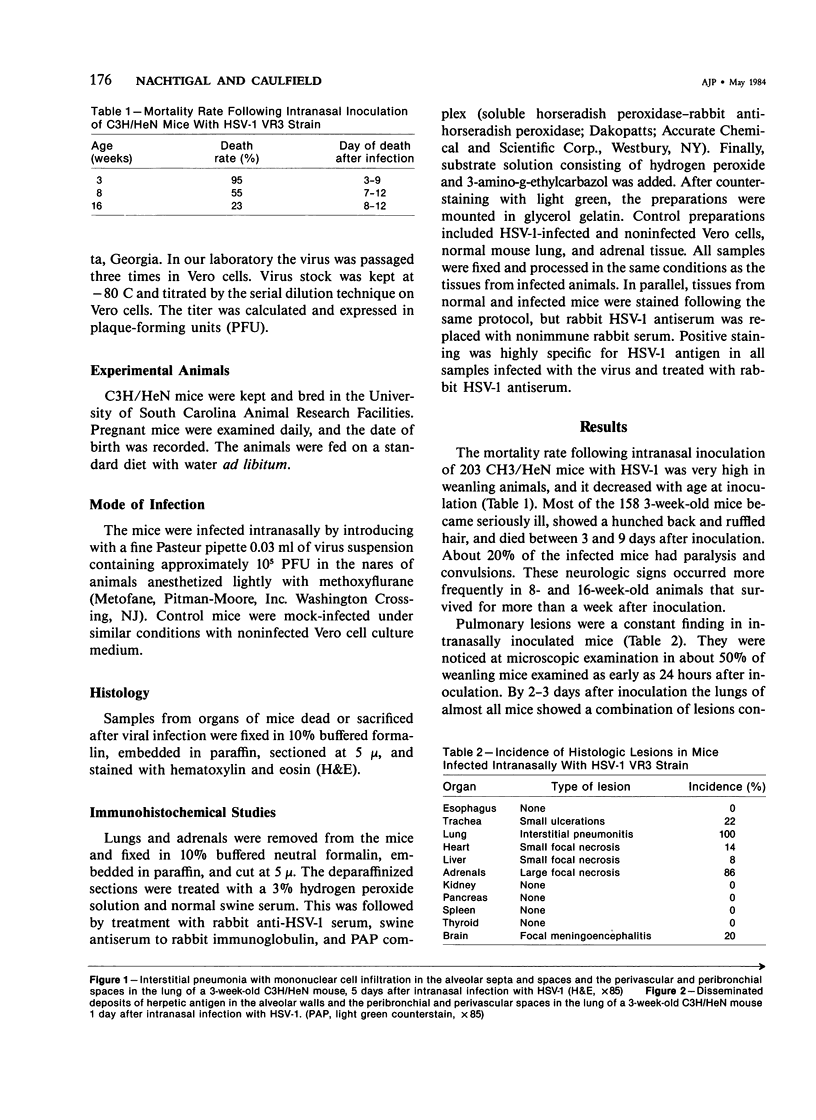
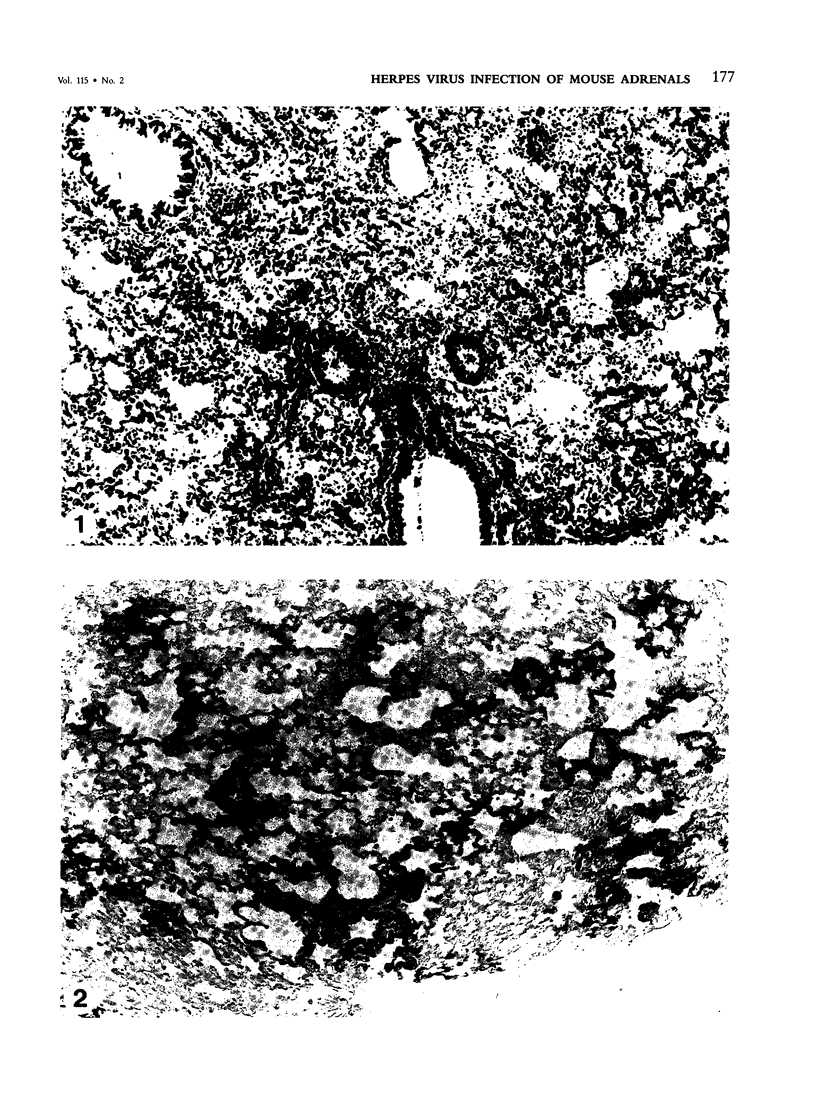
Abstract
Intranasal infection of CH/HeN mice with herpes simplex virus Type 1 (HSV-1), VR3 strain, caused a high death rate in the 3-12 days following inoculation. Acute interstitial pneumonia and focal adrenal necrosis developed in almost all the animals. About 20% of the mice showed meningoencephalitis and myocarditis. Viral antigen was demonstrated by immunoperoxidase staining in the alveolar walls, around bronchi and blood vessels, and in focal areas of the adrenal cortex as early as 24-48 hours after infection. The virus disseminated probably hematogenously from the lungs and seemed to localize preferentially in the adrenals. In mice surviving the acute stage of infection a subcapsular cell reaction developed in the adrenal cortex. The extensive pneumonia and adrenal necrosis contributed to the high mortality rate of weanling mice infected with HSV-1. The route of viral inoculation and the age of the host animal seemed to influence the localization and outcome of the pathologic process.
Full text
PDF
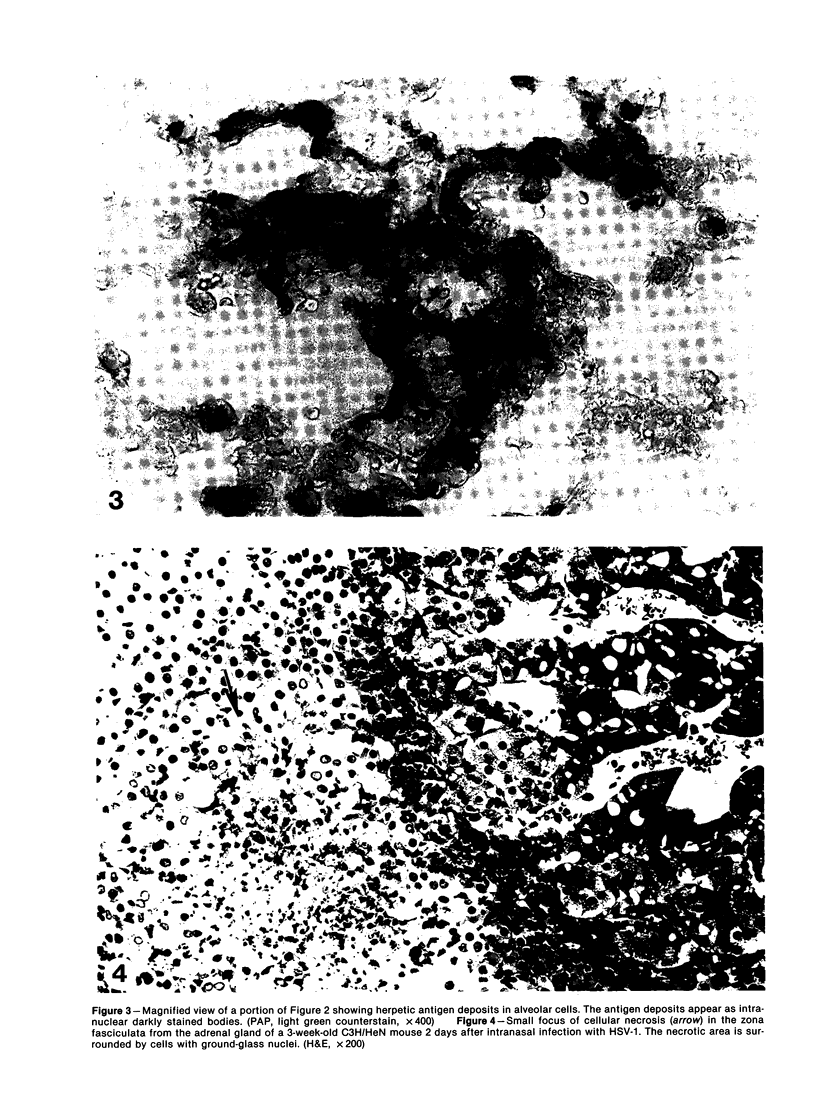

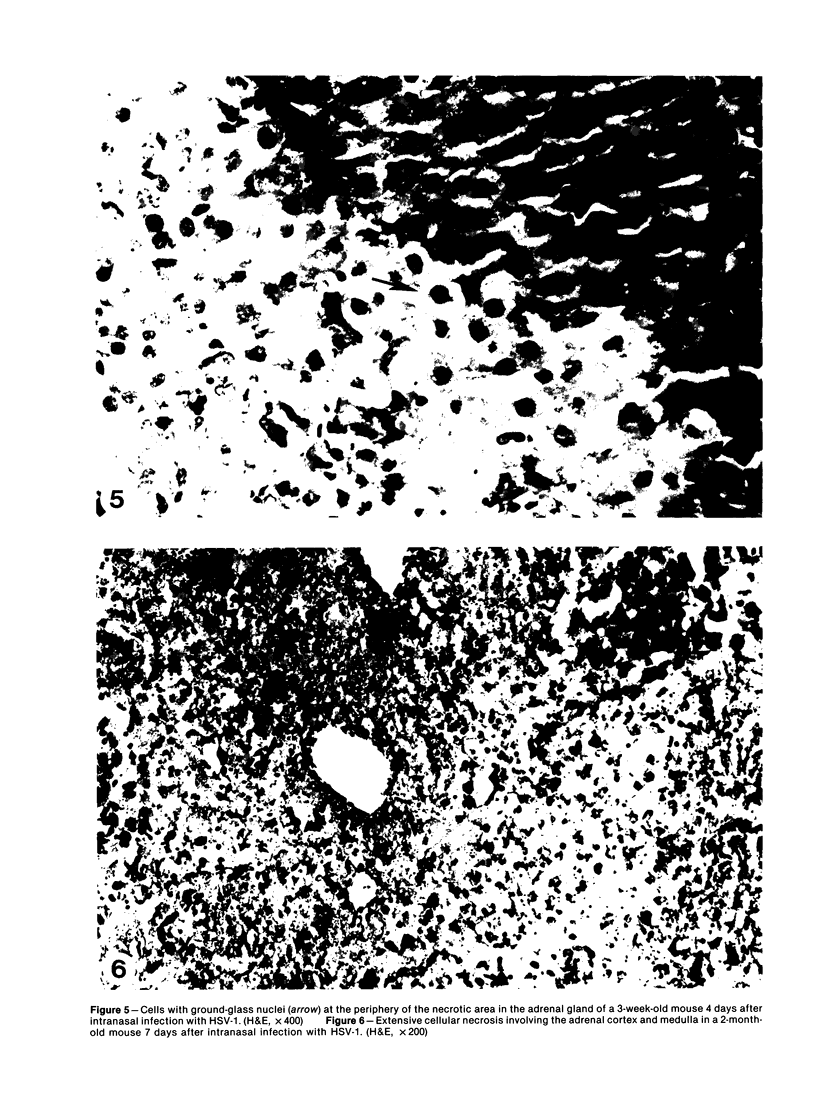
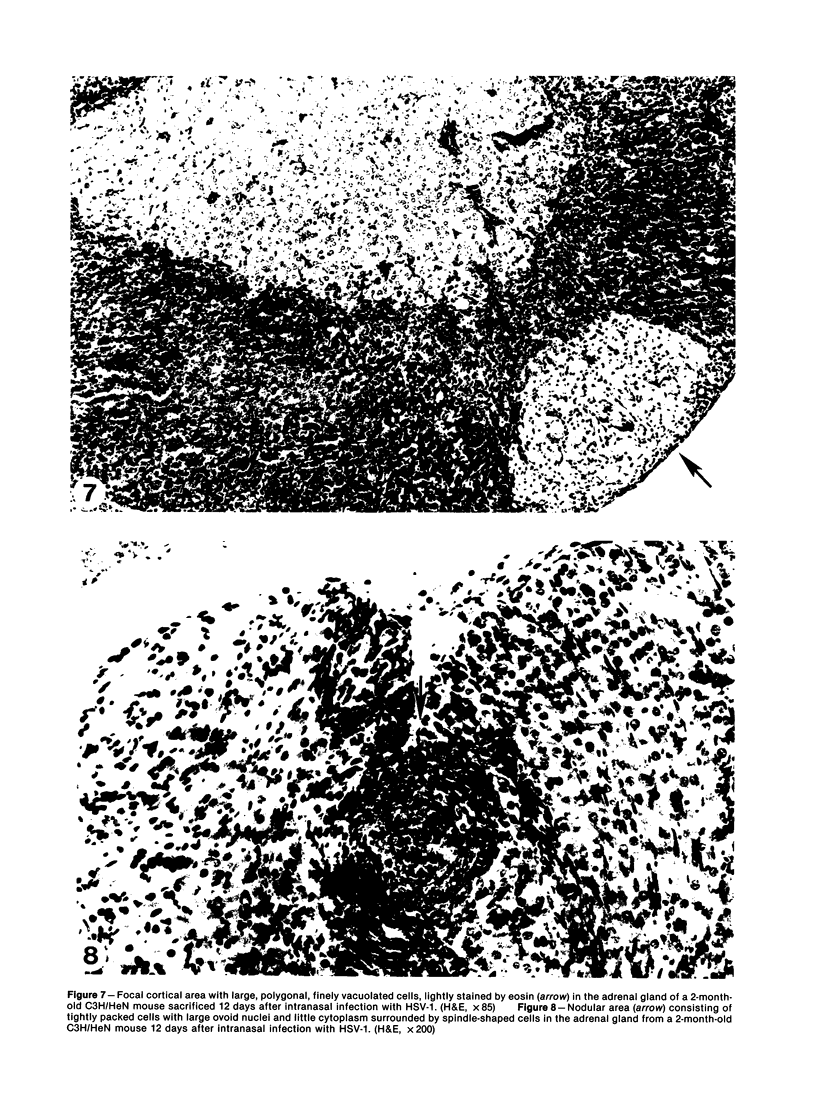
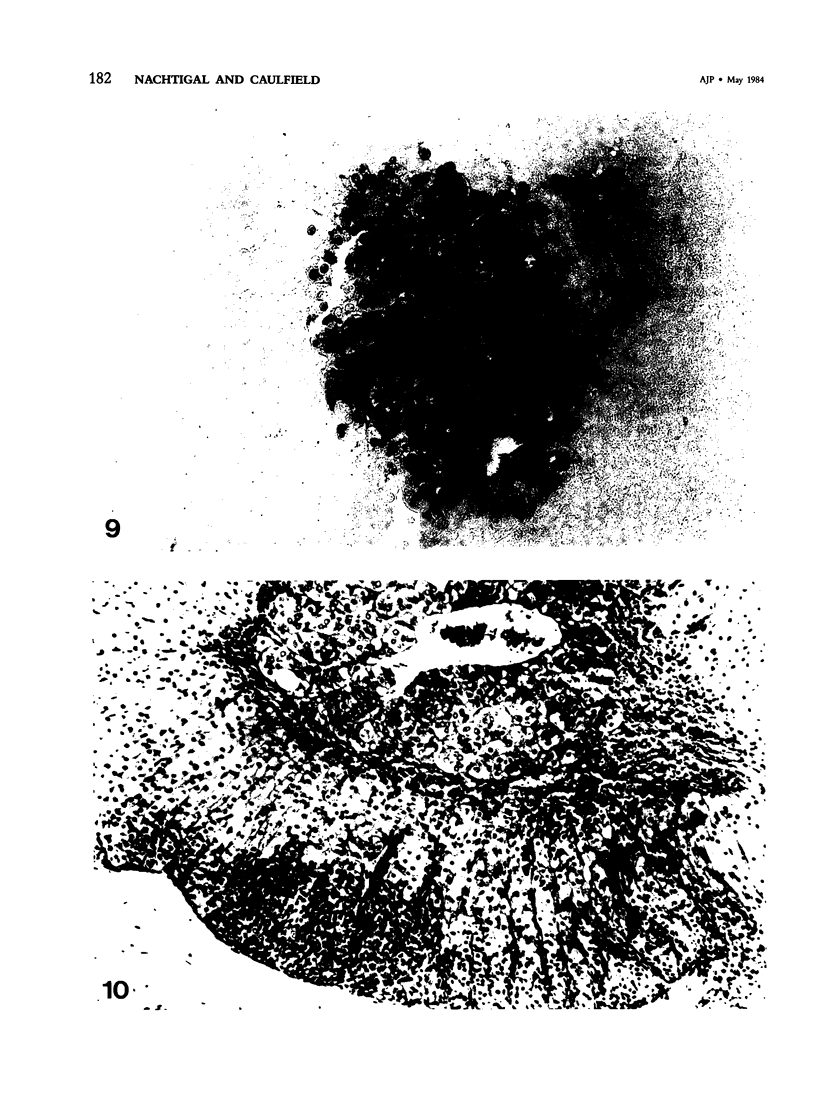



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERDJIS C. C. Structure of the zona glomerulosa of the adrenal cortex as an indication of age and sex variations in normal mice. Arch Ital Anat Istol Patol. 1958;63(3):223–232. [PubMed] [Google Scholar]
