Abstract
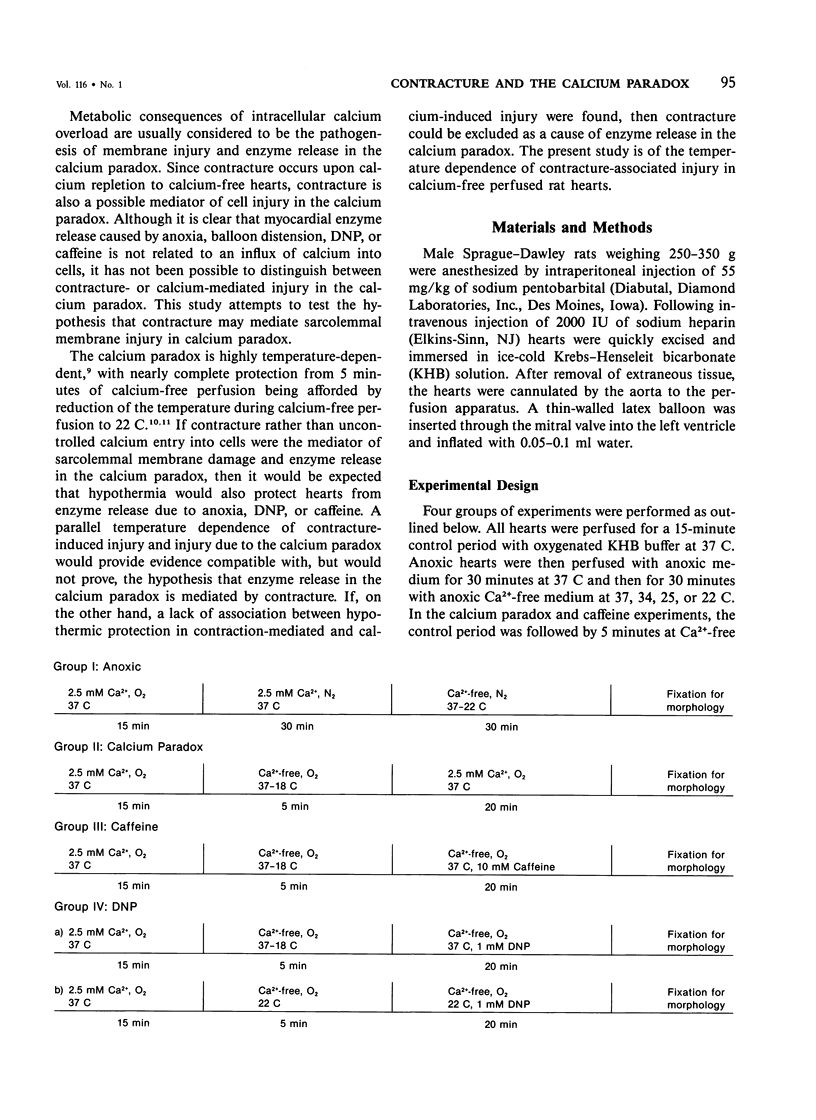
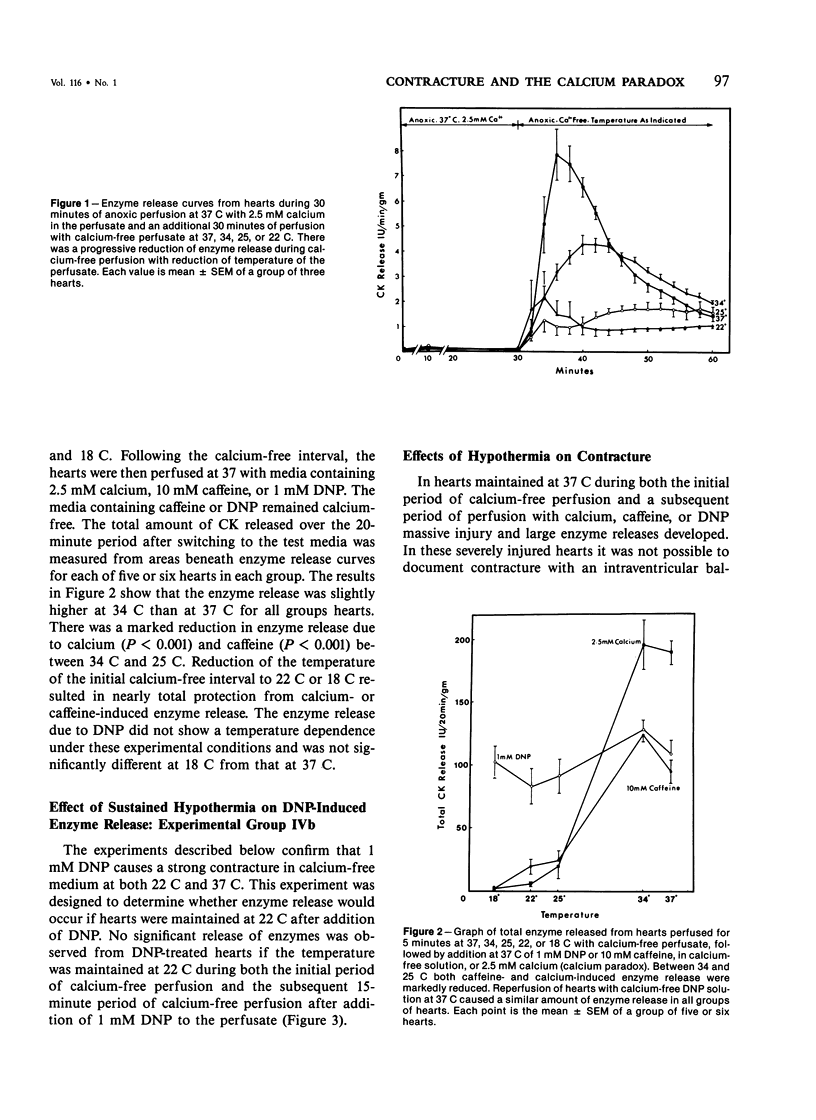
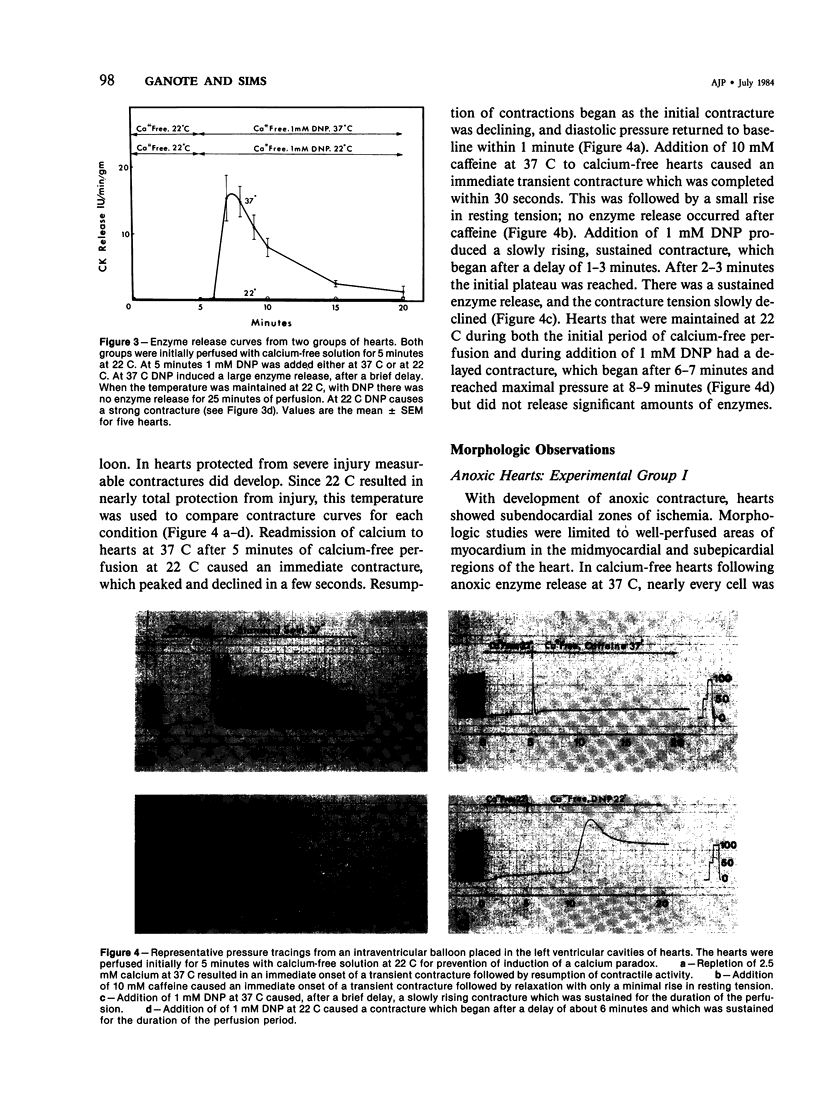
Hypothermia during calcium-free perfusion of hearts protects them from injury caused by subsequent calcium repletion at 37 C (calcium paradox). Injury to calcium-free hearts is also associated with contracture caused by anoxia, 2,4-dinitrophenol (DNP), or caffeine. This study was done for the purpose of determining whether hypothermia during calcium-free perfusions protects hearts from contracture-associated injury. Langendorff-perfused rat hearts were studied in four experimental groups: I) Anoxia: Thirty minutes of anoxic perfusion at 37 C was followed by thirty minutes of anoxic calcium-free perfusion at 37-18 C. II) Calcium paradox: Five minutes of calcium-free perfusion at 37-18 C was followed by calcium repletion at 37 C. III, IVa) Caffeine or DNP: Five minutes of calcium-free perfusion at 37-18 C was followed by addition of 10 mM caffeine or 1 mM DNP in calcium-free medium at 37 C or, IVb) 1 mM DNP in calcium-free medium at 22 C. Injury was assessed by measurement of serial releases of creatine kinase (CK) in effluents and by cellular morphology. The results show that progressive hypothermia to 22 C during calcium-free perfusion periods produced a progressive reduction of CK release and morphologic evidence of injury due to anoxia, caffeine, or DNP, which closely paralleled protection of hearts from the calcium paradox. Protection from injury in all experimental groups was associated with preservation of sarcolemmal membrane integrity and prevention of cell separations at intercalated disk junctions. It is proposed that weakening of intercalated disks occurs during calcium-free perfusions and may be a cause of mechanical fragility of the sarcolemma. Hypothermia may protect hearts from contracture-associated injury by preserving the integrity of intercalated disk junctions during periods of extracellular calcium depletion.