Abstract
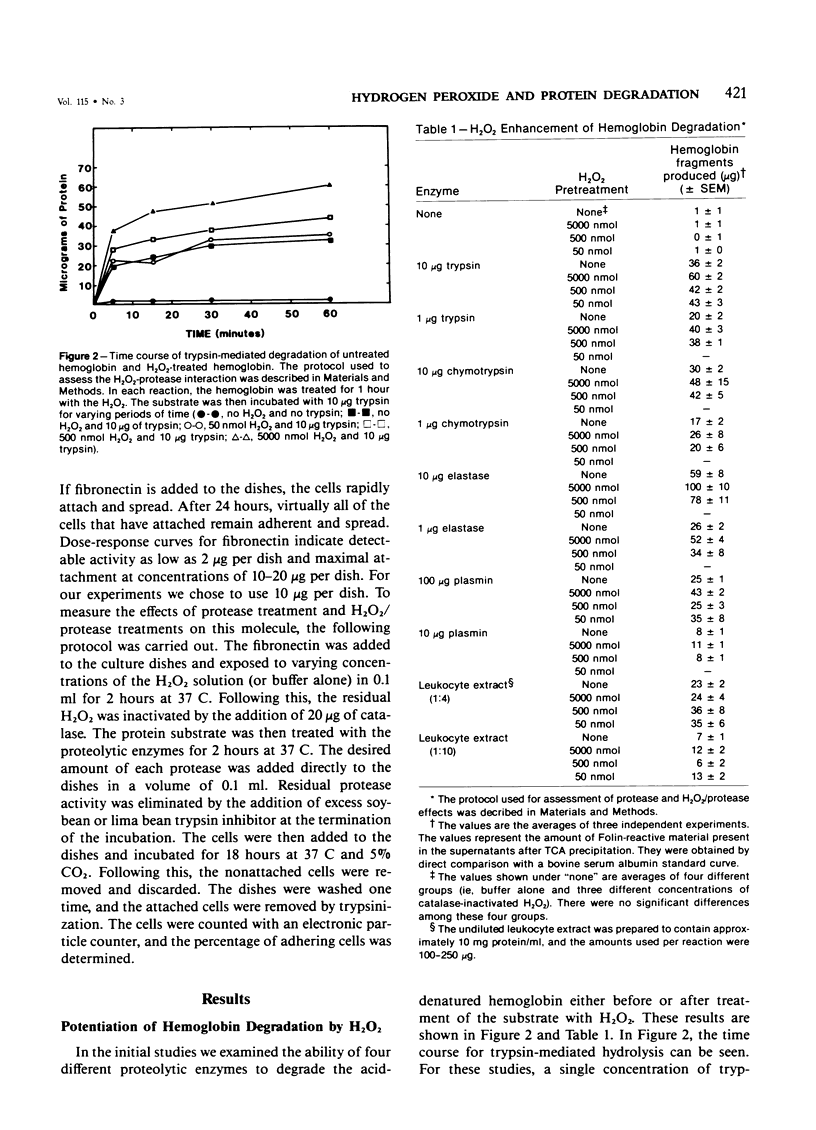
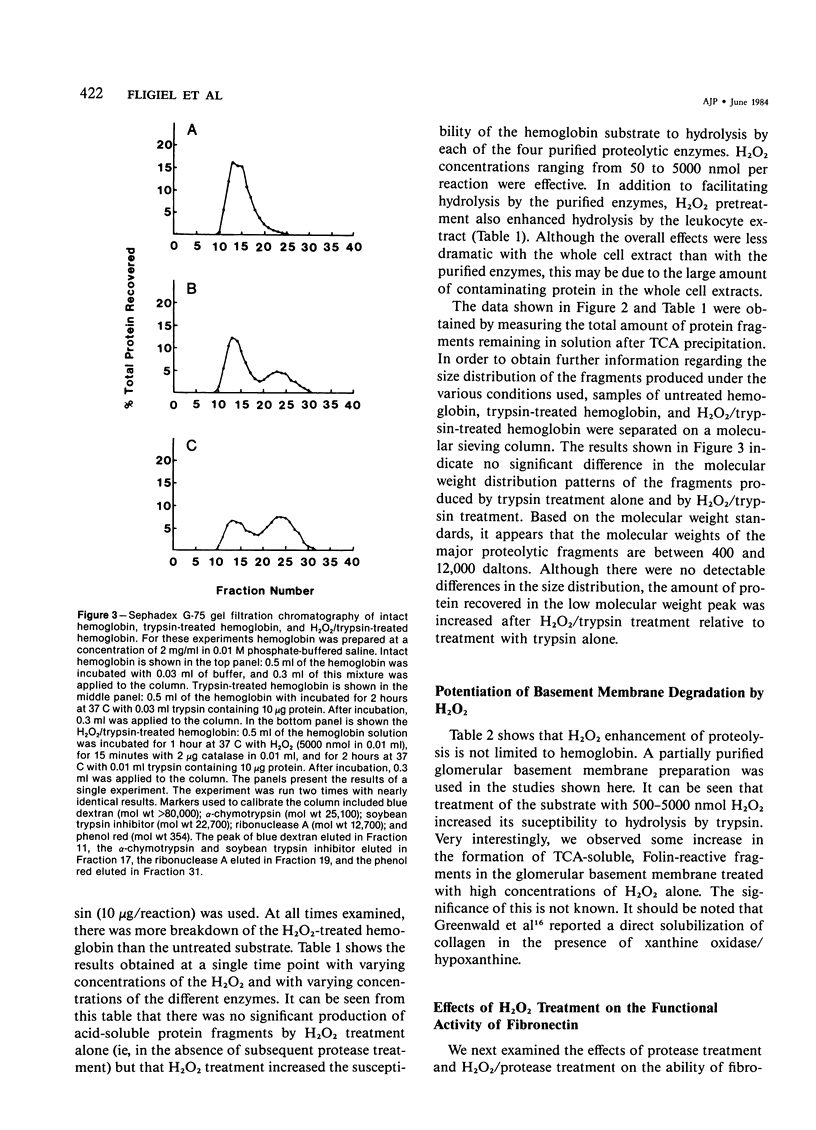
Pretreatment of hemoglobin with 50-5000 nmol hydrogen peroxide (H2O2) increased its susceptibility to proteolysis by a number of purified enzymes, including trypsin, chymotrypsin, elastase, and plasmin, and by the neutral protease of rat peritoneal leukocytes. Pretreatment of the protein substrate with catalase-inactivated H2O2 had no effect. Separation of the proteolytic fragments by G-75 Sephadex gel filtration indicated no apparent differences in the size distribution of the fragments produced by treatment with the H2O2/proteolytic enzyme combination as compared with enzyme treatment alone. A partially purified preparation of rat glomerular basement membrane was also treated with proteolytic enzyme alone or in combination with H2O2. As with the hemoglobin, pretreatment of the glomerular basement membrane with H2O2 increased its susceptibility to subsequent proteolytic attack. In addition, treatment of a basement membrane glycoprotein, fibronectin, with H2O2 also increased its sensitivity to subsequent proteolysis. These results suggest that in addition to their other proinflammatory activities, oxygen-derived metabolites may contribute to tissue destruction by altering the susceptibility of proteins to hydrolytic enzymes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979 Feb;63(2):177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Szot S., Venkatasubramanian K., Schiffmann E. Chemotactic factor inactivation by myeloperoxidase-mediated oxidation of methionine. J Immunol. 1980 Apr;124(4):2020–2026. [PubMed] [Google Scholar]
- Dahinden C. A., Fehr J., Hugli T. E. Role of cell surface contact in the kinetics of superoxide production by granulocytes. J Clin Invest. 1983 Jul;72(1):113–121. doi: 10.1172/JCI110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980 Apr;23(4):455–463. doi: 10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A., Moy W. W. Inhibition of collagen gelation by action of the superoxide radical. Arthritis Rheum. 1979 Mar;22(3):251–259. doi: 10.1002/art.1780220307. [DOI] [PubMed] [Google Scholar]
- Henderson W. R., Jörg A., Klebanoff S. J. Eosinophil peroxidase-mediated inactivation of leukotrienes B4, C4, and D4. J Immunol. 1982 Jun;128(6):2609–2613. [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A. Role of oxygen metabolites in immune complex injury of lung. J Immunol. 1981 Jun;126(6):2365–2369. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmeyer J. E., Snyderman R., Johnston R. B., Jr Stimulation of neutrophil oxidative metabolism by chemotactic peptides: influence of calcium ion concentration and cytochalasin B and comparison with stimulation by phorbol myristate acetate. Blood. 1979 Jul;54(1):35–45. [PubMed] [Google Scholar]