- Bahrani M., Boxerbaum B., Gilger A. P., Rosenthal M. S., Teree T. M. Generalized herpes simplex and hypoadrenocorticism. A case associated with adrenocortical insufficiency in a prematurely born male: clinical, virologic, ophthalmological, and metabolic studies. Am J Dis Child. 1966 Apr;111(4):437–445. doi: 10.1001/archpedi.1966.02090070135023. [DOI] [PubMed] [Google Scholar]
- Becker W. B., Kipps A., McKenzie D. Disseminated herpes simplex virus infection. Its pathogenesis based on virological and pathological studies in 33 cases. Am J Dis Child. 1968 Jan;115(1):1–8. doi: 10.1001/archpedi.1968.02100010003001. [DOI] [PubMed] [Google Scholar]
- Buss D. H., Scharyj M. Herpesvirus infection of the esophagus and other visceral organs in adults. Incidence and clinical significance. Am J Med. 1979 Mar;66(3):457–462. doi: 10.1016/0002-9343(79)91068-4. [DOI] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Latent herpetic infections following experimental viraemia. J Gen Virol. 1976 Apr;31(1):75–80. doi: 10.1099/0022-1317-31-1-75. [DOI] [PubMed] [Google Scholar]
- Darai G., Munk K. Human embryonic lung cells abortively infected with Herpes virus hominis type 2 show some properties of cell transformation. Nat New Biol. 1973 Feb 28;241(113):268–269. doi: 10.1038/newbio241268a0. