be more fully elucidated, we demonstrate that CD40L-bearing virions can promote homotypic aggregation of B lymphocytes isolated from human tonsils and induce secretion of IgG and IL-6 from such cells. Interestingly, none of the observed virus-anchored host CD40L-mediated effects was attributable to HIV-1 Env. At the intracellular level, a rapid nuclear translocation of NF-κB was detected following the exposure of tonsillar B cells to virions harboring host CD40L. In addition, we show that virus-associated CD40L increases the attachment of viral particles to B lymphocytes and facilitates their eventual transfer to autologous CD4+ T lymphocytes, thus enhancing virus propagation. In the past, a number of studies have highlighted the potential role that such host molecules might have in the pathogenesis of AIDS (reviewed in reference 35). However, this is, to the best of our knowledge, the first study connecting some of the numerous B-cell abnormalities seen in infected patients and a virus-anchored host cell membrane component.
Our results also indicate that CD40L, once embedded within HIV-1, triggers IgG synthesis in primary B lymphocytes. The amounts of IgG we observed varied among donors, which is perfectly in line with the results of a previous study (22). It can be proposed, based on the current observations and the previously reported presence of host CD40L in clinical isolates of HIV-1 produced in physiologic cell systems (44), that the hypergammaglobulinemia documented in HIV-1-infected patients might be linked to some extent to the insertion of host-derived CD40L within HIV-1. Interestingly, the hypergammaglobulinemia seen in C57BL/6 mice infected with the murine retrovirus LP-BM5 (also called the murine AIDS model) was alleviated after treatment with anti-CD40L monoclonal antibodies (28). Moreover, our data indicate that Env-deficient HIV-1 particles that bear CD40L are also efficient at inducing IgG production, an observation that contrasts with the previously reported gp120-mediated IgG production by B lymphocytes (30, 61). Differences in the experimental methodologies may account for the apparent discrepancy (e.g., purified recombinant gp120 versus fully competent HIV-1 particles).
In addition to the hypergammaglobulinemia syndrome, HIV-1-infected patients display a more-important production of some cytokines, including IL-1, IFN-γ, TNF-α, IL-2, IL-6, and IL-12, than uninfected individuals (3, 32, 61). More particularly, an increase in IL-6 secretion at the early stages of infection (8, 13) and its spontaneous production by B lymphocytes from HIV-1-infected individuals (32) were previously described. Thus, the augmentation in IL-6 synthesis that we observed in B lymphocyte cultures exposed to CD40L-bearing HIV-1 particles represents further evidence of the possible link that can be made between virus-anchored host CD40L and the B-cell dysfunction seen in advanced HIV-1 diseases. A previous study performed by Boue and colleagues has demonstrated that HIV-1 alone does not induce IL-6 secretion by B cells isolated from healthy donors (10). The virus preparations used in that work were produced in chronically infected CEM, an established T-cell line that does not constitutively express CD40L. Thus, the inability of CEM-derived HIV-1 particles to drive IL-6 production in B cells might be due to the absence of CD40L in the viral particle. It is noteworthy that IgG production seems to be dependent on IL-6, since a reduction in IgG occurs after treatment of HIV-1-infected individuals with an anti-IL-6 monoclonal antibody (42).
The homotypic B-cell adhesion that is seen in the presence of IL-4 and CD40L-bearing HIV-1 particles is in line with a report showing the formation of homotypic aggregates upon costimulation of the B-cell receptor and CD40 on the surface of B lymphocytes from HIV-1-infected persons (17). Whether the cellular aggregation we report here depends on the LFA-1/ICAM-1 interaction after the stimulation of human B lymphocytes with the monoclonal antibody G28.5, as described by Barrett and coworkers (6), is, however, unknown.
The main transcription factor triggered by CD40 is NF-κB (54, 55, 67, 72), which binds to the promoter region of many B-lymphocyte genes, including those involved in the synthesis of IgG (34) and the expression of IL-6 (40, 65) and ICAM-1 (71). It has been previously reported that CD40L and IL-4 act in a concerted manner to drive the human Ig heavy chain S gamma 3 region through the induction of p50/p65, p50/c-Rel, and p50/p50 NF-κB complexes and STAT-6 (62). We found that CD40L-bearing virions can strongly induce NF-κB in purified B lymphocytes. Moreover, the NF-κB activation that is seen following contact with CD40L-bearing virions is comparable to the activation resulting from the stimulation of resting tonsillar B cells with an anti-CD40 antibody (7). Our data also indicate that exposure of B cells to CD40L-bearing HIV-1 particles leads to the formation of complexes that include p50/p50 homodimers and p50/p65 heterodimers, as indicated by the supershifts seen in the EMSA results. These observations are in agreement with the results of previous studies performed with B cells that analyzed the signal transduction events triggered by cross-linking of the CD40 receptor (7, 59). It can thus be hypothesized that the detected IgG and IL-6 production is associated with the induction of NF-κB that results from the attachment of CD40L-bearing virions onto the surface of CD40-positive B lymphocytes.