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alto L. E., Dhalla N. S. Myocardial cation contents during induction of calcium paradox. Am J Physiol. 1979 Dec;237(6):H713–H719. doi: 10.1152/ajpheart.1979.237.6.H713. [DOI] [PubMed] [Google Scholar]
- Altschuld R., Gibb L., Ansel A., Hohl C., Kruger F. A., Brierley G. P. Calcium tolerance of isolated rat heart cells. J Mol Cell Cardiol. 1980 Dec;12(12):1383–1395. doi: 10.1016/0022-2828(80)90123-6. [DOI] [PubMed] [Google Scholar]
- Ashraf M. Correlative studies on sarcolemmal ultrastructure, permeability, and loss of intracellular enzymes in the isolated heart perfused with calcium-free medium. Am J Pathol. 1979 Nov;97(2):411–432. [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Bullock G. R., Hearse D. J. The temperature dependence of the calcium paradox: enzymatic, functional and morphological correlates of cellular injury. J Mol Cell Cardiol. 1983 Jun;15(6):393–411. doi: 10.1016/0022-2828(83)90323-1. [DOI] [PubMed] [Google Scholar]
- Boink A. B., Ruigrok T. J., de Moes D., Maas A. H., Zimmerman A. N. The effect of hypothermia on the occurrence of the calcium paradox. Pflugers Arch. 1980 May;385(2):105–109. doi: 10.1007/BF00588688. [DOI] [PubMed] [Google Scholar]
- Bulkley B. H., Nunnally R. L., Hollis D. P. "Calcium paradox" and the effect of varied temperature on its development: a phosphorus nuclear magnetic resonance and morphologic study. Lab Invest. 1978 Aug;39(2):133–140. [PubMed] [Google Scholar]
