Abstract
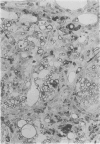
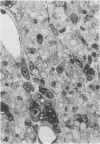
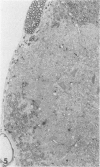
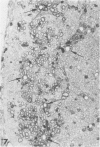
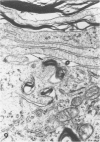
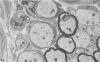
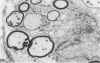
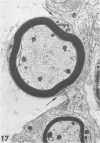
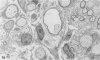
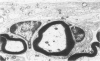
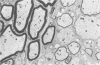
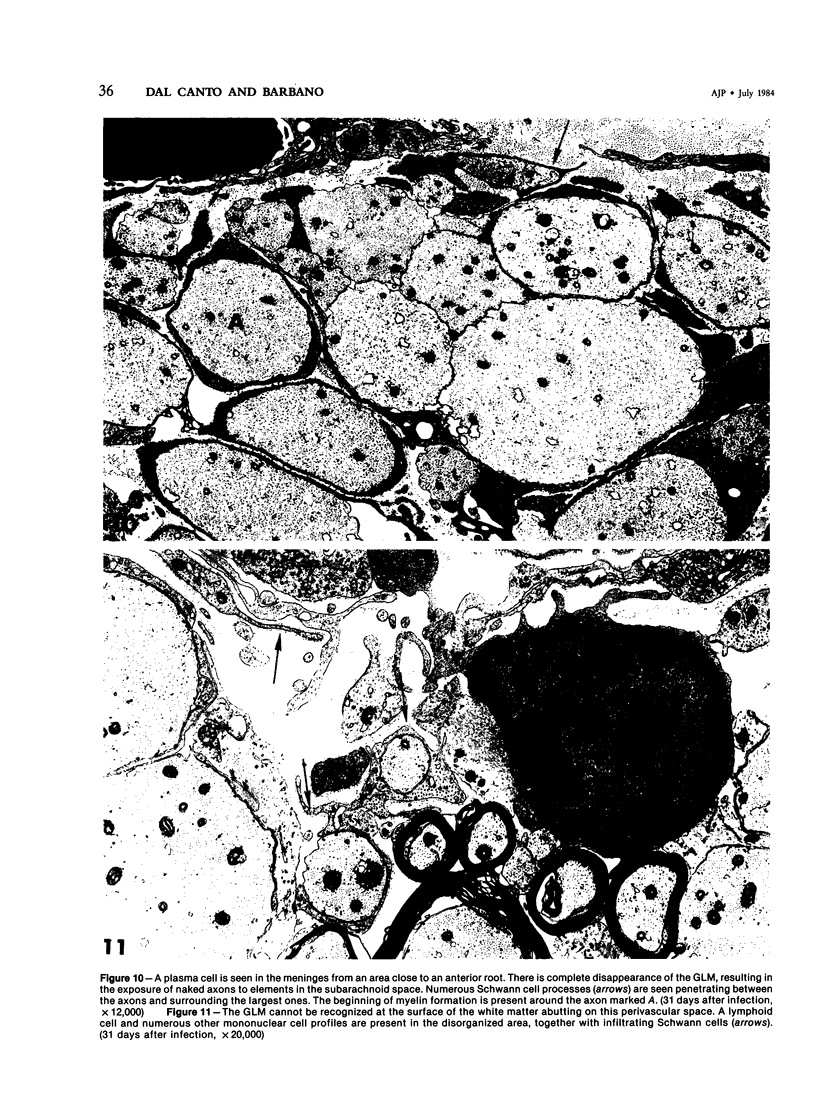
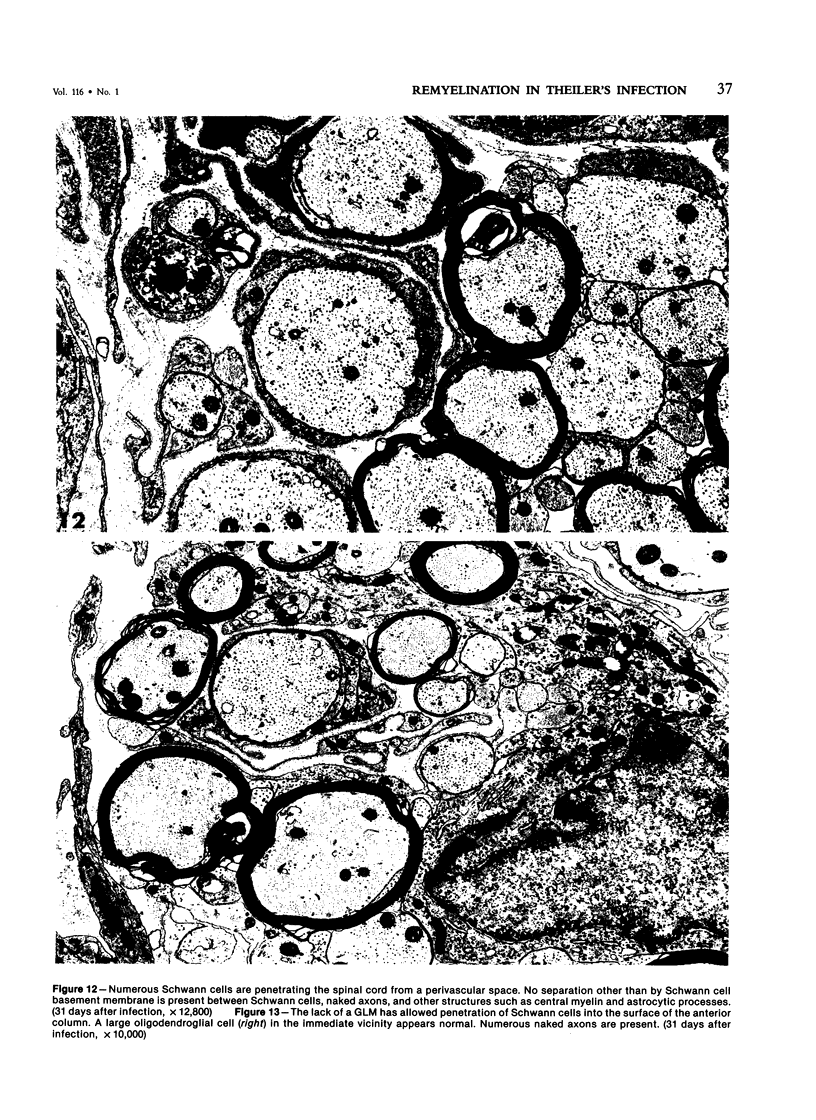
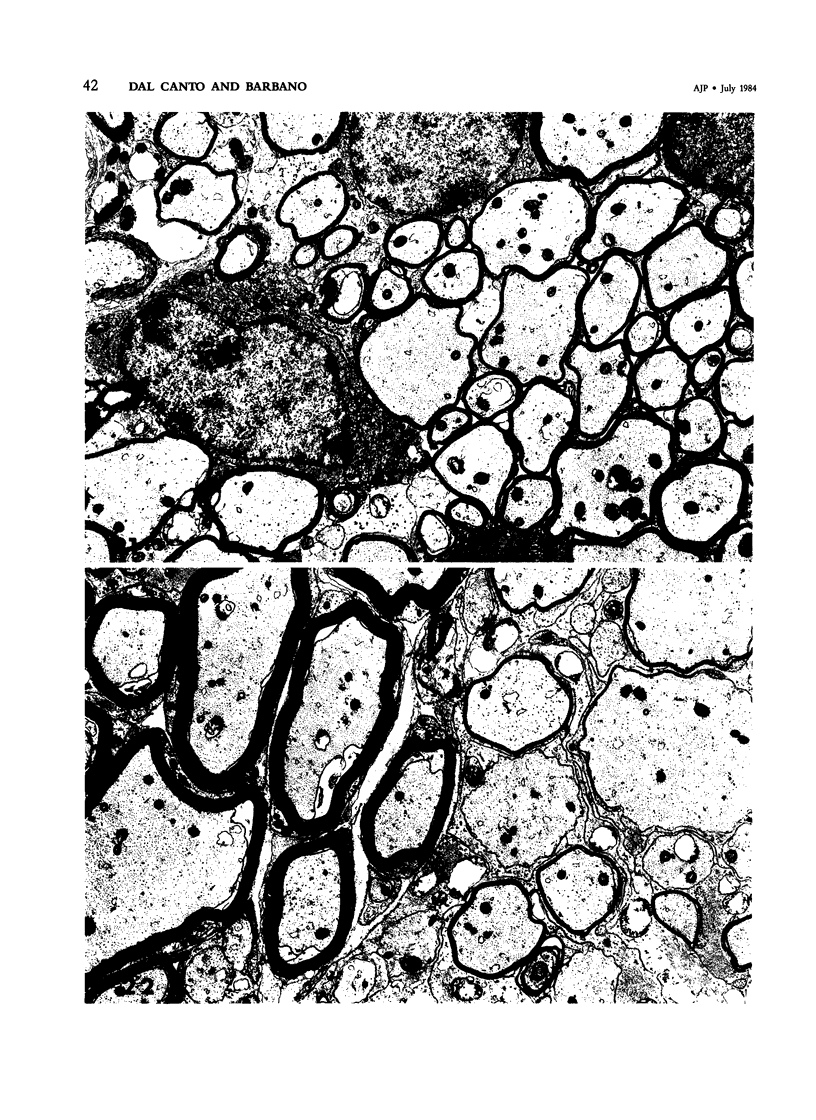
Inoculation of the cell-adapted WW strain of Theiler's virus into mice produces a chronic demyelinating infection of the central nervous system (CNS) characterized by a remitting relapsing course. During remission, extensive remyelination of spinal cord white matter is observed. Remyelination is carried out by both Schwann cells and oligodendrocytes. This paper examines the possible mechanisms of entry of Schwann cells into the CNS, their possible source in different regions of the white matter, their relations with various CNS elements, and