Abstract
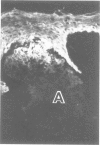
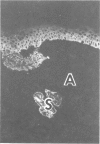
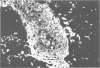
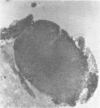
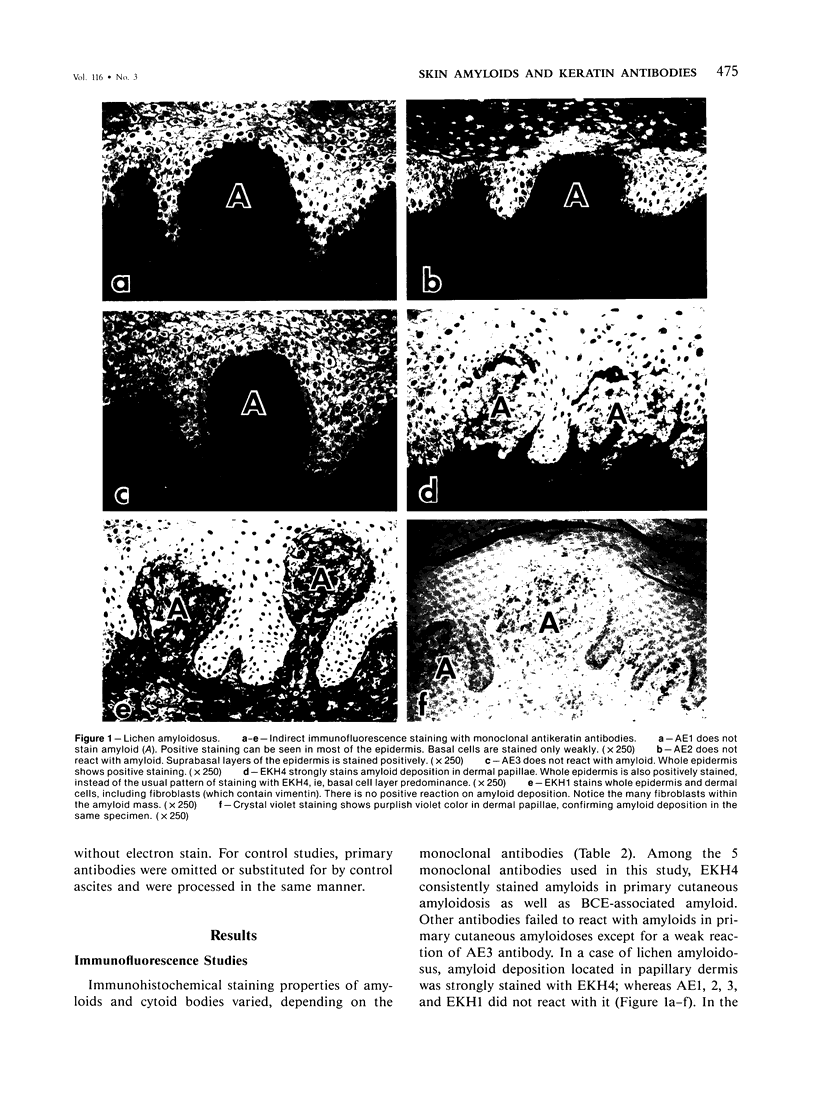
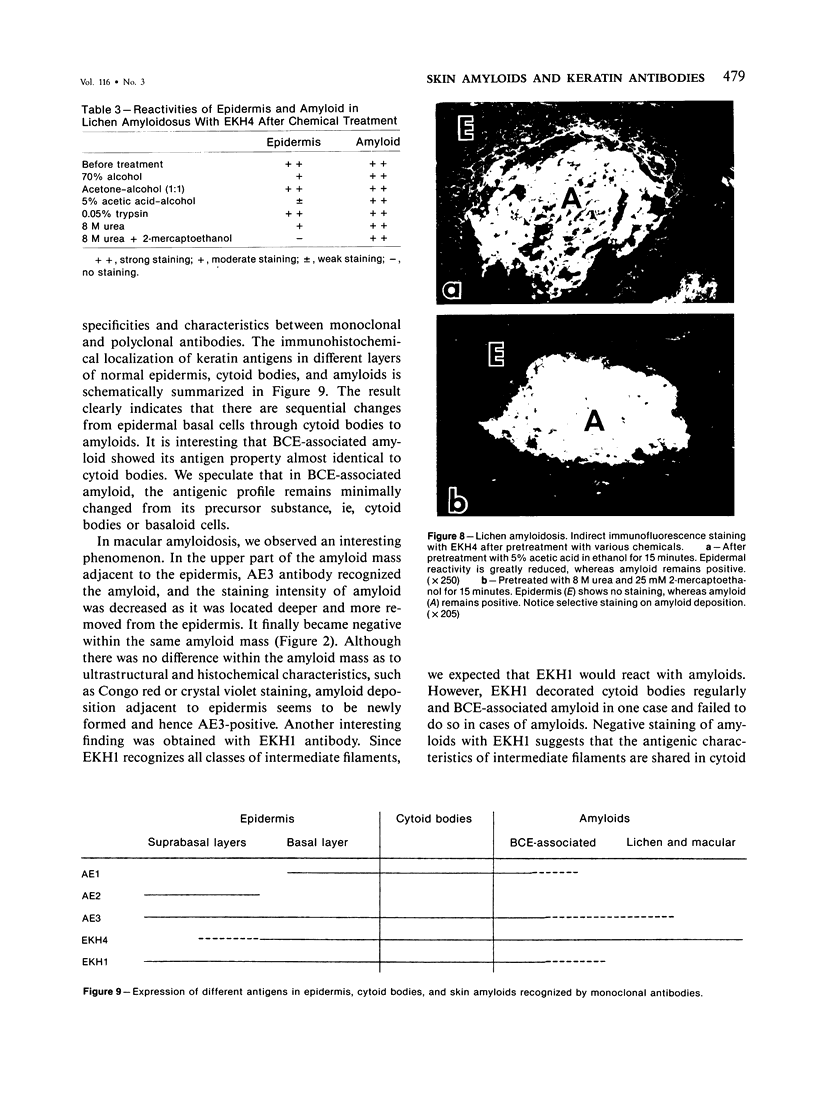
The authors have used 5 different monoclonal antikeratin antibodies to study the antigenic profiles of cytoid bodies and skin-limited amyloids. Monoclonal antibodies AE1 (which stains the basal cell layer in normal human epidermis), AE2 (suprabasal layers), AE3 (whole epidermis), EKH4 (lower 2-3 layers), and EKH1 (recognizes all classes of intermediate filaments) were used to stain frozen skin sections by the indirect immunofluorescent or indirect immunoperoxidase technique. Cytoid bodies in lichen planus (LP) and discoid lupus erythematosus (DLE) were strongly stained with AE1, AE3, EKH4, and EKH1 antibodies but were negative with AE2. In contrast, amyloids in lichen amyloidosus and macular amyloidosis were stained strongly with EKH4 but only weakly or not at all with AE1, AE2, AE3, and EKH1. Amyloid associated with epithelial tumors showed closer immunologic profiles to cytoid body. These findings suggest that epidermal keratins are the major precursor substance of skin-limited amyloids as well as cytoid bodies in LP and DLE. Sequential changes in antigenic profiles from basal cells to amyloids through cytoid bodies further suggest that cytoid bodies may represent one of the precursor substances of skin-limited amyloids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black M. M. The role of the epidermis in the histopathogenesis of lichen amyloidosus. Histochemical correlations. Br J Dermatol. 1971 Dec;85(6):524–530. doi: 10.1111/j.1365-2133.1971.tb14077.x. [DOI] [PubMed] [Google Scholar]
- Breathnach S. M., Melrose S. M., Bhogal B., de Beer F. C., Black M. M., Pepys M. B. Ultrastructural localization of amyloid P component in primary localized cutaneous amyloidosis. Clin Exp Dermatol. 1983 Jul;8(4):355–362. doi: 10.1111/j.1365-2230.1983.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Brownstein M. H., Helwig E. B. The cutaneous amyloidoses. I. Localized forms. Arch Dermatol. 1970 Jul;102(1):8–19. [PubMed] [Google Scholar]