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Luczak M. Intranasal challenge of mice with herpes simplex virus: an experimental model for evaluation of the efficacy of antiviral drugs. J Infect Dis. 1976 Jun;133 (Suppl):A226–A236. doi: 10.1093/infdis/133.supplement_2.a226. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster embryo cells after exposure to inactivated herpes simplex virus type 1. J Virol. 1973 Aug;12(2):209–217. doi: 10.1128/jvi.12.2.209-217.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff R., Rapp F. Properties of hamster embryo fibroblasts transformed in vitro after exposure to ultraviolet-irradiated herpes simplex virus type 2. J Virol. 1971 Oct;8(4):469–477. doi: 10.1128/jvi.8.4.469-477.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn T. B. Normal and pathologic anatomy of the adrenal gland of the mouse, including neoplasms. J Natl Cancer Inst. 1970 Jun;44(6):1323–1389. [PubMed] [Google Scholar]
- Dvorak A. M., Dvorak H. F., Karnovsky M. J. Uptake of horseradish peroxidase by guinea pig basophilic leukocytes. Lab Invest. 1972 Jan;26(1):27–39. [PubMed] [Google Scholar]
- Grodums E. I., Zbitnew A. Experimental herpes simplex virus carditis in mice. Infect Immun. 1976 Dec;14(6):1322–1331. doi: 10.1128/iai.14.6.1322-1331.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes R. E., Azimi P. H., Cramblett H. G. Fatal herpesvirus hominis (herpes simplex virus) infections in children. Clinical, pathologic, and virologic characteristics. JAMA. 1968 Oct 7;206(2):312–319. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan S. W., McLaren L. C., Crosby J. H. Herpetic tracheobronchitis. Cytologic and virologic detection. Arch Intern Med. 1975 Jun;135(6):784–788. doi: 10.1001/archinte.135.6.784. [DOI] [PubMed] [Google Scholar]