the relative activity of these cells versus that of oligodendrocytes. Observations suggest that Schwann cells, originating from peripheral roots and from perivascular areas, migrate into white matter through gaps in the glial limiting membrane ( GLM ), probably caused by active mononuclear inflammatory cells. Schwann cell invasion and axonal contact appear to be facilitated by the presence of collagen matrix along their pathway of migration. No alterations of astrocytes in the immediate vicinity of Schwann cells were observed, and free contact between Schwann cells and different neuroglial elements was present in the initial stages of Schwann cell migration. While Schwann cells were the predominant myelinating cells in the outer white matter, oligodendrocytes were numerous and very active in the inner portions of the spinal cord column. Although oligodendrocytes produced thinner myelin than normal, in most areas essentially complete remyelination by these cells was observed. These results contrast with those of previous studies of DA infected mice in which remyelination is sporadic in the presence of unabated inflammation which continues without remission for many months after infection. It is suggested that oligodendroglial cells are quite capable of extensive remyelinating activity in this infection, provided the noxa responsible for myelin injury subsides. The host inflammatory response appears to be the most likely noxa impeding remyelination in this model.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando K. A histochemical study on the innervation of the cerebral blood vessels in bats. Cell Tissue Res. 1981;217(1):55–64. doi: 10.1007/BF00233826. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F., Patterson R. C. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975 Oct;4(5):573–585. doi: 10.1007/BF01351538. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. Remyelination by Schwann cells of axons demyelinated by intraspinal injection of 6-aminonicotinamide in the rat. J Neurocytol. 1975 Dec;4(6):745–757. doi: 10.1007/BF01181634. [DOI] [PubMed] [Google Scholar]
- Bunge R. P., Bunge M. B., Cochran M. Some factors influencing the proliferation and differentiation of myelin-forming cells. Neurology. 1978 Sep;28(9 Pt 2):59–67. doi: 10.1212/wnl.28.9_part_2.59. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
