Abstract
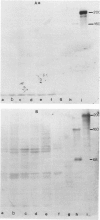
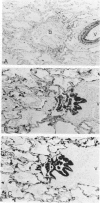
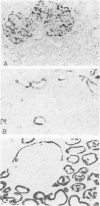
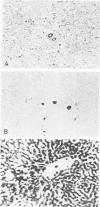
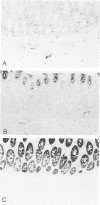
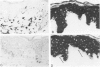
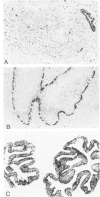
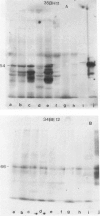
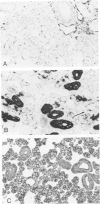
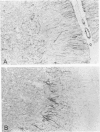
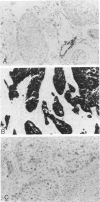
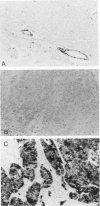
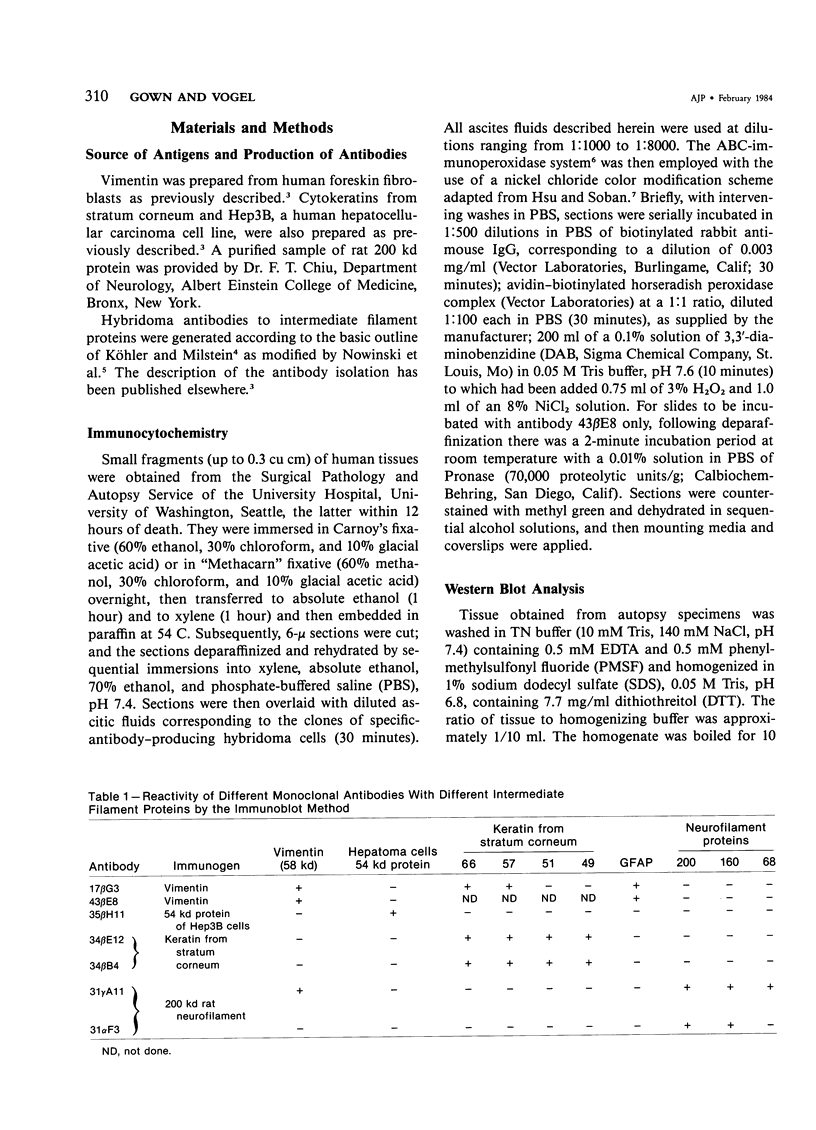
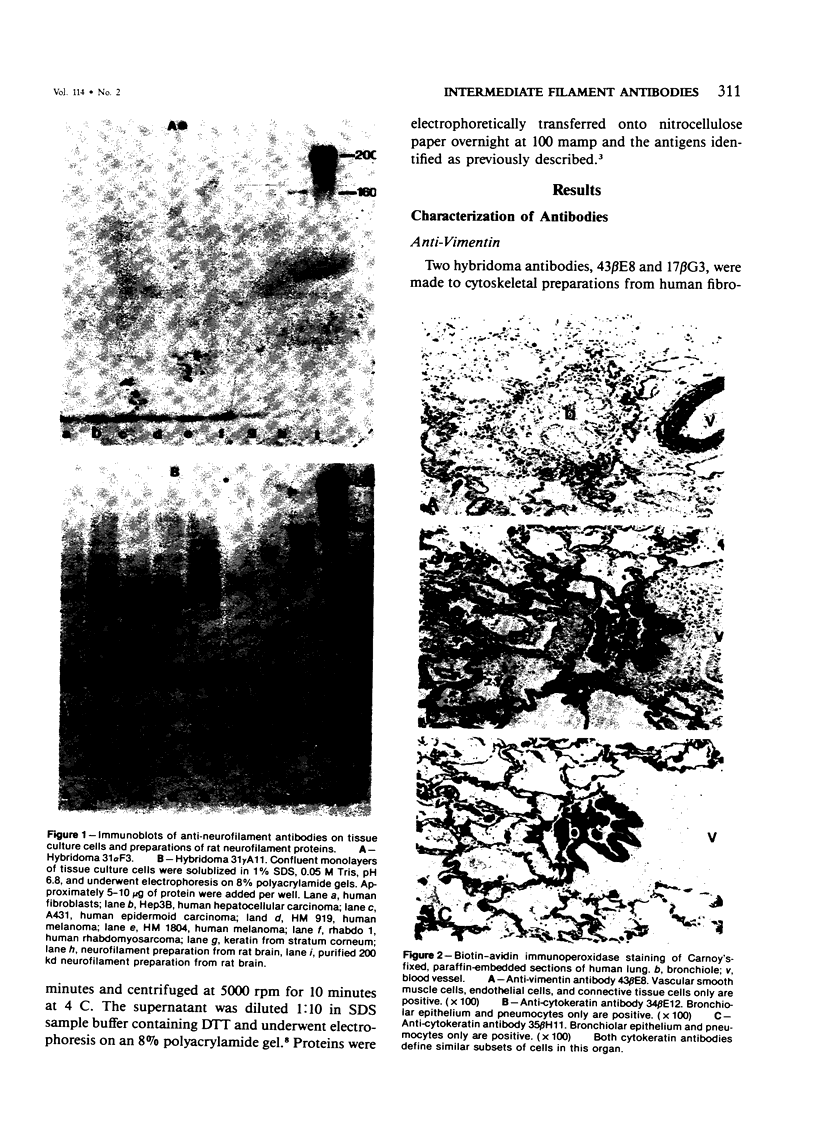
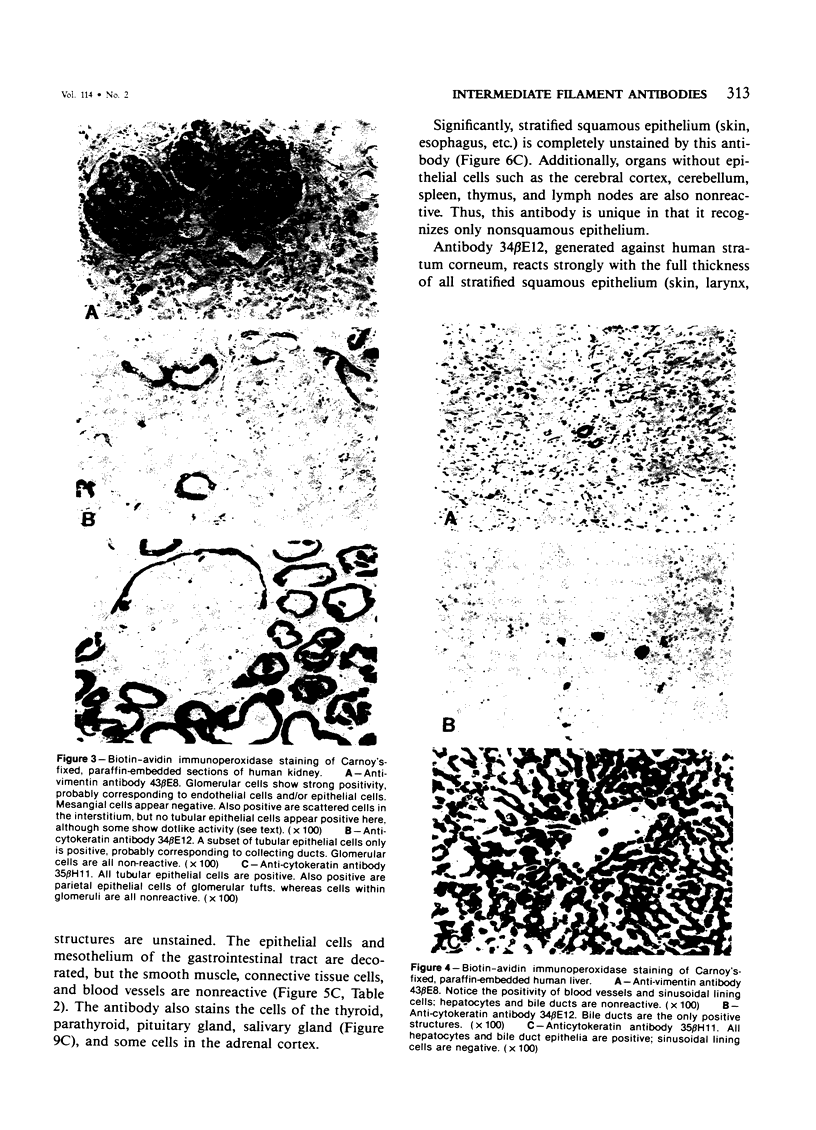