Concerning the phenomenon of HIV-1 transmission within an infected individual, it is now postulated that viral particles bound to cells not considered to be natural cellular reservoirs are very efficiently transferred following contact with more-susceptible target cells, such as CD4+ T cells. Interestingly, our group has demonstrated recently that in some cases, HIV-1 transmission is enhanced upon the incorporation of host-derived ICAM-1 (11). Here, we report another situation where virus transfer from a cell type not considered to be a natural reservoir of HIV-1 (namely, B lymphocytes) is augmented by a molecule of cellular origin incorporated within HIV-1 (i.e., CD40L). Of particular interest, we used B lymphocytes and CD4+ T cells that were isolated from human tonsils, a lymphoid tissue where these lymphocytes normally interact with each other. The physiological significance of the higher transmission of CD40L-bearing virions is confirmed by the idea that CD40 is expressed on B cells at all stages of development (reviewed in reference 68). Thus, the vast majority of B cells have the potential to capture HIV-1 particles that bear host-derived CD40L and transmit such viruses to CD4+ T cells once they are located in lymphoid tissues. In support of this hypothetical working model, Moir and coworkers described a model of transmission of B-cell-associated HIV-1 from chronically infected patients to activated CD4+ T lymphocytes from uninfected donors through an interaction between CD21 on B cells and complement breakdown product C3 attached to the surface of HIV-1 immune complexes (49).
In conclusion, our data indicate that virus-anchored host CD40L molecules are still functional once located on virions and can account for part of the described B-cell perturbation associated with HIV-1 infection. Our results thus suggest that the host-derived cell surface protein CD40L, once incorporated within emerging HIV-1 particles, might play a previously unrecognized role in some of the demonstrated B-cell deficiencies seen in HIV-1-infected patients, such as aberrant activation.
Acknowledgments
We thank Maurice Dufour for flow cytometry analyses and Sylvie Méthot for editorial assistance.
This study was made possible through a grant (HOP-14438) to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program. G.M. and J.R. each hold a CIHR doctoral award, C.G. holds a CIHR fellowship, and M.J.T. is the recipient of a senior Canada research chair in human immunoretrovirology.
Footnotes
Published ahead of print on 28 March 2007.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agresti, A., and D. Vercelli. 2002. c-Rel is a selective activator of a novel IL-4/CD40 responsive element in the human Ig gamma4 germline promoter. Mol. Immunol. 38:849-859. [DOI] [PubMed] [Google Scholar]
- 3.Alonso, K., P. Pontiggia, R. Medenica, and S. Rizzo. 1997. Cytokine patterns in adults with AIDS. Immunol. Investig. 26:341-350. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo, A., M. Farrington, D. Hollenbaugh, X. Li, A. Milatovich, S. Nonoyama, J. Bajorath, L. S. Grosmaire, R. Stenkamp, M. Neubauer, et al. 1993. The CD40 ligand, gp39, is defective in activated T cells from patients with X-linked hyper-IgM syndrome. Cell 72:291-300. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Bazan, D. Blanchard, F. Briere, J. P. Galizzi, C. van Kooten, Y. J. Liu, F. Rousset, and S. Saeland. 1994. The CD40 antigen and its ligand. Annu. Rev. Immunol. 12:881-922. [DOI] [PubMed] [Google Scholar]
- 6.Barrett, T. B., G. Shu, and E. A. Clark. 1991. CD40 signaling activates CD11a/CD18 (LFA-1)-mediated adhesion in B cells. J. Immunol. 146:1722-1729. [PubMed] [Google Scholar]
- 7.Berberich, I., G. L. Shu, and E. A. Clark. 1994. Cross-linking CD40 on B cells rapidly activates nuclear factor-kappa B. J. Immunol. 153:4357-4366. [PubMed] [Google Scholar]