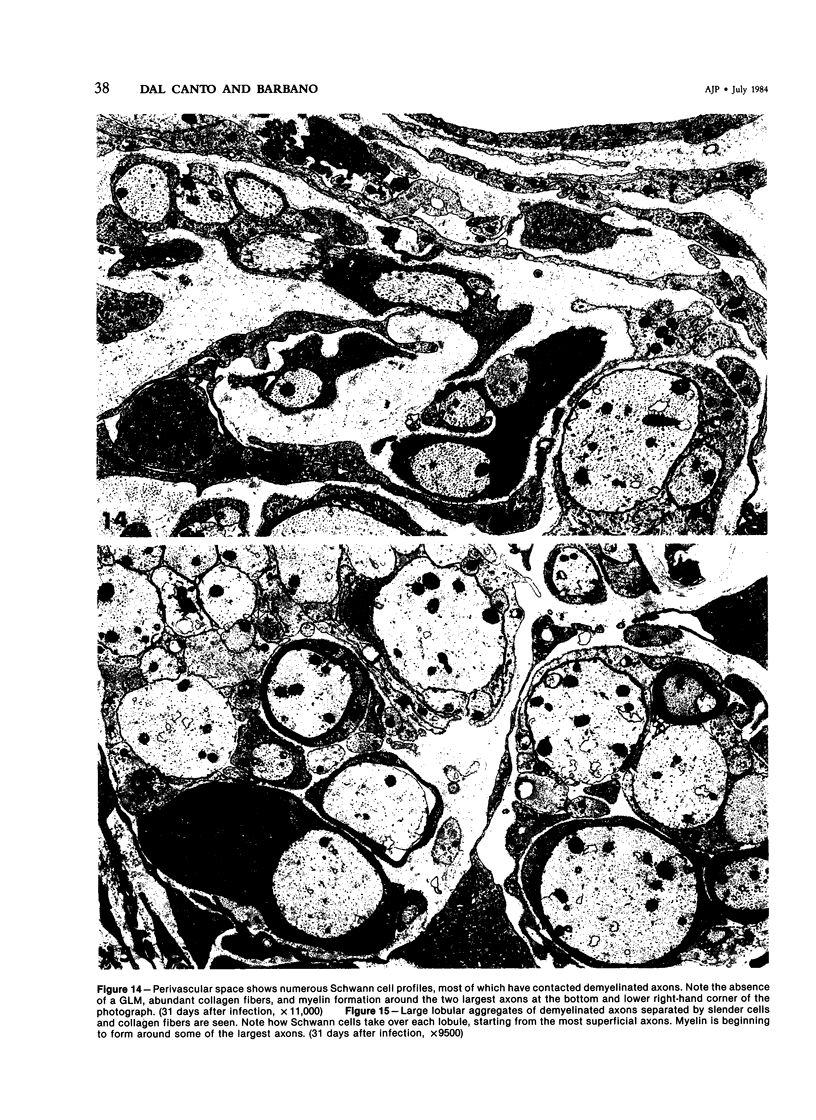
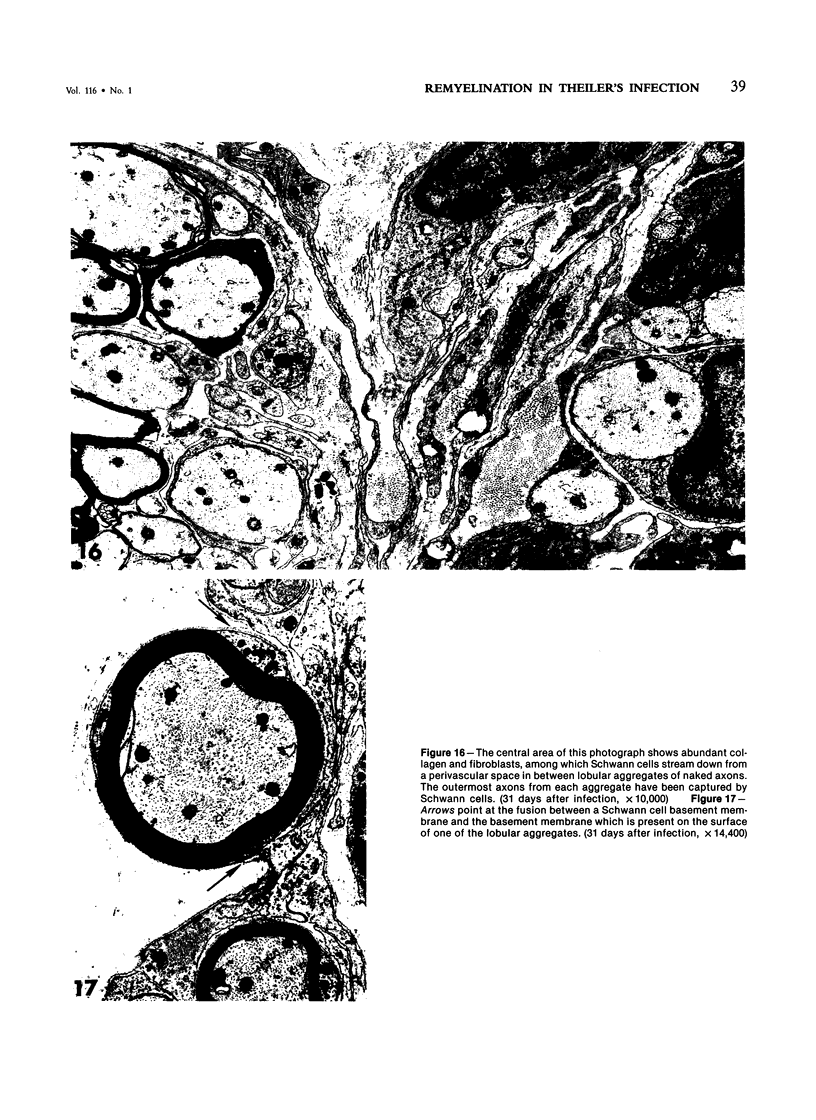
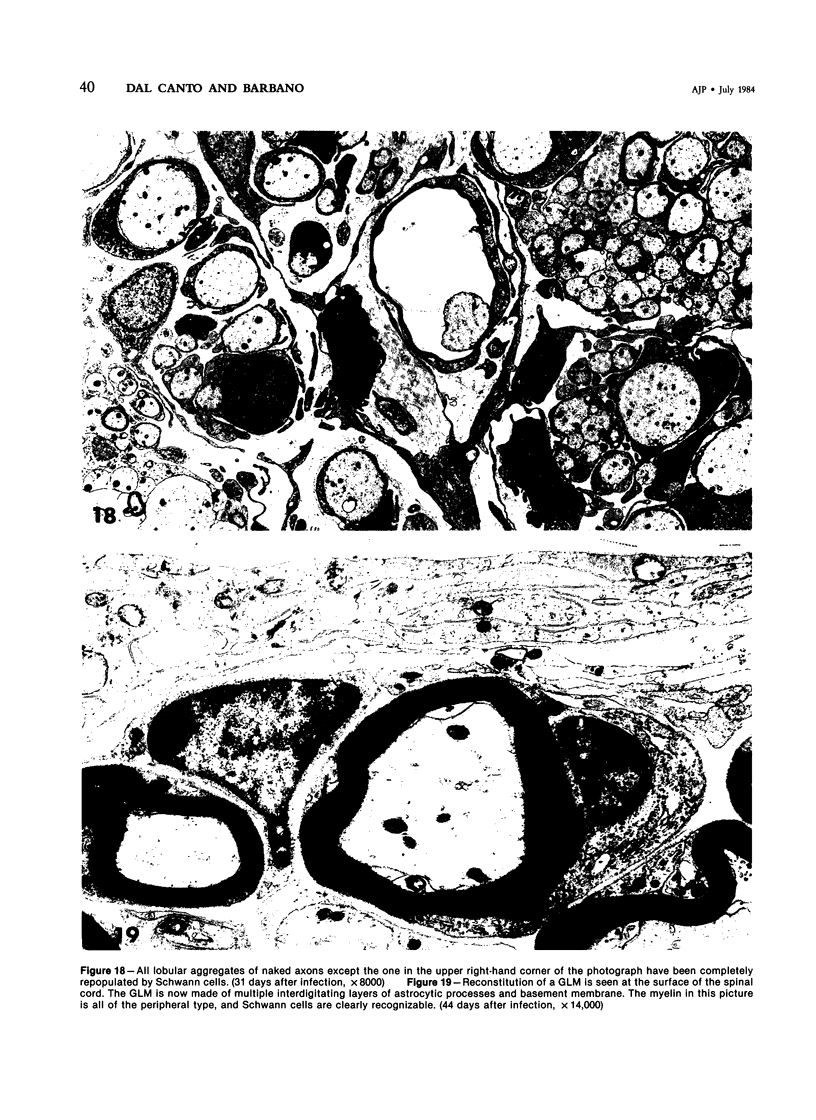
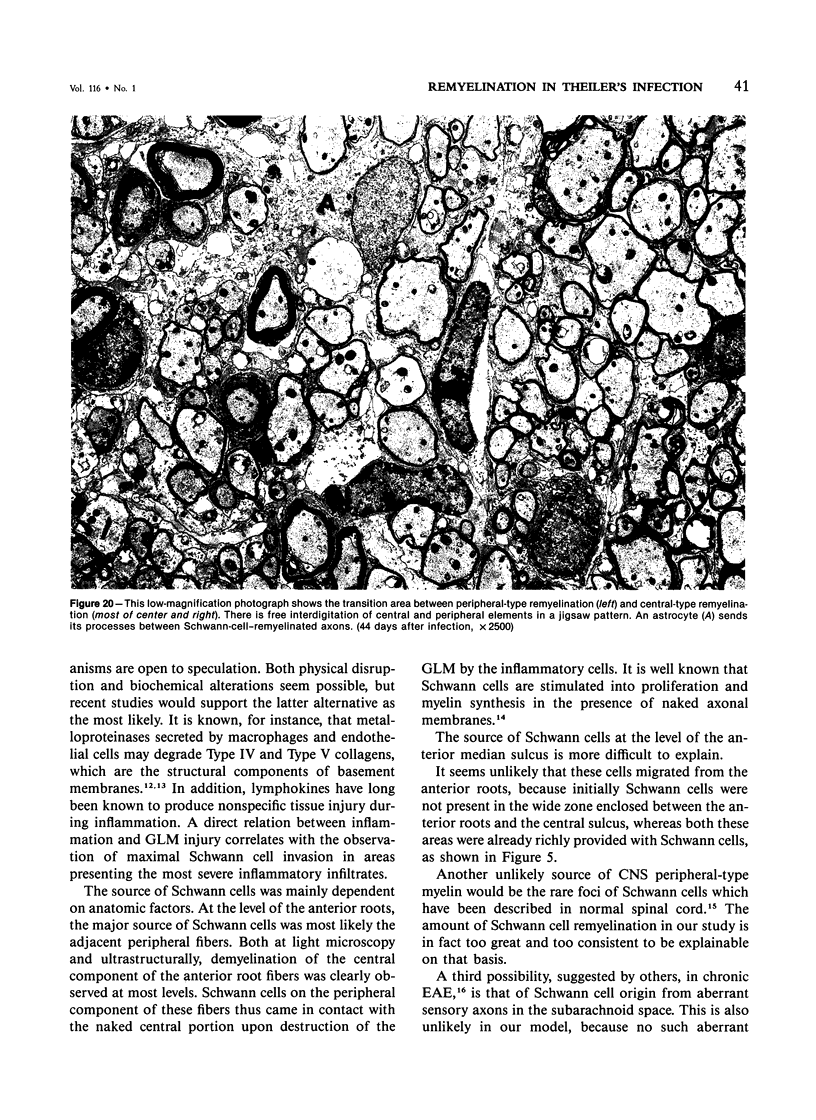
- Dal Canto M. C., Lipton H. L. Schwann cell remyelination and recurrent demyelination in the central nervous system of mice infected with attenuated Theiler's virus. Am J Pathol. 1980 Jan;98(1):101–122. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Ultrastructural immunohistochemical localization of virus in acute and chronic demyelinating Theiler's virus infection. Am J Pathol. 1982 Jan;106(1):20–29. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C. Uncoupled relationship between demyelination and primary infection of myelinating cells in Theiler's virus encephalomyelitis. Infect Immun. 1982 Mar;35(3):1133–1138. doi: 10.1128/iai.35.3.1133-1138.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries G. H., Salzer J. L., Bunge R. P. Axolemma-enriched fractions isolated from PNS and CNS are mitogenic for cultured Schwann cells. Brain Res. 1982 Feb;255(2):295–299. doi: 10.1016/0165-3806(82)90028-1. [DOI] [PubMed] [Google Scholar]