- Kapoor A. K., Nash A. A., Wildy P., Phelan J., McLean C. S., Field H. J. Pathogenesis of herpes simplex virus in congenitally athymic mice: the relative roles of cell-mediated and humoral immunity. J Gen Virol. 1982 Jun;60(Pt 2):225–233. doi: 10.1099/0022-1317-60-2-225. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Overall J. C., Jr, Glasgow L. A. Herpesvirus hominis infection in newborn mice. I. An experimental model and therapy with iododeoxyuridine. J Infect Dis. 1973 Sep;128(3):290–299. doi: 10.1093/infdis/128.3.290. [DOI] [PubMed] [Google Scholar]
- Kern E. R., Overall J. C., Jr, Glasgow L. A. Herpesvirus hominis infection in newborn mice: comparison of the therapeutic efficacy of 1-beta-D-arabinofuranosylcytosine and 9-beta-D-arabinofuranosyladenine. Antimicrob Agents Chemother. 1975 May;7(5):587–595. doi: 10.1128/aac.7.5.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R., Overall J. C., Jr, Glasgow L. A. Herpesvirus hominis infection in newborn mice: treatment with interferon inducer polyinosinic-polycytidylic acid. Antimicrob Agents Chemother. 1975 Jun;7(6):793–800. doi: 10.1128/aac.7.