- 8.Birx, D. L., R. R. Redfield, K. Tencer, A. Fowler, D. S. Burke, and G. Tosato. 1990. Induction of interleukin-6 during human immunodeficiency virus infection. Blood 76:2303-2310. [PubMed] [Google Scholar]
- 9.Bishop, G. A., and B. S. Hostager. 2003. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 14:297-309. [DOI] [PubMed] [Google Scholar]
- 10.Boue, F., C. Wallon, C. Goujard, F. Barre-Sinoussi, P. Galanaud, and J. F. Delfraissy. 1992. HIV induces IL-6 production by human B lymphocytes. Role of IL-4. J. Immunol. 148:3761-3767. [PubMed] [Google Scholar]
- 11.Bounou, S., J. F. Giguere, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 18:1294-1296. [DOI] [PubMed] [Google Scholar]
- 12.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breen, E. C., A. R. Rezai, K. Nakajima, G. N. Beall, R. T. Mitsuyasu, T. Hirano, T. Kishimoto, and O. Martinez-Maza. 1990. Infection with HIV is associated with elevated IL-6 levels and production. J. Immunol. 144:480-484. [PubMed] [Google Scholar]
- 14.Briere, F., C. Servet-Delprat, J. M. Bridon, J. M. Saint-Remy, and J. Banchereau. 1994. Human interleukin 10 induces naive surface immunoglobulin D+ (sIgD+) B cells to secrete IgG1 and IgG3. J. Exp. Med. 179:757-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark, E. A., T. C. Yip, J. A. Ledbetter, H. Yukawa, H. Kikutani, T. Kishimoto, and M. H. Ng. 1988. CDw40 and BLCa-specific monoclonal antibodies detect two distinct molecules which transmit progression signals to human B lymphocytes. Eur. J. Immunol. 18:451-457. [DOI] [PubMed] [Google Scholar]
- 16.Cognasse, F., S. Acquart, L. Beniguel, O. Sabido, P. Chavarin, C. Genin, and O. Garraud. 2005. Differential production of immunoglobulin classes and subclasses by mucosal-type human B-lymphocytes exposed in vitro to CpG oligodeoxynucleotides. Clin. Chem. Lab. Med. 43:22-31. [DOI] [PubMed] [Google Scholar]
- 17.Conge, A. M., K. Tarte, J. Reynes, M. Segondy, J. Gerfaux, M. Zembala, and J. P. Vendrell. 1998. Impairment of B-lymphocyte differentiation induced by dual triggering of the B-cell antigen receptor and CD40 in advanced HIV-1-disease. AIDS 12:1437-1449. [DOI] [PubMed] [Google Scholar]
- 18.De Boer, R. J., H. Mohri, D. D. Ho, and A. S. Perelson. 2003. Turnover rates of B cells, T cells, and NK cells in simian immunodeficiency virus-infected and uninfected rhesus macaques. J. Immunol. 170:2479-2487. [DOI] [PubMed] [Google Scholar]
- 19.De Milito, A., A. Nilsson, K. Titanji, R. Thorstensson, E. Reizenstein, M. Narita, S. Grutzmeier, A. Sonnerborg, and F. Chiodi. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180-2186. [DOI] [PubMed] [Google Scholar]
- 20.Dornadula, G., H. Zhang, S. Shetty, and R. J. Pomerantz. 1999. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: implications for pathogenesis and transmission. Virology 253:10-16. [DOI] [PubMed] [Google Scholar]
- 21.Fauci, A. S., A. M. Macher, D. L. Longo, H. C. Lane, A. H. Rook, H. Masur, and E. P. Gelmann. 1984. NIH conference. Acquired immunodeficiency syndrome: epidemiologic, clinical, immunologic, and therapeutic considerations. Ann. Intern. Med. 100:92-106. [DOI] [PubMed] [Google Scholar]
- 22.Fear, D. J., N. McCloskey, B. O'Connor, G. Felsenfeld, and H. J. Gould. 2004. Transcription of Ig germline genes in single human B cells and the role of cytokines in isotype determination. J. Immunol. 173:4529-4538. [DOI] [PubMed] [Google Scholar]
- 23.Fortin, J. F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foy, T. M., D. M. Shepherd, F. H. Durie, A. Aruffo, J. A. Ledbetter, and R. J. Noelle. 1993. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J. Exp. Med. 178:1567-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gascan, H., J. F. Gauchat, G. Aversa, P. Van Vlasselaer, and J. E. de Vries. 1991. Anti-CD40 monoclonal antibodies or CD4+ T cell clones and IL-4 induce IgG4 and IgE switching in purified human B cells via different signaling pathways. J. Immunol. 147:8-13. [PubMed] [Google Scholar]
