Abstract
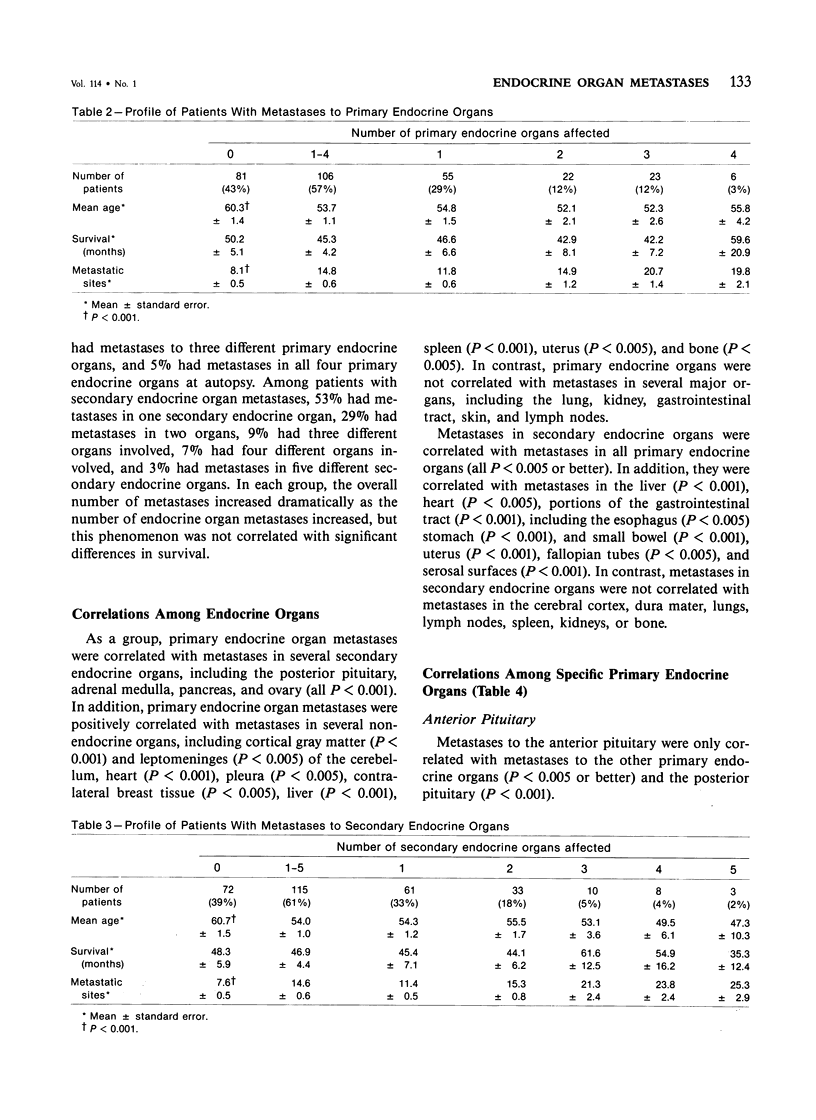
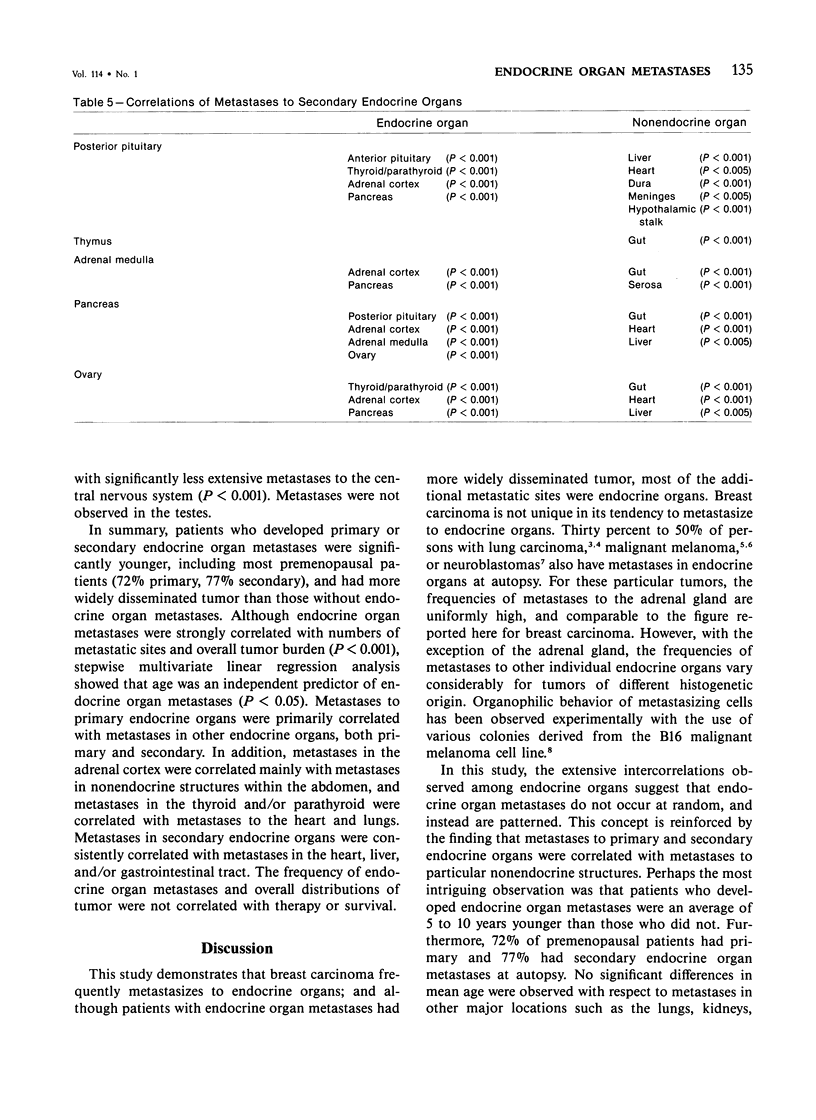
Breast carcinoma frequently metastasizes to endocrine organs, a behavior which may have prognostic or therapeutic relevance. Whether endocrine organ involvement represents a trophic influence on some carcinomas or is simply a "mass effect" of tumor dissemination is uncertain. To investigate this question, the authors reviewed the clinical and pathologic features of 187 subjects with metastatic breast carcinoma, all of whom had been subjected to complete autopsy at The Johns Hopkins Hospital. Metastases to primary endocrine organs, ie, the anterior pituitary, thyroid, parathyroid, or adrenal cortex, occurred in 57%, and metastases to secondary endocrine organs, ie, the pineal, posterior pituitary, thymus, adrenal medulla, or pancreas, occurred in 62% of patients. In general, patients with endocrine organ metastases were significantly younger and had significantly greater numbers of metastases and greater overall tumor burden than those without endocrine organ metastases (all P less than 0.001). There was no correlation between endocrine organ metastases and survival, therapy, histologic type of tumor, or grade of anaplasia or desmoplasia. Metastases to primary endocrine organs were correlated with one another and with metastases in secondary endocrine organs. Metastases in secondary endocrine organs were intercorrelated and also correlated with several nonendocrine organs, chiefly the heart, liver, and gut (all P less than 0.005). These findings indicate that metastases of breast carcinoma to endocrine organs occur in a setting of widely disseminated tumor. However, the observed correlations among metastatic sites suggest that the distributions are nonrandom; these distributions may reflect fundamental properties of some breast carcinomas with respect to hormone receptors, biologic behavior, or environmental growth requirements.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUDINGER J. M. Untreated bronchogenic carcinoma; a clinicopathological study of 250 autopsied cases. Cancer. 1958 Jan-Feb;11(1):106–116. doi: 10.1002/1097-0142(195801/02)11:1<106::aid-cncr2820110121>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Beall P. T., Szopa B., Burzynski S. R., Hazlewood C. F. Polypeptides that inhibit human breast cancer cell division. Cancer Biochem Biophys. 1979;3(2):93–96. [PubMed] [Google Scholar]
- Cerbon M. A., Pichon M. F., Milgrom E. Thyroid hormone receptors in human breast cancer. Cancer Res. 1981 Oct;41(10):4167–4173. [PubMed] [Google Scholar]