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern E. R., Richards J. T., Overall J. C., Jr, Glasgow L. A. Alteration of mortality and pathogenesis of three experimental Herpesvirus hominis infections of mice with adenine arabinoside 5'-monophosphate, adenine arabinoside, and phosphonoacetic acid. Antimicrob Agents Chemother. 1978 Jan;13(1):53–60. doi: 10.1128/aac.13.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano E. F., Berria M. I. Histological study of the progression of herpes simplex virus in mice. Arch Virol. 1980;64(1):67–79. doi: 10.1007/BF01317392. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Fortuny I. E. Adult herpes simplex hepatitis. Hum Pathol. 1972 Jun;3(2):277–281. doi: 10.1016/s0046-8177(72)80081-9. [DOI] [PubMed] [Google Scholar]
- Lopez C., Simmons R. L., Mauer S. M., Najarian J. S., Good R. A., Gentry S. Association of renal allograft rejection with virus infections. Am J Med. 1974 Mar;56(3):280–289. doi: 10.1016/0002-9343(74)90609-3. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Transformation of rat embryo cells by temperature-sensitive mutants of herpes simplex virus. J Gen Virol. 1974 Jul;24(1):143–153. doi: 10.1099/0022-1317-24-1-143. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Courtney R. J., Powell K. L., Schaffer P. A., Melnick M. B., Dreesman G. R., Anzai T., Adam E. Studies on herpes simplex virus and cancer. Cancer Res. 1976 Feb;36(2 Pt 2):845–856. [PubMed] [Google Scholar]