- 26.Gordon, J., M. J. Millsum, G. R. Guy, and J. A. Ledbetter. 1988. Resting B lymphocytes can be triggered directly through the CDw40 (Bp50) antigen. A comparison with IL-4-mediated signaling. J. Immunol. 140:1425-1430. [PubMed] [Google Scholar]
- 27.Gould, H. J., R. L. Beavil, and D. Vercelli. 2000. IgE isotype determination: epsilon-germline gene transcription, DNA recombination and B-cell differentiation. Br. Med. Bull. 56:908-924. [DOI] [PubMed] [Google Scholar]
- 28.Green, K. A., K. M. Crassi, J. D. Laman, A. Schoneveld, R. R. Strawbridge, T. M. Foy, R. J. Noelle, and W. R. Green. 1996. Antibody to the ligand for CD40 (gp39) inhibits murine AIDS-associated splenomegaly, hypergammaglobulinemia, and immunodeficiency in disease-susceptible C57BL/6 mice. J. Virol. 70:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haswell, L. E., M. J. Glennie, and A. Al-Shamkhani. 2001. Analysis of the oligomeric requirement for signaling by CD40 using soluble multimeric forms of its ligand, CD154. Eur. J. Immunol. 31:3094-3100. [DOI] [PubMed] [Google Scholar]
- 30.He, B., X. Qiao, P. J. Klasse, A. Chiu, A. Chadburn, D. M. Knowles, J. P. Moore, and A. Cerutti. 2006. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 176:3931-3941. [DOI] [PubMed] [Google Scholar]
- 31.Kansas, G. S., and T. F. Tedder. 1991. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-1-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J. Immunol. 147:4094-4102. [PubMed] [Google Scholar]
- 32.Kehrl, J. H., P. Rieckmann, E. Kozlow, and A. S. Fauci. 1992. Lymphokine production by B cells from normal and HIV-infected individuals. Ann. N. Y. Acad. Sci. 651:220-227. [DOI] [PubMed] [Google Scholar]
- 33.Kekow, J., G. Hobusch, and W. L. Gross. 1988. Predominance of the IgG1 subclass in the hypergammaglobulinemia observed in pre-AIDS and AIDS. Cancer Detect. Prev. 12:211-216. [PubMed] [Google Scholar]
- 34.Kenter, A. L., R. Wuerffel, R. Sen, C. E. Jamieson, and G. V. Merkulov. 1993. Switch recombination breakpoints occur at nonrandom positions in the S gamma tandem repeat. J. Immunol. 151:4718-4731. [PubMed] [Google Scholar]
- 35.Kolegraff, K., P. Bostik, and A. A. Ansari. 2006. Characterization and role of lentivirus-associated host proteins. Exp. Biol. Med. (Maywood) 231:252-263. [DOI] [PubMed] [Google Scholar]
- 36.Kornbluth, R. S., K. Kee, and D. D. Richman. 1998. CD40 ligand (CD154) stimulation of macrophages to produce HIV-1-suppressive beta-chemokines. Proc. Natl. Acad. Sci. USA 95:5205-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kroczek, R. A., D. Graf, D. Brugnoni, S. Giliani, U. Korthuer, A. Ugazio, G. Senger, H. W. Mages, A. Villa, and L. D. Notarangelo. 1994. Defective expression of CD40 ligand on T cells causes “X-linked immunodeficiency with hyper-IgM (HIGM1).” Immunol. Rev. 138:39-59. [DOI] [PubMed] [Google Scholar]
- 38.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 39.Lederman, S., M. J. Yellin, G. Inghirami, J. J. Lee, D. M. Knowles, and L. Chess. 1992. Molecular interactions mediating T-B lymphocyte collaboration in human lymphoid follicles. Roles of T cell-B-cell-activating molecule (5c8 antigen) and CD40 in contact-dependent help. J. Immunol. 149:3817-3826. [PubMed] [Google Scholar]
- 40.Libermann, T. A., and D. Baltimore. 1990. Activation of interleukin-6 gene expression through the NF-κB transcription factor. Mol. Cell. Biol. 10:2327-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malaspina, A., S. Moir, S. Kottilil, C. W. Hallahan, L. A. Ehler, S. Liu, M. A. Planta, T. W. Chun, and A. S. Fauci. 2003. Deleterious effect of HIV-1 plasma viremia on B cell costimulatory function. J. Immunol. 170:5965-5972. [DOI] [PubMed] [Google Scholar]