- Burt J. M. Electrical and contractile consequences of Na+ or Ca2+ gradient reduction in cultured heart cells. J Mol Cell Cardiol. 1982 Feb;14(2):99–110. doi: 10.1016/0022-2828(82)90198-5. [DOI] [PubMed] [Google Scholar]
- Carlson E. C., Grosso D. S., Romero S. A., Frangakis C. J., Byus C. V., Bressler R. Ultrastructural studies of metabolically active isolated adult rat heart myocytes. J Mol Cell Cardiol. 1978 May;10(5):449–459. doi: 10.1016/0022-2828(78)90366-8. [DOI] [PubMed] [Google Scholar]
- De Leiris J., Feuvray D. Factors affecting the release of lactate dehydrogenase from isolated rat heart after calcium and magnesium free perfusions. Cardiovasc Res. 1973 May;7(3):383–390. doi: 10.1093/cvr/7.3.383. [DOI] [PubMed] [Google Scholar]
- Frank J. S., Rich T. L., Beydler S., Kreman M. Calcium depletion in rabbit myocardium. Ultrastructure of the sarcolemma and correlation with the calcium paradox. Circ Res. 1982 Aug;51(2):117–130. doi: 10.1161/01.res.51.2.117. [DOI] [PubMed] [Google Scholar]
- Ganote C. E. Contraction band necrosis and irreversible myocardial injury. J Mol Cell Cardiol. 1983 Feb;15(2):67–73. doi: 10.1016/0022-2828(83)90283-3. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Liu S. Y., Safavi S., Kaltenbach J. P. Anoxia, calcium and contracture as mediators of myocardial enzyme release. J Mol Cell Cardiol. 1981 Jan;13(1):93–106. doi: 10.1016/0022-2828(81)90231-5. [DOI] [PubMed] [Google Scholar]
- Ganote C. E., Sims M. A. Physical stress-mediated enzyme release from calcium-deficient hearts. J Mol Cell Cardiol. 1983 Jul;15(7):421–429. doi: 10.1016/0022-2828(83)90262-6. [DOI] [PubMed] [Google Scholar]
- Grinwald P. M., Nayler W. G. Calcium entry in the calcium paradox. J Mol Cell Cardiol. 1981 Oct;13(10):867–880. doi: 10.1016/0022-2828(81)90286-8. [DOI] [PubMed] [Google Scholar]