- Morrison R. E., Miller M. H., Lyon L. W., Griffiss J. M., Artenstein M. S. Adult meningoencephalitis caused by herpesvirus hominis type 2. Am J Med. 1974 Apr;56(4):540–544. doi: 10.1016/0002-9343(74)90486-0. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Alford C. A., Korones S. B. Infection of the newborn with herpesvirus hominis. Adv Pediatr. 1970;17:185–226. [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E. Cytology and histopathology of cervical herpes simplex infection. Cancer. 1966 Jul;19(7):1026–1031. doi: 10.1002/1097-0142(196607)19:7<1026::aid-cncr2820190718>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Naib Z. M., Nahmias A. J., Josey W. E., Kramer J. H. Genital herpetic infection. Association with cervical dysplasia and carcinoma. Cancer. 1969 Apr;23(4):940–945. doi: 10.1002/1097-0142(196904)23:4<940::aid-cncr2820230432>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Nash G., Foley F. D. Herpetic infection of the middle and lower respiratory tract. Am J Clin Pathol. 1970 Dec;54(6):857–863. doi: 10.1093/ajcp/54.6.857. [DOI] [PubMed] [Google Scholar]
- Nash G. Necrotizing tracheobronchitis and bronchopneumonia consistent with herpetic infection. Hum Pathol. 1972 Jun;3(2):283–291. doi: 10.1016/s0046-8177(72)80082-0. [DOI] [PubMed] [Google Scholar]
- Patrizi G., Middelkamp J. N., Reed C. A. Fine structure of herpes simplex virus hepatoadrenal necrosis in the newborn. Am J Clin Pathol. 1968 Mar;49(3):325–341. doi: 10.1093/ajcp/49.3.325. [DOI] [PubMed] [Google Scholar]
