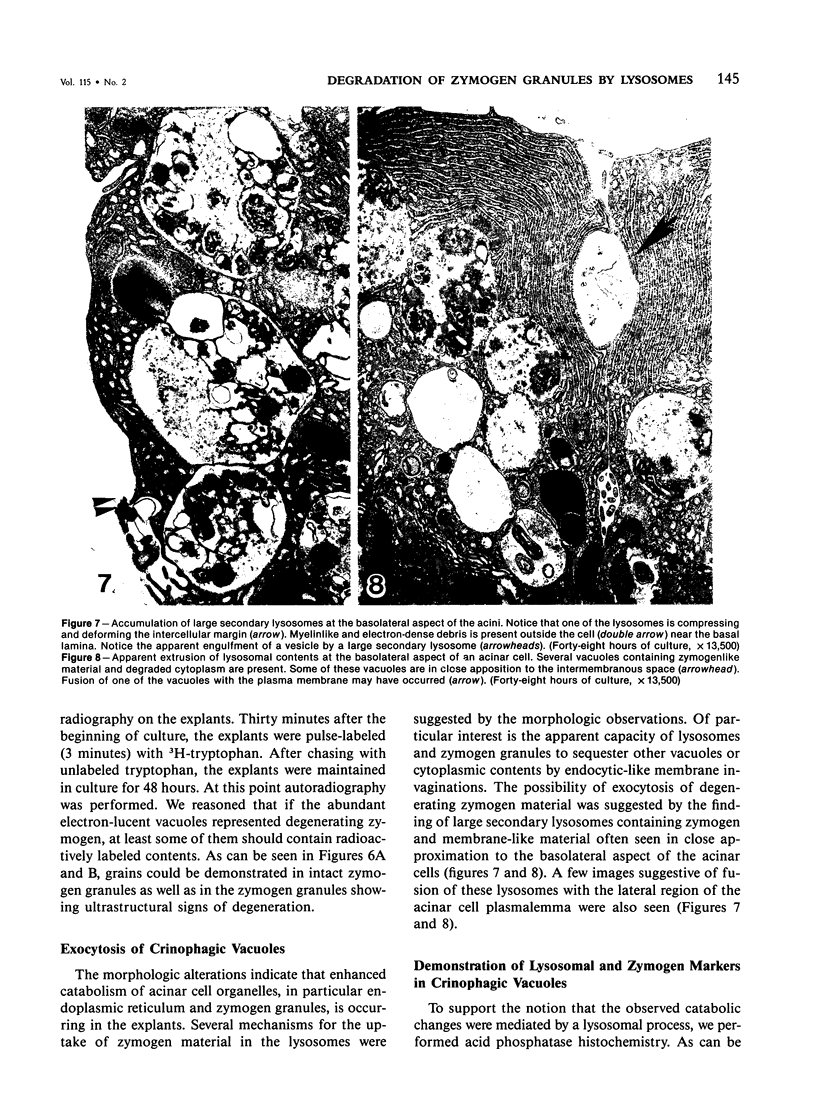
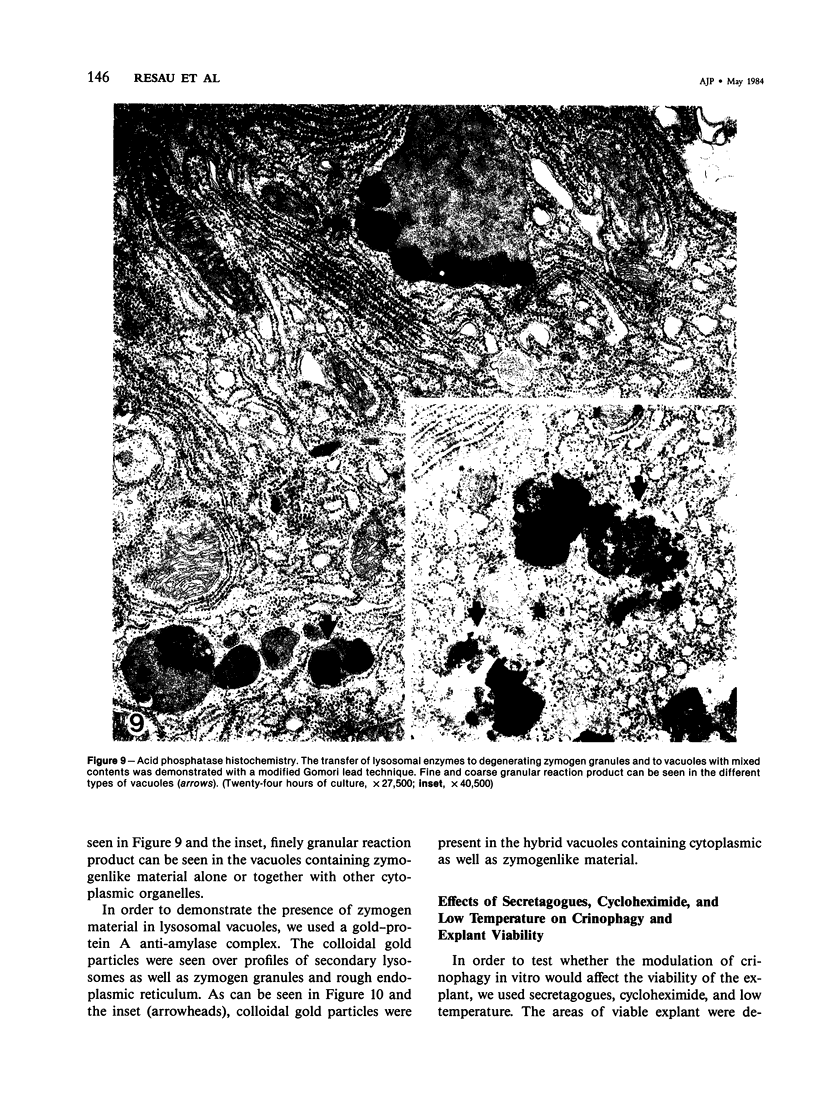
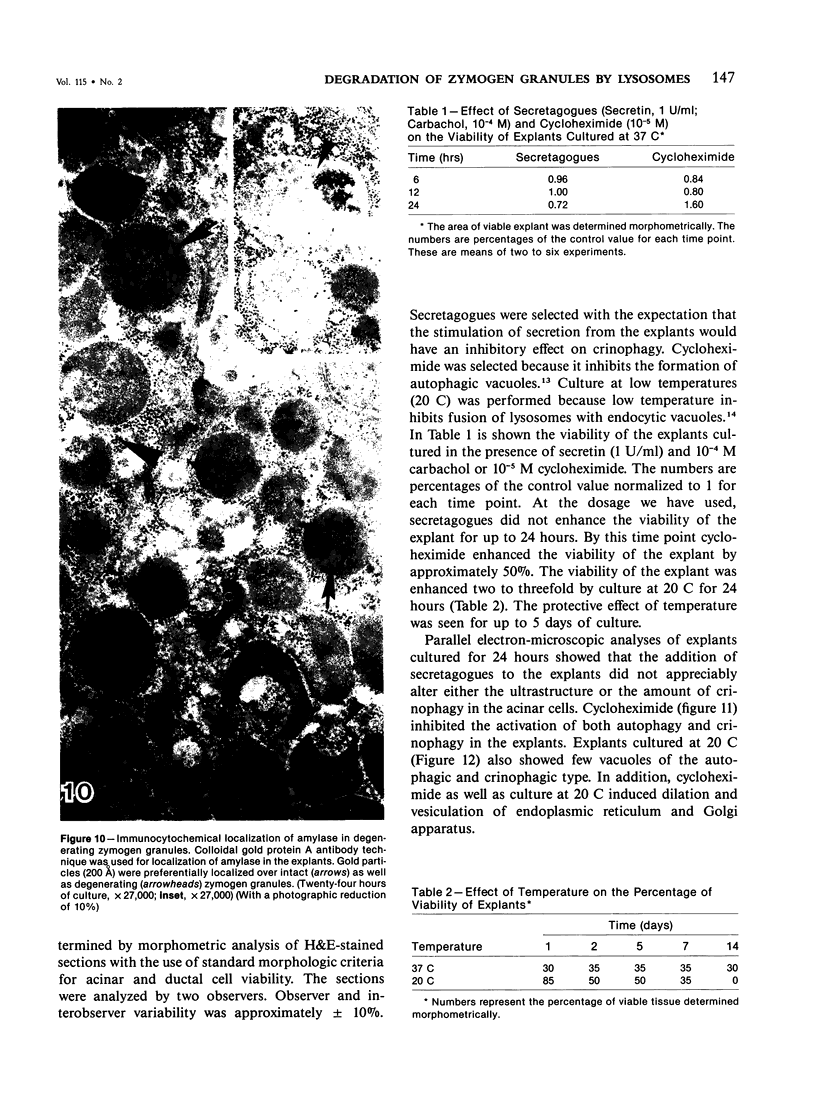
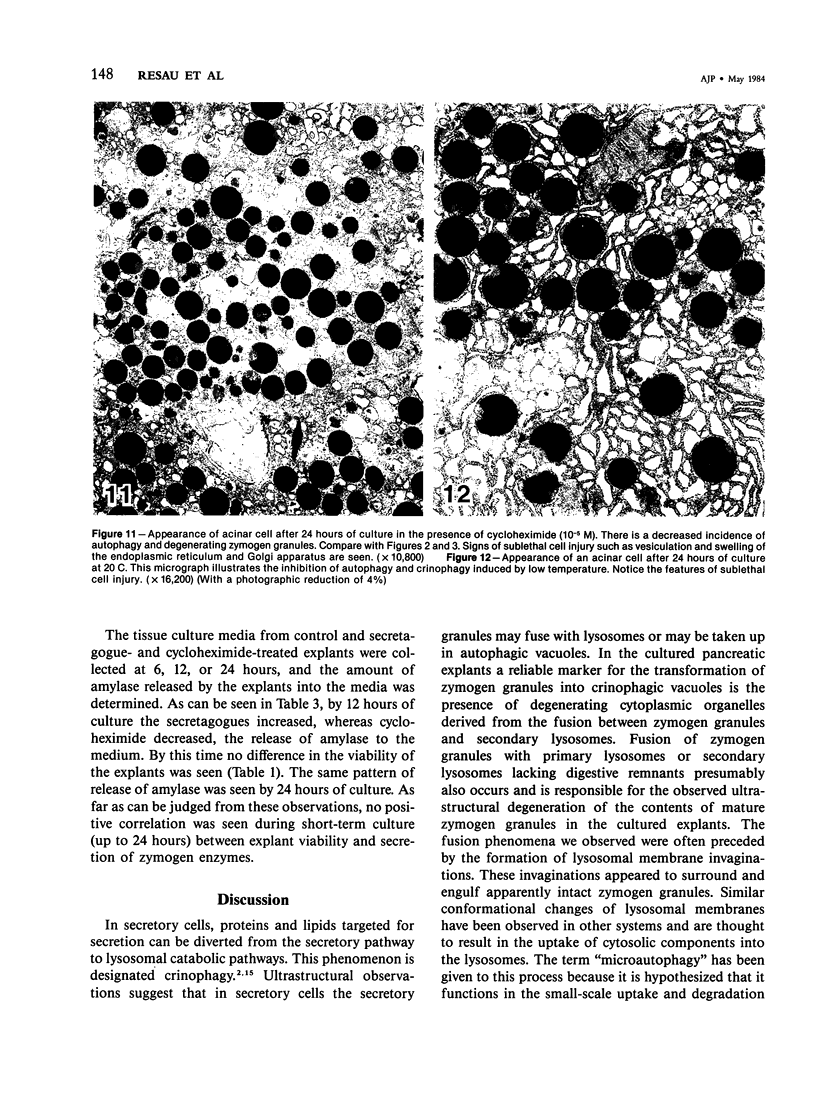
Abstract
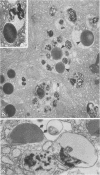
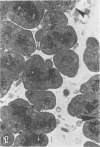
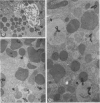
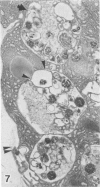
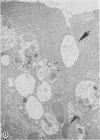
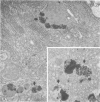
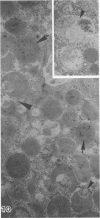
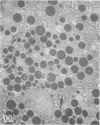
Pancreatic explants from Syrian hamsters were maintained in culture for determination of the fate of the zymogen granules in the acinar cells. By 6 hours of culture the zymogen granule matrix in many cells becomes nonhomogeneous, and electron-lucent spaces appear. Autoradiography performed 48 hours after pulse labeling shows that several of the altered zymogen granules contain radioactively labeled proteins. Hence these altered granules are mature zymogen granules undergoing regression. Ultrastructural analyses indicate