- 42.Marfaing-Koka, A., J. T. Aubin, L. Grangeot-Keros, A. Portier, C. Benattar, D. Merrien, H. Agut, P. Aucouturier, B. Autran, J. Wijdenes, et al. 1996. In vivo role of IL-6 on the viral load and on immunological abnormalities of HIV-infected patients. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 11:59-68. [DOI] [PubMed] [Google Scholar]
- 43.Martin, G., Y. Beausejour, J. Thibodeau, and M. J. Tremblay. 2005. Envelope glycoproteins are dispensable for insertion of host HLA-DR molecules within nascent human immunodeficiency virus type 1 particles. Virology 335:286-290. [DOI] [PubMed] [Google Scholar]
- 44.Martin, G., and M. J. Tremblay. 2004. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin. Immunol. 111:275-285. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Maza, O., and E. C. Breen. 2002. B-cell activation and lymphoma in patients with HIV. Curr. Opin. Oncol. 14:528-532. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Maza, O., E. Crabb, R. T. Mitsuyasu, J. L. Fahey, and J. V. Giorgi. 1987. Infection with the human immunodeficiency virus (HIV) is associated with an in vivo increase in B lymphocyte activation and immaturity. J. Immunol. 138:3720-3724. [PubMed] [Google Scholar]
- 47.Miedema, F., A. J. Petit, F. G. Terpstra, J. K. Schattenkerk, F. de Wolf, B. J. Al, M. Roos, J. M. Lange, S. A. Danner, J. Goudsmit, et al. 1988. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J. Clin. Investig. 82:1908-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizuma, H., S. Zolla-Pazner, S. Litwin, W. el-Sadr, S. Sharpe, B. Zehr, S. Weiss, W. C. Saxinger, and M. Marmor. 1987. Serum IgD elevation is an early marker of B cell activation during infection with the human immunodeficiency viruses. Clin. Exp. Immunol. 68:5-14. [PMC free article] [PubMed] [Google Scholar]
- 49.Moir, S., A. Malaspina, Y. Li, T. W. Chun, T. Lowe, J. Adelsberger, M. Baseler, L. A. Ehler, S. Liu, R. T. Davey, Jr., J. A. Mican, and A. S. Fauci. 2000. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J. Exp. Med. 192:637-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moir, S., A. Malaspina, K. M. Ogwaro, E. T. Donoghue, C. W. Hallahan, L. A. Ehler, S. Liu, J. Adelsberger, R. Lapointe, P. Hwu, M. Baseler, J. M. Orenstein, T. W. Chun, J. A. Mican, and A. S. Fauci. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. USA 98:10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris, L., J. M. Binley, B. A. Clas, S. Bonhoeffer, T. P. Astill, R. Kost, A. Hurley, Y. Cao, M. Markowitz, D. D. Ho, and J. P. Moore. 1998. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J. Exp. Med. 188:233-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholson, J. K., J. S. McDougal, T. J. Spira, G. D. Cross, B. M. Jones, and E. L. Reinherz. 1984. Immunoregulatory subsets of the T helper and T suppressor cell populations in homosexual men with chronic unexplained lymphadenopathy. J. Clin. Investig. 73:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ott, D. E., E. N. Chertova, L. K. Busch, L. V. Coren, T. D. Gagliardi, and D. G. Johnson. 1999. Mutational analysis of the hydrophobic tail of the human immunodeficiency virus type 1 p6Gag protein produces a mutant that fails to package its envelope protein. J. Virol. 73:19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pahl, H. L. 1999. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18:6853-6866. [DOI] [PubMed] [Google Scholar]
- 55.Pullen, S. S., T. T. Dang, J. J. Crute, and M. R. Kehry. 1999. CD40 signaling through tumor necrosis factor receptor-associated factors (TRAFs). Binding site specificity and activation of downstream pathways by distinct TRAFs. J. Biol. Chem. 274:14246-14254. [DOI] [PubMed] [Google Scholar]