- Haworth R. A., Hunter D. R., Berkoff H. A. The isolation of Ca2+-resistant myocytes from the adult rat. J Mol Cell Cardiol. 1980 Jul;12(7):715–723. doi: 10.1016/0022-2828(80)90101-7. [DOI] [PubMed] [Google Scholar]
- Hearse D. J., Humphrey S. M., Bullock G. R. The oxygen paradox and the calcium paradox: two facets of the same problem? J Mol Cell Cardiol. 1978 Jul;10(7):641–668. doi: 10.1016/s0022-2828(78)80004-2. [DOI] [PubMed] [Google Scholar]
- Holland C. E., Jr, Olson R. E. Prevention by hypothermia of paradoxical calcium necrosis in cardiac muscle. J Mol Cell Cardiol. 1975 Dec;7(12):917–928. doi: 10.1016/0022-2828(75)90152-2. [DOI] [PubMed] [Google Scholar]
- Hunter D. R., Haworth R. A., Berkoff H. A. Cellular calcium turnover in the perfused rat heart: modulation by caffeine and procaine. Circ Res. 1982 Sep;51(3):363–370. doi: 10.1161/01.res.51.3.363. [DOI] [PubMed] [Google Scholar]
- Maunsbach A. B. The influence of different fixatives and fixation methods on the ultrastructure of rat kidney proximal tubule cells. I. Comparison of different perfusion fixation methods and of glutaraldehyde, formaldehyde and osmium tetroxide fixatives. J Ultrastruct Res. 1966 Jun;15(3):242–282. doi: 10.1016/s0022-5320(66)80109-0. [DOI] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich T. L., Langer G. A. Calcium depletion in rabbit myocardium. Calcium paradox protection by hypothermia and cation substitution. Circ Res. 1982 Aug;51(2):131–141. doi: 10.1161/01.res.51.2.131. [DOI] [PubMed] [Google Scholar]
- Yates J. C., Dhalla N. S. Structural and functional changes associated with failure and recovery of hearts after perfusion with Ca2+-free medium. J Mol Cell Cardiol. 1975 Feb;7(2):91–103. doi: 10.1016/0022-2828(75)90011-5. [DOI] [PubMed] [Google Scholar]