that the zymogen granules are degraded intracellularly in the lysosomes by autophagy, by direct fusion between the lysosomes and the zymogen granules, as well as by engulfment of intact zymogen granules by the lysosomes. Budding of cytoplasm into zymogen granules is also frequently observed. Acid phosphatase histochemistry and anti-amylase immunohistochemistry were used for demonstration of presence of hydrolases and of secretory material in the degenerating zymogen granules. Cycloheximide and low temperature inhibit the degradation of zymogen granules and enhance the short-term viability of the explant. Secretagogues stimulate secretion but have little or no effect on the degradation of zymogen granules or on explant viability. The lysosomes participate in the intracellular degradation of zymogen granules in acinar cells of pancreatic explants. The induction of these lysosomal catabolic processes correlates with the structural alteration of pancreatic acinar cells. The intracellular degradation of secretory membranes and products by the lysosomes may play a role in the adaptation of pancreatic acinar cells to injury.
Full text
PDF


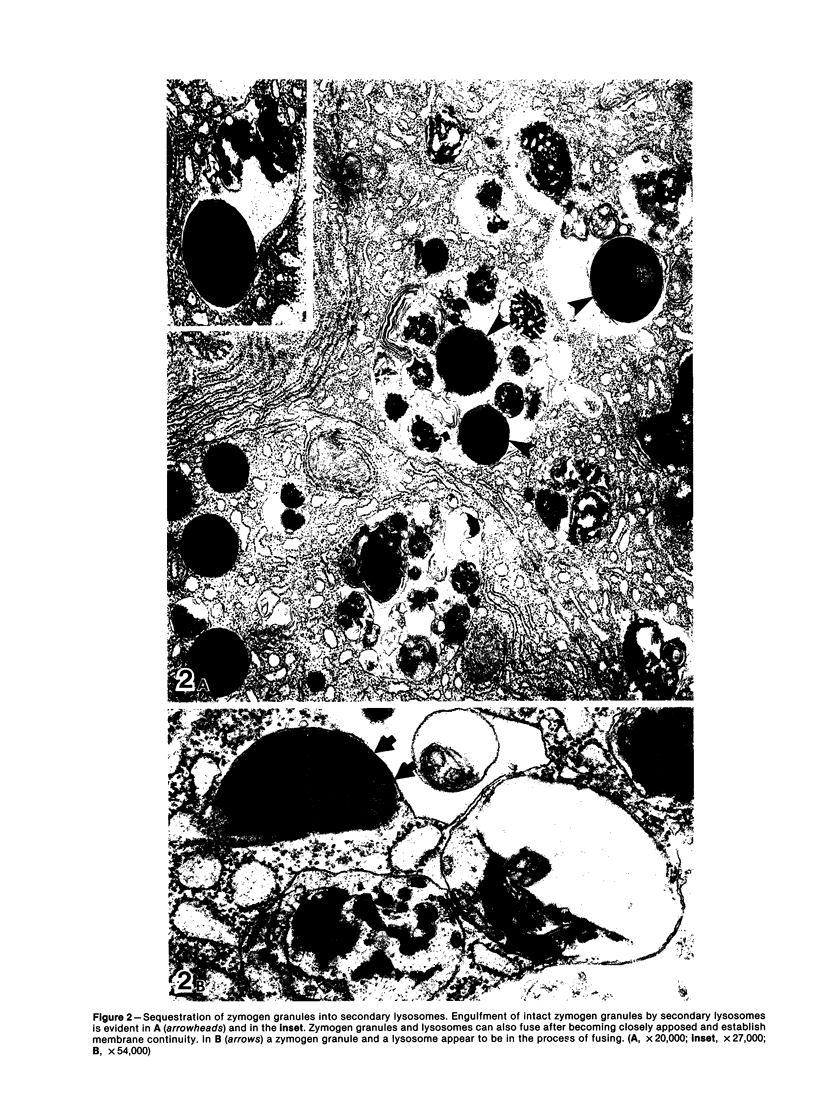
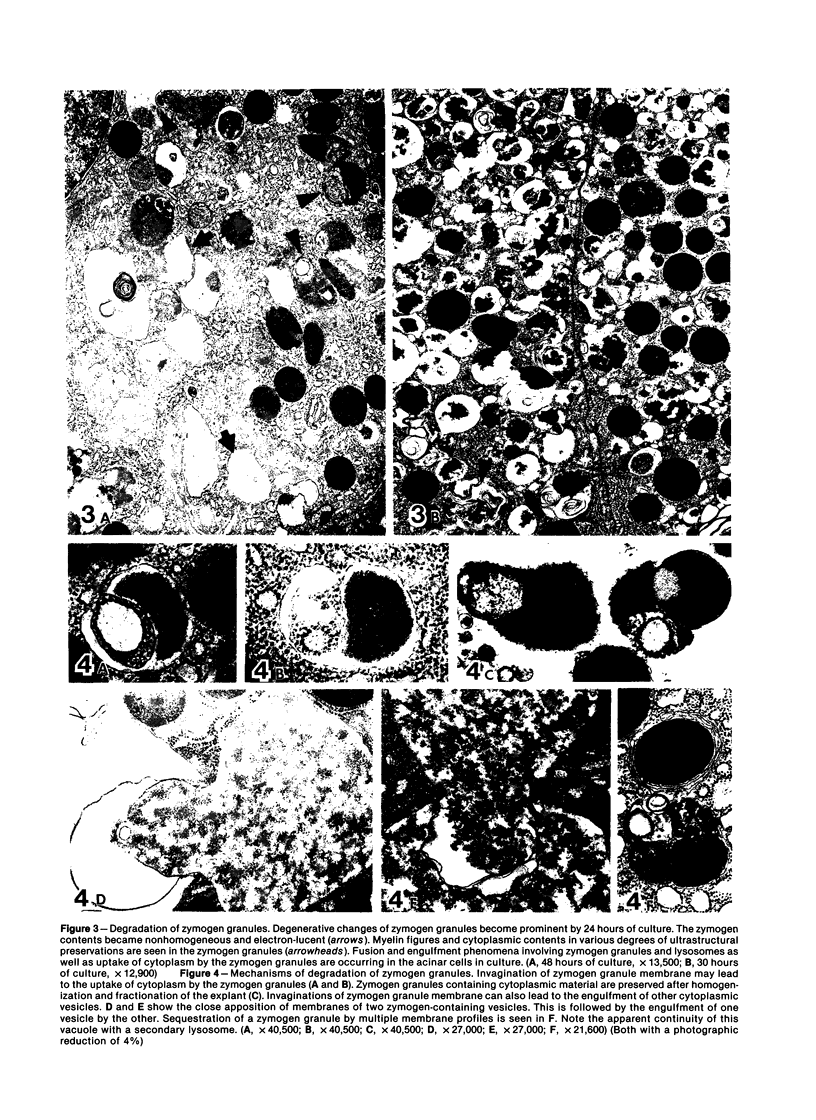
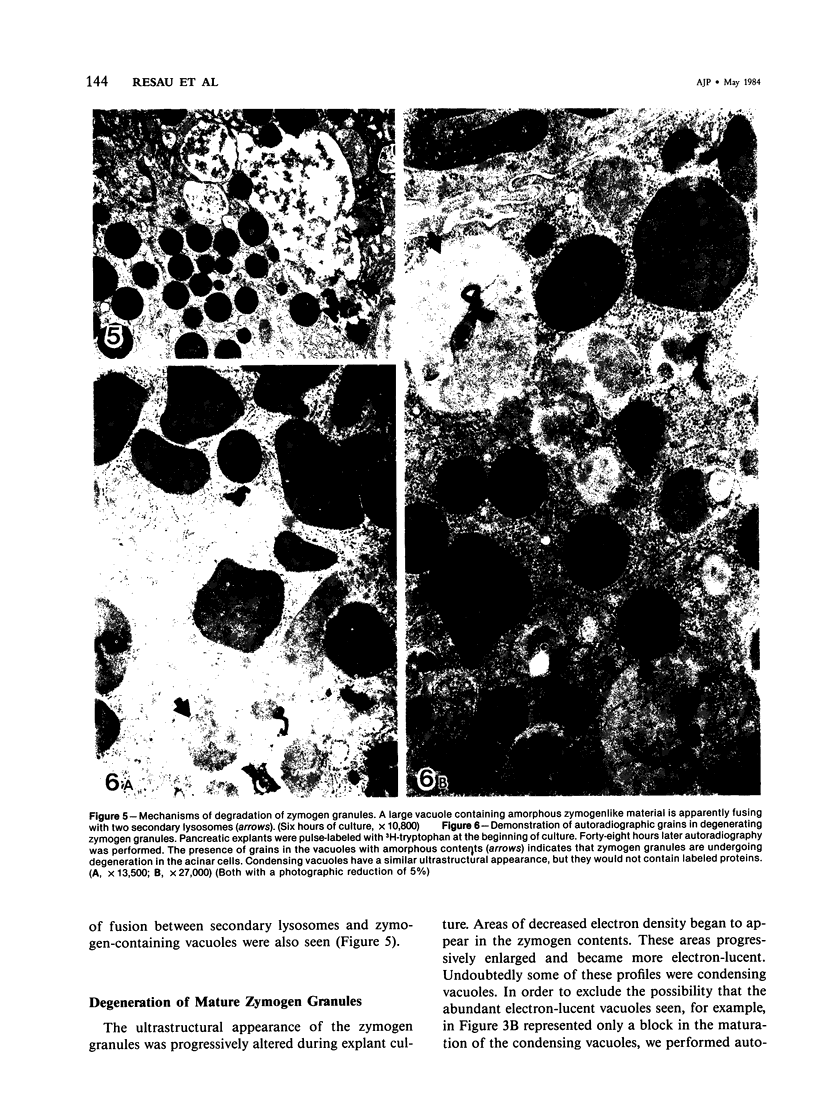


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Rohr G., Kern H. F. Alteration of membrane fusion as a cause of acute pancreatitis in the rat. Dig Dis Sci. 1982 Nov;27(11):993–1002. doi: 10.1007/BF01391745. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S. Intracellular degradation of newly synthesized secretory proteins. Biochem J. 1983 Jul 15;214(1):1–10. doi: 10.1042/bj2140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARAWAY W. T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am J Clin Pathol. 1959 Jul;32(1):97–99. doi: 10.1093/ajcp/32.1_ts.97. [DOI] [PubMed] [Google Scholar]
- Dunn W. A., Hubbard A. L., Aronson N. N., Jr Low temperature selectively inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused rat liver. J Biol Chem. 1980 Jun 