- Martin W. J., 2nd, Gadek J. E., Hunninghake G. W., Crystal R. G. Oxidant injury of lung parenchymal cells. J Clin Invest. 1981 Nov;68(5):1277–1288. doi: 10.1172/JCI110374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Free radicals and inflammation: protection of synovial fluid by superoxide dismutase. Science. 1974 Aug 9;185(4150):529–531. doi: 10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McCormick J. R., Harkin M. M., Johnson K. J., Ward P. A. Suppression by superoxide dismutase of immune-complex--induced pulmonary alveolitis and dermal inflammation. Am J Pathol. 1981 Jan;102(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Oyanagui Y. Participation of superoxide anions at the prostaglandin phase of carrageenan foot-oedema. Biochem Pharmacol. 1976 Jul 1;25(13):1465–1472. [PubMed] [Google Scholar]
- Petrone W. F., English D. K., Wong K., McCord J. M. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Parellada P., Planas J. M. Synovial fluid degradation induced by free radicals. In vitro action of several free radical scavengers and anti-inflammatory drugs. Biochem Pharmacol. 1978 Feb 15;27(4):535–537. doi: 10.1016/0006-2952(78)90390-8. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Smith D. C., Klebanoff S. J. A uterine fluid-mediated sperm-inhibitory system. Biol Reprod. 1970 Oct;3(2):229–235. doi: 10.1093/biolreprod/3.2.229. [DOI] [PubMed] [Google Scholar]
- Thurman R. G., Ley H. G., Scholz R. Hepatic microsomal ethanol oxidation. Hydrogen peroxide formation and the role of catalase. Eur J Biochem. 1972 Feb;25(3):420–430. doi: 10.1111/j.1432-1033.1972.tb01711.x. [DOI] [PubMed] [Google Scholar]
- Varani J., Lovett E. J., 3rd, Wicha M., Malinoff H., McCoy J. P., Jr Cell surface alpha-D-galactopyranosyl end groups: use as markers in the isolation of murine tumor cell lines with different cancer-causing potentials. J Natl Cancer Inst. 1983 Dec;71(6):1281–1287. [PubMed] [Google Scholar]
- Varani J., Ward D., Johnson K. J. Nonspecific protease and elastase activities in rat leukocytes. Inflammation. 1982 Jun;6(2):177–187. doi: 10.1007/BF00916242. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Duque R. E., Sulavik M. C., Johnson K. J. In vitro and in vivo stimulation of rat neutrophils and alveolar macrophages by immune complexes. Production of O-2 and H2O2. Am J Pathol. 1983 Mar;110(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane. Preparation and composition. Biochemistry. 1970 Sep 15;9(19):3837–3846. doi: 10.1021/bi00821a025. [DOI] [PubMed] [Google Scholar]