- Brownstein M. H., Helwig E. B. The cutaneous amyloidoses. II. Systemic forms. Arch Dermatol. 1970 Jul;102(1):20–28. [PubMed] [Google Scholar]
- Cornwell G. G., 3rd, Husby G., Westermark P., Natvig J. B., Michaelsen T. E., Skogen B. Identification and characterization of different amyloid fibril proteins in tissue sections. Scand J Immunol. 1977;6(11):1071–1080. doi: 10.1111/j.1365-3083.1977.tb00344.x. [DOI] [PubMed] [Google Scholar]
- Danno K., Horio T. Sulphhydryl crosslinking in cutaneous apoptosis: a review. J Cutan Pathol. 1982 Jun;9(3):123–132. doi: 10.1111/j.1600-0560.1982.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Eady R. A., Cowen T. Half-and-half cells in lichen planus. A possible clue to the origin and early formation of colloid bodies. Br J Dermatol. 1978 Apr;98(4):417–423. [PubMed] [Google Scholar]
- Ebner H., Gebhart W. Light and electron microscopic studies on colloid and other cytoid bodies. Clin Exp Dermatol. 1977 Dec;2(4):311–322. doi: 10.1111/j.1365-2230.1977.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis: the beta-fibrilloses (second of two parts). N Engl J Med. 1980 Jun 12;302(24):1333–1343. doi: 10.1056/NEJM198006123022403. [DOI] [PubMed] [Google Scholar]
- Gomes M. A., Staquet M. J., Thivolet J. Staining of colloid bodies by keratin antisera in lichen planus. Am J Dermatopathol. 1981 Winter;3(4):341–347. doi: 10.1097/00000372-198100340-00002. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Apoptosis in lichen planus and several other dermatoses. Intra-epidermal cell death with filamentous degeneration. Acta Derm Venereol. 1976;56(3):187–210. [PubMed] [Google Scholar]
- Hashimoto K., Kobayashi H. Histogenesis of amyloid in the skin. Am J Dermatopathol. 1980 Summer;2(2):165–171. doi: 10.1097/00000372-198000220-00014. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Kumakiri M. Colloid-amyloid bodies in PUVA-treated human psoriatic patients. J Invest Dermatol. 1979 Feb;72(2):70–80. doi: 10.1111/1523-1747.ep12530290. [DOI] [PubMed] [Google Scholar]