- Ghatak N. R., Hirano A., Doron Y., Zimmerman H. M. Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol. 1973 Oct;29(4):262–267. doi: 10.1001/archneur.1973.00490280074011. [DOI] [PubMed] [Google Scholar]
- Gilmore S. A., Duncan D. On the presence of peripheral-like nervous and connective tissue within irradiated spinal cord. Anat Rec. 1968 Apr;160(4):675–690. doi: 10.1002/ar.1091600403. [DOI] [PubMed] [Google Scholar]
- Herndon R. M., Price D. L., Weiner L. P. Regeneration of oligodendroglia during recovery from demyelinating disease. Science. 1977 Feb 18;195(4279):693–694. doi: 10.1126/science.190678. [DOI] [PubMed] [Google Scholar]
- Iijima T., Wasano T., Tagawa T., Ando K. A histochemical study of the innervation of cerebral blood vessels in the snake. Cell Tissue Res. 1977 Apr 7;179(2):143–155. doi: 10.1007/BF00219792. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Garbisa S., Glaser B., Liotta L. A. Basement membrane collagen: degradation by migrating endothelial cells. Science. 1983 Jul 15;221(4607):281–283. doi: 10.1126/science.6190230. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. The TO strains of Theiler's viruses cause "slow virus-like" infections in mice. Ann Neurol. 1979 Jul;6(1):25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin S. K. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am J Pathol. 1979 Jun;95(3):683–696. [PMC free article] [PubMed] [Google Scholar]
- Ludwin S. K. Chronic demyelination inhibits remyelination in the central nervous system. An analysis of contributing factors. Lab Invest. 1980 Oct;43(4):382–387. [PubMed] [Google Scholar]
- Mainardi C. L., Seyer J. M., Kang A. H. Type-specific collagenolysis: a type V collagen-degrading enzyme from macrophages. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1108–1115. doi: 10.1016/0006-291x(80)91490-4. [DOI] [PubMed] [Google Scholar]
- Nelson E., Takayanagi T., Rennels M. L., Kawamura J. The innervation of human intracranial arteries: a study by scanning and transmission electron microscopy. J Neuropathol Exp Neurol. 1972 Jul;31(3):526–534. doi: 10.1097/00005072-197207000-00010. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979 Jan;5(1):22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Prineas J. W., Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978 Sep;28(9 Pt 2):68–75. doi: 10.1212/wnl.28.9_part_2.68. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Brown A. M., McFarlin D. E. Heterotopic regeneration of peripheral nerve fibres into the subarachnoid space. J Neurocytol. 1982 Feb;11(1):109–118. doi: 10.1007/BF01258007. [DOI] [PubMed] [Google Scholar]
- Raine C. S. On the occurrence of Schwann cells within the normal central nervous system. J Neurocytol. 1976 Jun;5(3):371–380. doi: 10.1007/BF01175122. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Leibowitz J. L., Lampert P. W. Persistent infection of oligodendrocytes in Theiler's virus-induced encephalomyelitis. Ann Neurol. 1983 Apr;13(4):426–433. doi: 10.1002/ana.410130409. [DOI] [PubMed] [Google Scholar]
- Tagawa T., Ando K., Wasano T. A histochemical study of the innervation of the cerebral blood vessels in the domestic fowl. Cell Tissue Res. 1979 Apr 30;198(1):43–51. doi: 10.1007/BF00234833. [DOI] [PubMed] [Google Scholar]