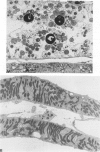
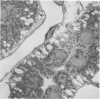
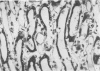Abstract
The hypothesis that decrease in energy demand may prevent anoxic cell damage has been examined in the medullary thick ascending limb of isolated perfused rat kidneys exposed to oxygen deprivation. The effects of decreasing active reabsorptive transport in the medullary thick ascending limb were observed on the extensive damage regularly induced by hypoxic perfusion (gassed with no oxygen) or potassium cyanide. Anoxic injury was consistently attenuated or abolished if reabsorptive transport was decreased with ouabain or furosemide or by halting the glomerular filtration rate with the use of a hyperoncotic medium (nonfiltering kidney). Comparison of the injury generated by warm ischemia for identical time periods showed that complete ischemia does not reproduce the severe lesions seen during hypoxic perfusion. These results suggest that transport activity is a determining factor of anoxic cell death in the thick ascending limb of Henle's loop.
Full text
PDF
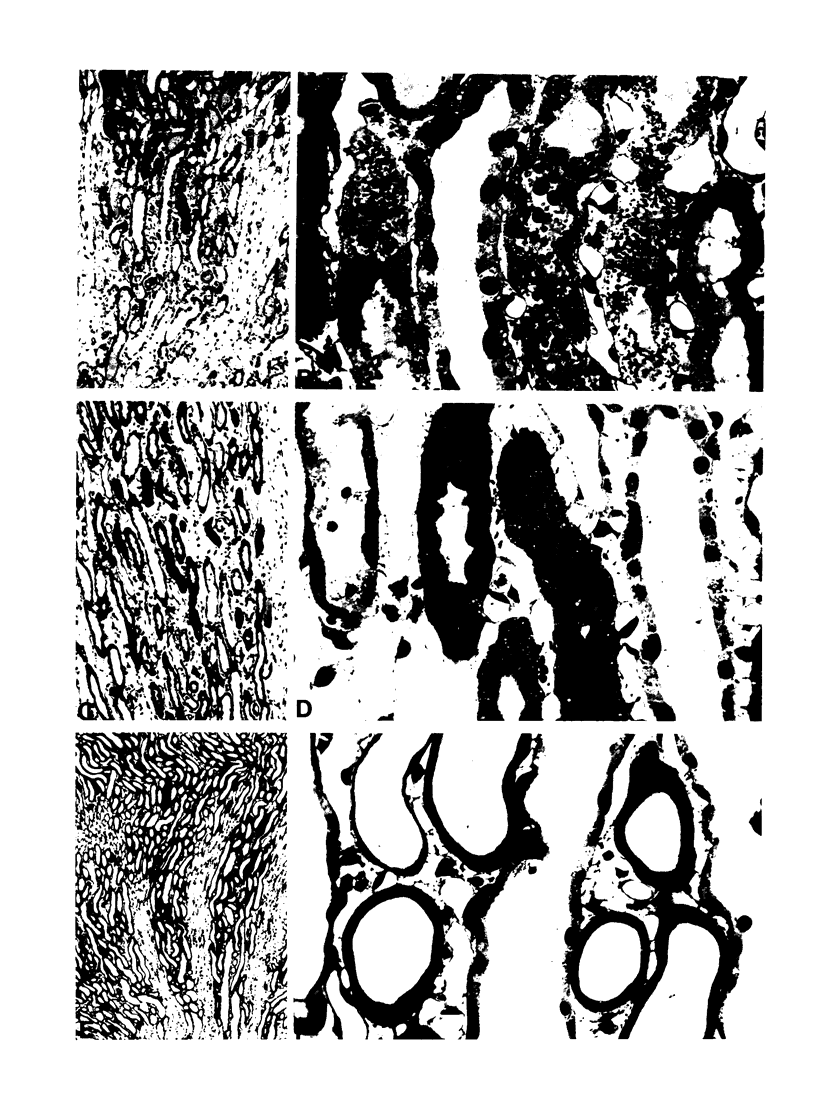








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn D., Emslie K. R., Ross B. D., Ryan G. B., Tange J. D. Selective distal nephron damage during isolated kidney perfusion. Kidney Int. 1981 May;19(5):638–647. doi: 10.1038/ki.1981.63. [DOI] [PubMed] [Google Scholar]
- Baumgärtl H., Leichtweiss H. P., Lübbers D. W., Weiss C., Huland H. The oxygen supply of the dog kidney: measurements of intrarenal pO 2 . Microvasc Res. 1972 Jul;4(3):247–257. doi: 10.1016/0026-2862(72)90036-2. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Selective glutathione depletion on function and structure of the isolated perfused rat kidney. Kidney Int. 1983 Aug;24(2):178–184. doi: 10.1038/ki.1983.142. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest. 1984 Jan;73(1):182–190. doi: 10.1172/JCI111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Transport activity modifies thick ascending limb damage in the isolated perfused kidney. Kidney Int. 1984 Jan;25(1):65–72. doi: 10.1038/ki.1984.9. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Spokes K., Epstein F. H. Polyene toxicity in renal medulla: injury mediated by transport activity. Science. 1984 Apr 6;224(4644):66–68. doi: 10.1126/science.6322305. [DOI] [PubMed] [Google Scholar]
- Chaudry I. H. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983 Aug;245(2):R117–R134. doi: 10.1152/ajpregu.1983.245.2.R117. [DOI] [PubMed] [Google Scholar]