- Dao T. L. Ablation therapy for hormone-dependent tumors. Annu Rev Med. 1972;23:1–18. doi: 10.1146/annurev.me.23.020172.000245. [DOI] [PubMed] [Google Scholar]
- Eisman J. A., Martin T. J., MacIntyre I., Moseley J. M. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979 Dec 22;2(8156-8157):1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- Freake H. C., Marcocci C., Iwasaki J., MacIntyre I. 1,25-dihydroxyvitamin D3 specifically binds to a human breast cancer cell line (T47D) and stimulates growth. Biochem Biophys Res Commun. 1981 Aug 31;101(4):1131–1138. doi: 10.1016/0006-291x(81)91565-5. [DOI] [PubMed] [Google Scholar]
- Israel N., Saez S. Relation between steroid receptor content and the response to hormone addition in isolated human breast cancer cells in short-term culture. Cancer Res. 1978 Nov;38(11 Pt 2):4314–4317. [PubMed] [Google Scholar]
- Kelsey J. L. A review of the epidemiology of human breast cancer. Epidemiol Rev. 1979;1:74–109. doi: 10.1093/oxfordjournals.epirev.a036215. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- McGuire W. L., Horwitz K. B., Pearson O. H., Segaloff A. Current status of estrogen and progesterone receptors in breast cancer. Cancer. 1977 Jun;39(6 Suppl):2934–2947. doi: 10.1002/1097-0142(197706)39:6<2934::aid-cncr2820390680>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Custead S. E. Tumor metastasis is not due to adaptation of cells to a new organ environment. Science. 1982 Jan 8;215(4529):176–178. doi: 10.1126/science.7053568. [DOI] [PubMed] [Google Scholar]
- Patel J. K., Didolkar M. S., Pickren J. W., Moore R. H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978 Jun;135(6):807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- Pearson O. H., Manni A., Chambers M., Brodkey J., Marshall J. S. Role of pituitary hormones in the growth of human breast cancer. Cancer Res. 1978 Nov;38(11 Pt 2):4323–4326. [PubMed] [Google Scholar]
- Rowbottom L. A., Whitehead R. H., Roberts G. P., Hughes L. E. A study of the growth requirements of human tumour cells in tissue culture. Aust J Exp Biol Med Sci. 1981 Feb;59(1):91–100. doi: 10.1038/icb.1981.5. [DOI] [PubMed] [Google Scholar]
- Welsch C. W., Dombroske S. E., McManus M. J. Effects of insulin, human placental lactogen and human growth hormone of DNA synthesis in organ cultures of benign human breast tumours. Br J Cancer. 1978 Aug;38(2):258–262. doi: 10.1038/bjc.1978.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte S. M., Moore G. W., Hutchins G. M. Patterned distribution of metastases from malignant melanoma in humans. Cancer Res. 1983 Jul;43(7):3427–3433. [PubMed] [Google Scholar]