- Rabin E. R., Jenson A. B., Melnick J. L. Herpes simplex virus in mice: electron microscopy of neural spread. Science. 1968 Oct 4;162(3849):126–127. doi: 10.1126/science.162.3849.126. [DOI] [PubMed] [Google Scholar]
- Rosen P., Hajdu S. I. Visceral herpesvirus infections in patients with cancer. Am J Clin Pathol. 1971 Oct;56(4):459–465. doi: 10.1093/ajcp/56.4.459. [DOI] [PubMed] [Google Scholar]
- Sutton A. L., Smithwick E. M., Seligman S. J., Kim D. S. Fatal disseminated herpesvirus hominis type 2 infection in an adult with associated thymic dysplasia. Am J Med. 1974 Apr;56(4):545–553. doi: 10.1016/0002-9343(74)90487-2. [DOI] [PubMed] [Google Scholar]
- Templeton A. C. Generalized herpes simplex in malnourished children. J Clin Pathol. 1970 Feb;23(1):24–30. doi: 10.1136/jcp.23.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxen D. V., Cade J. F., McDonald M. I., Buchanan M. R., Clark R. J., Pain M. C. Herpes simplex virus from the lower respiratory tract in adult respiratory distress syndrome. Am Rev Respir Dis. 1982 Sep;126(3):416–419. doi: 10.1164/arrd.1982.126.3.416. [DOI] [PubMed] [Google Scholar]
- Vernon S. E. Herpetic tracheobronchitis: immunohistologic demonstration of herpes simplex virus antigen. Hum Pathol. 1982 Jul;13(7):683–686. doi: 10.1016/s0046-8177(82)80017-8. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Nahmias A. J., Visintine A. M., Fleming C. L., Alford C. A. The natural history of herpes simplex virus infection of mother and newborn. Pediatrics. 1980 Oct;66(4):489–494. [PubMed] [Google Scholar]