- Hosokawa M., Masu S., Seiji M. Immunofluorescence studies on civatte bodies and dyskeratotic cells with anti-keratin antibody. Tohoku J Exp Med. 1981 Nov;135(3):219–229. doi: 10.1620/tjem.135.219. [DOI] [PubMed] [Google Scholar]
- Ishibashi Y., Tsuru N., Kukita A. The "colloid body"--its nature and pathogenesis. J Dermatol. 1978 Oct;5(5):199–208. doi: 10.1111/j.1346-8138.1978.tb01854.x. [DOI] [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kanzaki T., Eto H., Umezawa A., Maeda T., Iwase H., Ito M. Morphological, biological, and biochemical characteristics of a benign human trichilemmoma cell line in vivo and in vitro. Cancer Res. 1981 Jun;41(6):2468–2475. [PubMed] [Google Scholar]
- Kanzaki T., Kanamaru T., Nishiyama S., Eto H., Kobayashi H., Hashimoto K. Three-dimensional hair follicular differentiation of a trichilemmoma cell line in vitro. Dev Biol. 1983 Oct;99(2):324–330. doi: 10.1016/0012-1606(83)90282-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Hashimoto K. Amyloidogenesis in organ-limited cutaneous amyloidosis: an antigenic identity between epidermal keratin and skin amyloid. J Invest Dermatol. 1983 Jan;80(1):66–72. doi: 10.1111/1523-1747.ep12531130. [DOI] [PubMed] [Google Scholar]
- Kumakiri M., Hashimoto K. Histogenesis of primary localized cutaneous amyloidosis: sequential change of epidermal keratinocytes to amyloid via filamentous degeneration. J Invest Dermatol. 1979 Aug;73(2):150–162. doi: 10.1111/1523-1747.ep12581609. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Linke R. P., Sipe J. D., Pollock P. S., Ignaczak T. F., Glenner G. G. Isolation of a low-molecular-weight serum component antigenically related to an amyloid fibril protein of unknown origin. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1473–1476. doi: 10.1073/pnas.72.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald D. M., Black M. M., Ramnarain N. Immunofluorescence studies in primary localized cutaneous amyloidosis. Br J Dermatol. 1977 Jun;96(6):635–641. doi: 10.1111/j.1365-2133.1977.tb05208.x. [DOI] [PubMed] [Google Scholar]
- Maeda H., Ohta S., Saito Y., Nameki H., Ishikawa H. Epidermal origin of the amyloid in localized cutaneous amyloidosis. Br J Dermatol. 1982 Mar;106(3):345–351. doi: 10.1111/j.1365-2133.1982.tb01734.x. [DOI] [PubMed] [Google Scholar]