25;255(12):5971–5978. [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelbrand S., Coleman R., Silbermann M. The exocrine pancreas in triamcinolone-treated mice. A light and electron microscopy study. Acta Anat (Basel) 1978;102(4):348–357. doi: 10.1159/000145657. [DOI] [PubMed] [Google Scholar]
- Glaumann H., Sandberg P. O., Marzella L. Degradation of secretory content in Golgi-enriched fractions from rat liver after vinblastine administration. Exp Cell Res. 1982 Jul;140(1):201–213. doi: 10.1016/0014-4827(82)90170-7. [DOI] [PubMed] [Google Scholar]
- Helminen H. J., Ericsson J. L. On the mechanism of lysosomal enzyme secretion. Electron microscopic and histochemical studies on the epithelial cells of the rat's ventral prostate lobe. J Ultrastruct Res. 1970 Dec;33(5):528–549. doi: 10.1016/s0022-5320(70)90179-6. [DOI] [PubMed] [Google Scholar]
- Koike H., Steer M. L., Meldolesi J. Pancreatic effects of ethionine: blockade of exocytosis and appearance of crinophagy and autophagy precede cellular necrosis. Am J Physiol. 1982 Apr;242(4):G297–G307. doi: 10.1152/ajpgi.1982.242.4.G297. [DOI] [PubMed] [Google Scholar]