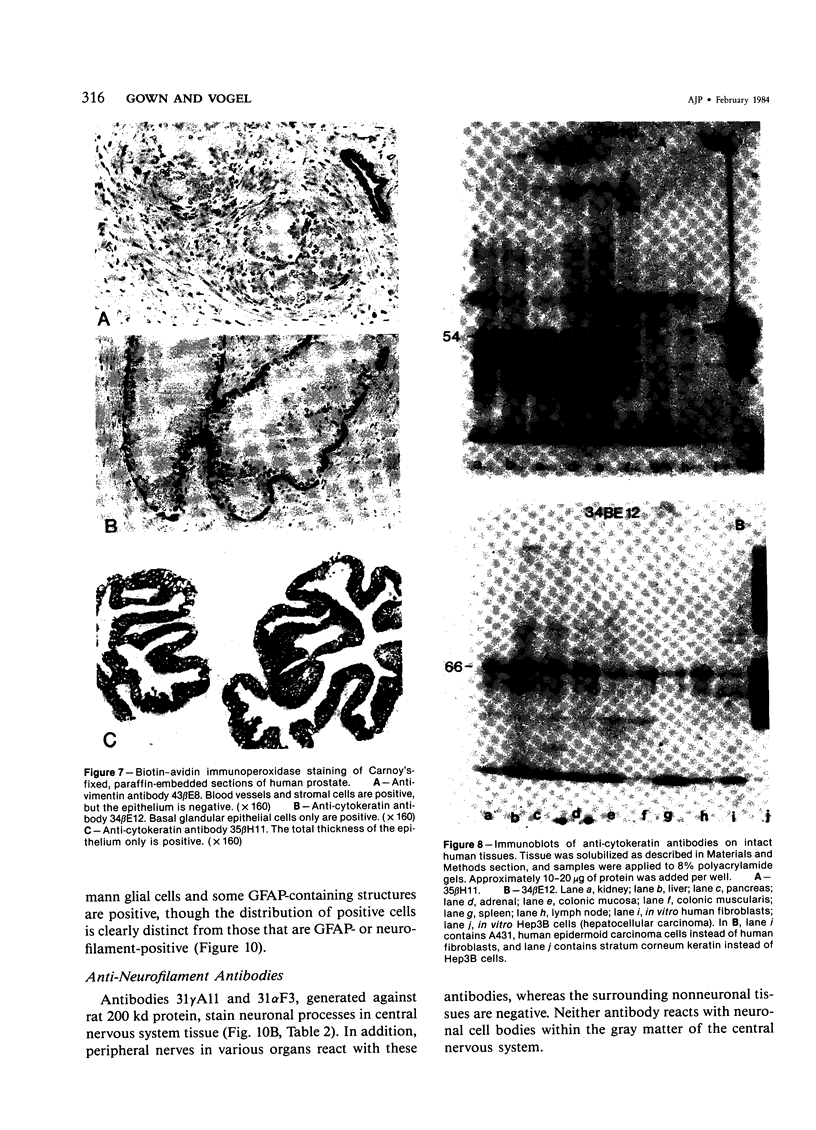
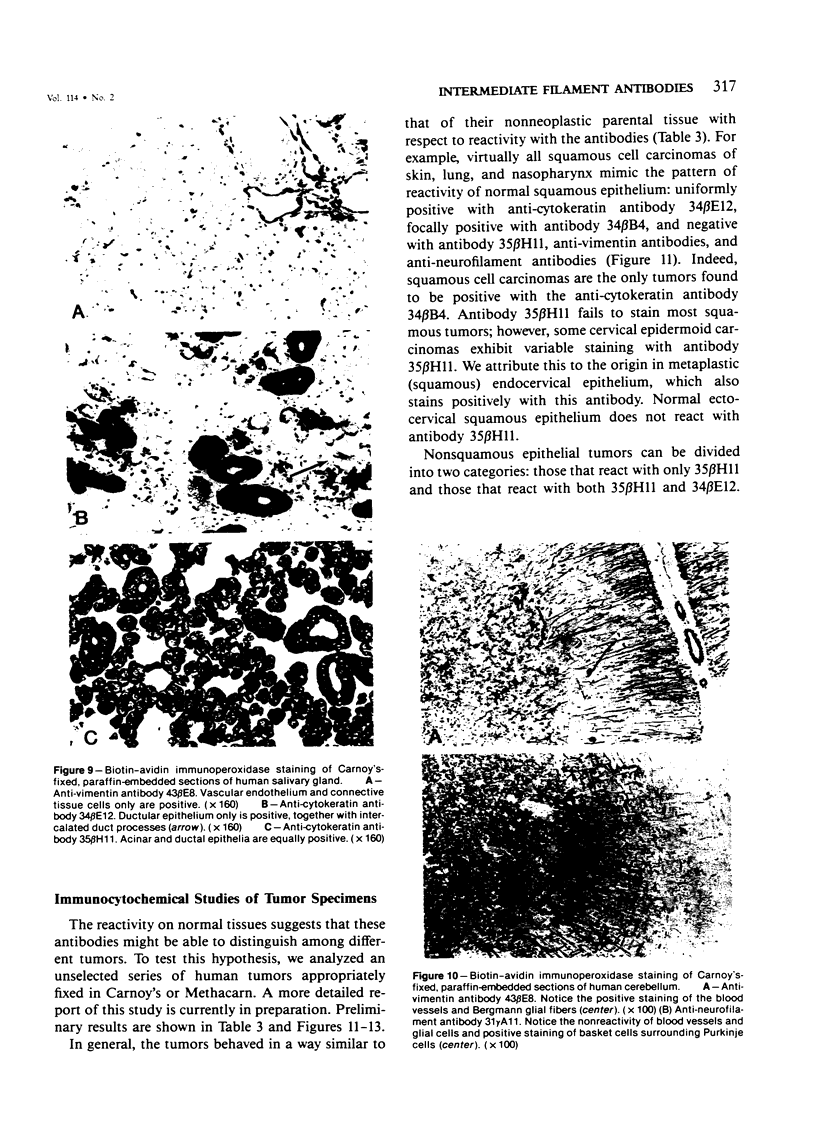
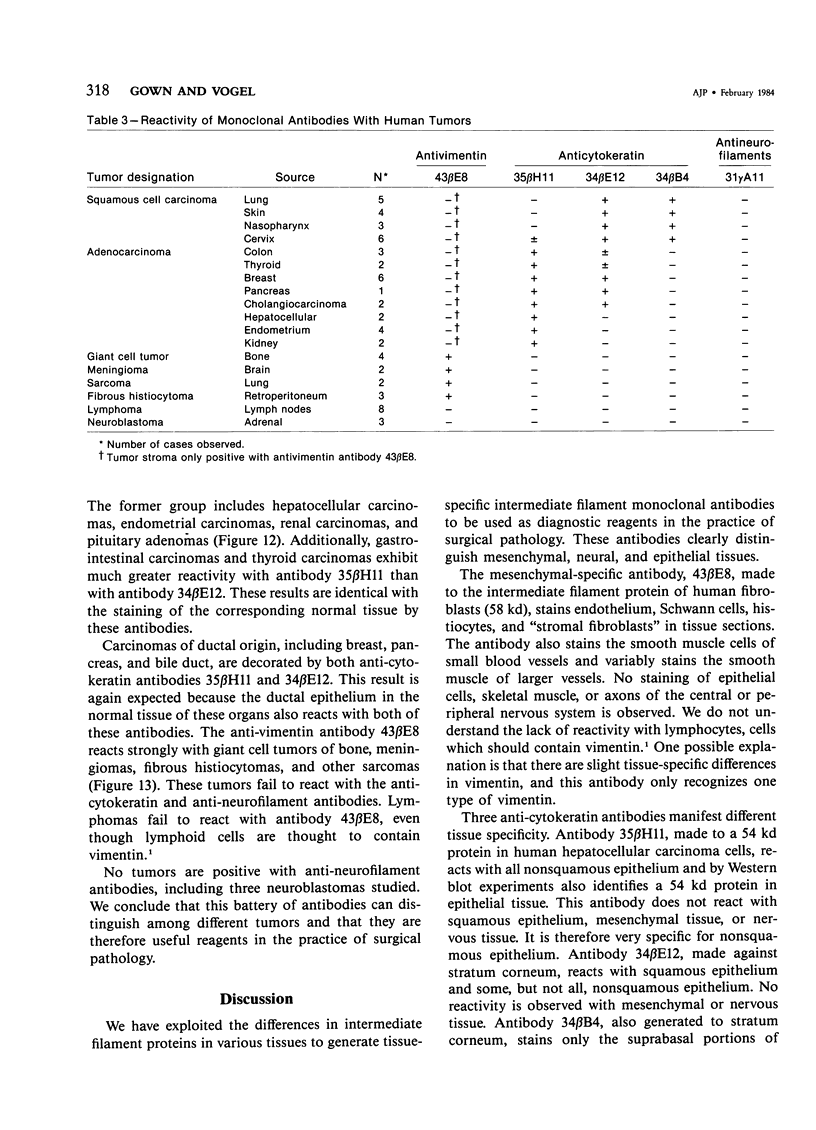
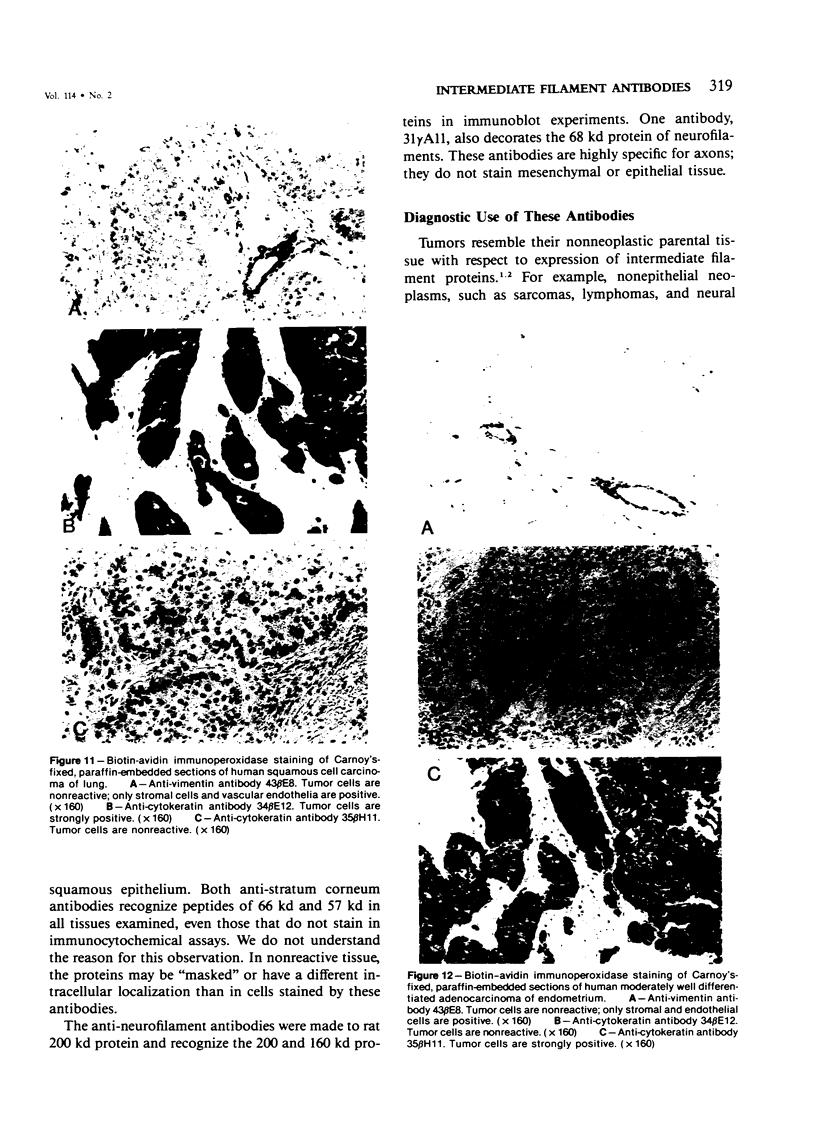
Monoclonal antibodies generated against different human intermediate filament (IF) proteins were assayed on fixed, embedded tissue by the biotin-avidin-immunoperoxidase method for evaluation of the tissue specificity of these antibodies. An antibody (43 beta E8) made to fibroblast IF protein stains mesenchymal tissue such as endothelium, histiocytes, stromal fibroblasts, and Schwann cells but does not stain epithelium, skeletal muscle, lymphocytes, or