- Donohoe J. F., Venkatachalam M. A., Bernard D. B., Levinsky N. G. Tubular leakage and obstruction after renal ischemia: structural-functional correlations. Kidney Int. 1978 Mar;13(3):208–222. doi: 10.1038/ki.1978.31. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Balaban R. S., Ross B. D. Redox state of cytochrome aa3 in isolated perfused rat kidney. Am J Physiol. 1982 Oct;243(4):F356–F363. doi: 10.1152/ajprenal.1982.243.4.F356. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Brosnan J. T., Tange J. D., Ross B. D. Improved function with amino acids in the isolated perfused kidney. Am J Physiol. 1982 Sep;243(3):F284–F292. doi: 10.1152/ajprenal.1982.243.3.F284. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Silva P., Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflugers Arch. 1981 Mar;389(3):263–270. doi: 10.1007/BF00584788. [DOI] [PubMed] [Google Scholar]
- Farber J. L. Biology of disease: membrane injury and calcium homeostasis in the pathogenesis of coagulative necrosis. Lab Invest. 1982 Aug;47(2):114–123. [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Biology of disease: free radicals and tissue injury. Lab Invest. 1982 Nov;47(5):412–426. [PubMed] [Google Scholar]
- Kessler R. J., Vande Zande H., Tyson C. A., Blondin G. A., Fairfield J., Glasser P., Green D. E. Uncouplers and the molecular mechanism of uncoupling in mitochondria. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2241–2245. doi: 10.1073/pnas.74.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer H. J., Schürmann J., Wassermann C., Düsing R. Prostaglandin-independent protection by furosemide from oliguric ischemic renal failure in conscious rats. Kidney Int. 1980 Apr;17(4):455–464. doi: 10.1038/ki.1980.53. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Lübbers D. W., Weiss C., Baumgärtl H., Reschke W. The oxygen supply of the rat kidney: measurements of int4arenal pO2. Pflugers Arch. 1969 Jun 19;309(4):328–349. doi: 10.1007/BF00587756. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Piantadosi C. A., Sylvia A. L., Jöbsis F. F. Cyanide-induced cytochrome a,a3 oxidation-reduction responses in rat brain in vivo. J Clin Invest. 1983 Oct;72(4):1224–1233. doi: 10.1172/JCI111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. Synaptic activity mediates death of hypoxic neurons. Science. 1983 Apr 29;220(4596):536–537. doi: 10.1126/science.6836300. [DOI] [PubMed] [Google Scholar]
- Rude R. E., Muller J. E., Braunwald E. Efforts to limit the size of myocardial infarcts. Ann Intern Med. 1981 Dec;95(6):736–761. doi: 10.7326/0003-4819-95-6-736. [DOI] [PubMed] [Google Scholar]
- Shlafer M., Kane P. F., Wiggins V. Y., Kirsh M. M. Possible role for cytotoxic oxygen metabolites in the pathogenesis of cardiac ischemic injury. Circulation. 1982 Aug;66(2 Pt 2):I85–I92. [PubMed] [Google Scholar]
- Thurau K., Boylan J. W. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med. 1976 Sep;61(3):308–315. doi: 10.1016/0002-9343(76)90365-x. [DOI] [PubMed] [Google Scholar]