- Marzella L., Ahlberg J., Glaumann H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;36(2-3):219–234. doi: 10.1007/BF02912068. [DOI] [PubMed] [Google Scholar]
- Marzella L., Glaumann H. Increased degradation in rat liver induced by vinblastine. I. Biochemical characterization. Lab Invest. 1980 Jan;42(1):8–17. [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Journey to the center of the cell: role of the receptosome. Science. 1981 Oct 30;214(4520):504–509. doi: 10.1126/science.6170111. [DOI] [PubMed] [Google Scholar]
- Pfeifer U. Kinetic and subcellular aspects of hypertrophy and atrophy. Int Rev Exp Pathol. 1982;23:1–45. [PubMed] [Google Scholar]
- Pound A. W., Walker N. I. Involution of the pancreas after ligation of the pancreatic ducts. I: a histological study. Br J Exp Pathol. 1981 Dec;62(6):547–558. [PMC free article] [PubMed] [Google Scholar]
- Rao K. N., Tuma J., Lombardi B. Acute hemorrhagic pancreatic necrosis in mice. Intraparenchymal activation of zymogens, and other enzyme changes in pancreas and serum. Gastroenterology. 1976 May;70(5 PT1):720–726. [PubMed] [Google Scholar]
- Rao M. S., Reddy M. K., Reddy J. K., Scarpelli D. G. Response of chemically induced hepatocytelike cells in hamster pancreas to methyl clofenapate, a peroxisome proliferator. J Cell Biol. 1982 Oct;95(1):50–56. doi: 10.1083/jcb.95.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. S., Subbarao V., Luetteke N., Scarpelli D. G. Further characterization of carcinogen-induced hepatocytelike cells in hamster pancreas. Am J Pathol. 1983 Jan;110(1):89–94. [PMC free article] [PubMed] [Google Scholar]
- Resau J. H., Hudson E. A., Jones R. T. Organ explant culture of adult Syrian golden hamster pancreas. In Vitro. 1983 Apr;19(4):315–325. doi: 10.1007/BF02619510. [DOI] [PubMed] [Google Scholar]
- Roth J., Bendayan M., Orci L. Ultrastructural localization of intracellular antigens by the use of protein A-gold complex. J Histochem Cytochem. 1978 Dec;26(12):1074–1081. doi: 10.1177/26.12.366014. [DOI] [PubMed] [Google Scholar]
- Rundell J. O., Sato T., Wetzelberger E., Ueda H., Brandes D. Lysosomal enzyme release by vitamin A in L1210 leukemia cells. J Natl Cancer Inst. 1974 Apr;52(4):1237–1244. doi: 10.1093/jnci/52.4.1237. [DOI] [PubMed] [Google Scholar]
- Scheele G., Jacoby R., Carne T. Mechanism of compartmentation of secretory proteins: transport of exocrine pancreatic proteins across the microsomal membrane. J Cell Biol. 1980 Dec;87(3 Pt 1):611–628. doi: 10.1083/jcb.87.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISBLUM B., HERMAN L., FITZGERALD P. J. Changes in pancreatic acinar cells during protein deprivation. J Cell Biol. 1962 Feb;12:313–327. doi: 10.1083/jcb.12.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeligs J. D., Janoff A., Dumont A. E. The course and nature of acinar cell death following pancreatic ligation in the guinea pig. Am J Pathol. 1975 Aug;80(2):203–226. [PMC free article] [PubMed] [Google Scholar]