- 56.Pullen, S. S., M. E. Labadia, R. H. Ingraham, S. M. McWhirter, D. S. Everdeen, T. Alber, J. J. Crute, and M. R. Kehry. 1999. High-affinity interactions of tumor necrosis factor receptor-associated factors (TRAFs) and CD40 require TRAF trimerization and CD40 multimerization. Biochemistry 38:10168-10177. [DOI] [PubMed] [Google Scholar]
- 57.Punnonen, J., M. K. Viljanen, E. Eerola, and J. Eskola. 1988. The tonsils are superior to the peripheral blood as sources of mononuclear cells for B-cell purification. J. Clin. Lab. Immunol. 27:29-33. [PubMed] [Google Scholar]
- 58.Reimer, C. B., C. M. Black, R. C. Holman, T. W. Wells, R. M. Ramirez, J. A. Sa-Ferreira, J. K. Nicholson, and J. S. McDougal. 1988. Hypergammaglobulinemia associated with human immunodeficiency virus infection. Monogr. Allergy 23:83-96. [PubMed] [Google Scholar]
- 59.Revy, P., C. Hivroz, G. Andreu, P. Graber, C. Martinache, A. Fischer, and A. Durandy. 1999. Activation of the Janus kinase 3-STAT5a pathway after CD40 triggering of human monocytes but not of resting B cells. J. Immunol. 163:787-793. [PubMed] [Google Scholar]
- 60.Rice, N. R., M. L. MacKichan, and A. Israel. 1992. The precursor of NF-kappa B p50 has I kappa B-like functions. Cell 71:243-253. [DOI] [PubMed] [Google Scholar]
- 61.Rieckmann, P., G. Poli, J. H. Kehrl, and A. S. Fauci. 1991. Activated B lymphocytes from human immunodeficiency virus-infected individuals induce virus expression in infected T cells and a promonocytic cell line, U1. J. Exp. Med. 173:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schaffer, A., A. Cerutti, S. Shah, H. Zan, and P. Casali. 1999. The evolutionarily conserved sequence upstream of the human Ig heavy chain S gamma 3 region is an inducible promoter: synergistic activation by CD40 ligand and IL-4 via cooperative NF-kappa B and STAT-6 binding sites. J. Immunol. 162:5327-5336. [PubMed] [Google Scholar]
- 63.Schnittman, S. M., H. C. Lane, S. E. Higgins, T. Folks, and A. S. Fauci. 1986. Direct polyclonal activation of human B lymphocytes by the acquired immune deficiency syndrome virus. Science 233:1084-1086. [DOI] [PubMed] [Google Scholar]
- 64.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts, ’ prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu, H., K. Mitomo, T. Watanabe, S. Okamoto, and K. Yamamoto. 1990. Involvement of a NF-κB-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol. Cell. Biol. 10:561-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terpstra, F. G., B. J. Al, M. T. Roos, F. De Wolf, J. Goudsmit, P. T. Schellekens, and F. Miedema. 1989. Longitudinal study of leukocyte functions in homosexual men seroconverted for HIV: rapid and persistent loss of B cell function after HIV infection. Eur. J. Immunol. 19:667-673. [DOI] [PubMed] [Google Scholar]
- 67.Tsukamoto, N., N. Kobayashi, S. Azuma, T. Yamamoto, and J. Inoue. 1999. Two differently regulated nuclear factor kappaB activation pathways triggered by the cytoplasmic tail of CD40. Proc. Natl. Acad. Sci. USA 96:1234-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Kooten, C., and J. Banchereau. 1996. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv. Immunol. 61:1-77. [DOI] [PubMed] [Google Scholar]
- 69.van Kooten, C., and J. Banchereau. 1997. Functional role of CD40 and its ligand. Int. Arch. Allergy Immunol. 113:393-399. [DOI] [PubMed] [Google Scholar]
- 70.Werneburg, B. G., S. J. Zoog, T. T. Dang, M. R. Kehry, and J. J. Crute. 2001. Molecular characterization of CD40 signaling intermediates. J. Biol. Chem. 276:43334-43342. [DOI] [PubMed] [Google Scholar]
- 71.Xia, Y. F., L. P. Liu, C. P. Zhong, and J. G. Geng. 2001. NF-kappaB activation for constitutive expression of VCAM-1 and ICAM-1 on B lymphocytes and plasma cells. Biochem. Biophys. Res. Commun. 289:851-856. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto, Y., and R. B. Gaynor. 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem. Sci. 29:72-79. [DOI] [PubMed] [Google Scholar]