neurons. Three different anti-cytokeratin antibodies decorate epithelium in three unique patterns. One (35 beta H11) stains all nonsquamous epithelium but fails to recognize squamous epithelium. Antibody 34 beta E12 stains the full thickness of squamous epithelium and ductular epithelium but does not react with hepatocytes, pancreatic acinar cells, proximal renal tubules, or endometrial glands. Antibody 34 beta B4 stains only the suprabasal portion of squamous epithelium. None of these three anti-cytokeratin antibodies reacts with nerve or mesenchymal tissue. Two anti-neurofilament antibodies recognize only neurons, failing to react with epithelial or mesenchymal tissue. We conclude that these anti-intermediate filament antibodies can be used as tissue-specific markers. Neoplasms retain the same intermediate filament patterns as the normal parental tissue; therefore, these antibodies can be used as diagnostic aids in surgical pathology.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Schauer A., Weber K. Antibodies to different intermediate filament proteins. Cell type-specific markers on paraffin-embedded human tissues. Lab Invest. 1981 Nov;45(5):427–434. [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kapanci Y., Barazzone P., Franke W. W. Immunochemical identification of intermediate-sized filaments in human neoplastic cells. A diagnostic aid for the surgical pathologist. Am J Pathol. 1981 Sep;104(3):206–216. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Vogel A. M. Monoclonal antibodies to intermediate filament proteins of human cells: unique and cross-reacting antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):414–424. doi: 10.1083/jcb.95.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Soban E. Color modification of diaminobenzidine (DAB) precipitation by metallic ions and its application for double immunohistochemistry. J Histochem Cytochem. 1982 Oct;30(10):1079–1082. doi: 10.1177/30.10.6182185. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Puchtler H., Waldrop F. S., Meloan S. N., Terry M. S., Conner H. M. Methacarn (methanol-Carnoy) fixation. Practical and theoretical considerations. Histochemie. 1970;21(2):97–116. doi: 10.1007/BF00306176. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Banks-Schlegel S., Pinkus G. S. Immunohistochemical localization of keratin in normal human tissues. Lab Invest. 1980 Jan;42(1):91–96. [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]