- Masu S., Hosokawa M., Seiji M. Immunofluorescence studies on cutaneous amyloidosis with anti-keratin antibody. Tohoku J Exp Med. 1980 Sep;132(1):121–122. doi: 10.1620/tjem.132.121. [DOI] [PubMed] [Google Scholar]
- Masu S., Sato A., Seiji M. Electron microscopic studies on Civatte body in Riehl's melanosis. Tohoku J Exp Med. 1980 Jun;131(2):177–196. doi: 10.1620/tjem.131.177. [DOI] [PubMed] [Google Scholar]
- Medenica M., Lorincz A. Lichen planus: an ultrastructural study. Acta Derm Venereol. 1977;57(1):55–62. [PubMed] [Google Scholar]
- Nagao S., Iijima S. Light and electron microscopic study of Riehl's melanosis. Possible mode of its pigmentary incontinence. J Cutan Pathol. 1974;1(4):165–175. doi: 10.1111/j.1600-0560.1974.tb00622.x. [DOI] [PubMed] [Google Scholar]
- THYRESSON N., MOBERGER G. Cytologic studies in lichen ruber planus. Acta Derm Venereol. 1957;37(2):191–204. [PubMed] [Google Scholar]
- Taylor C. R. Immunoperoxidase techniques: practical and theoretical aspects. Arch Pathol Lab Med. 1978 Mar;102(3):113–121. [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Weedon D., Shand E. Amyloid in basal cell carcinomas. Br J Dermatol. 1979 Aug;101(2):141–146. doi: 10.1111/j.1365-2133.1979.tb05598.x. [DOI] [PubMed] [Google Scholar]
- Weiss R. A., Guillet G. Y., Freedberg I. M., Farmer E. R., Small E. A., Weiss M. M., Sun T. T. The use of monoclonal antibody to keratin in human epidermal disease: alterations in immunohistochemical staining pattern. J Invest Dermatol. 1983 Sep;81(3):224–230. doi: 10.1111/1523-1747.ep12518198. [DOI] [PubMed] [Google Scholar]
- Westermark P. Amyloidosis of the skin: a comparison between localized and systemic amyloidosis. Acta Derm Venereol. 1979;59(4):341–345. [PubMed] [Google Scholar]
- Woodcock-Mitchell J., Eichner R., Nelson W. G., Sun T. T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982 Nov;95(2 Pt 1):580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Labban N. G., Kramer I. R. Civatte bodies and the actively dividing epithelial cells in oral lichen planus. Br J Dermatol. 1974 Jan;90(1):13–23. doi: 10.1111/j.1365-2133.1974.tb06357.x. [DOI] [PubMed] [Google Scholar]