Abstract
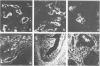
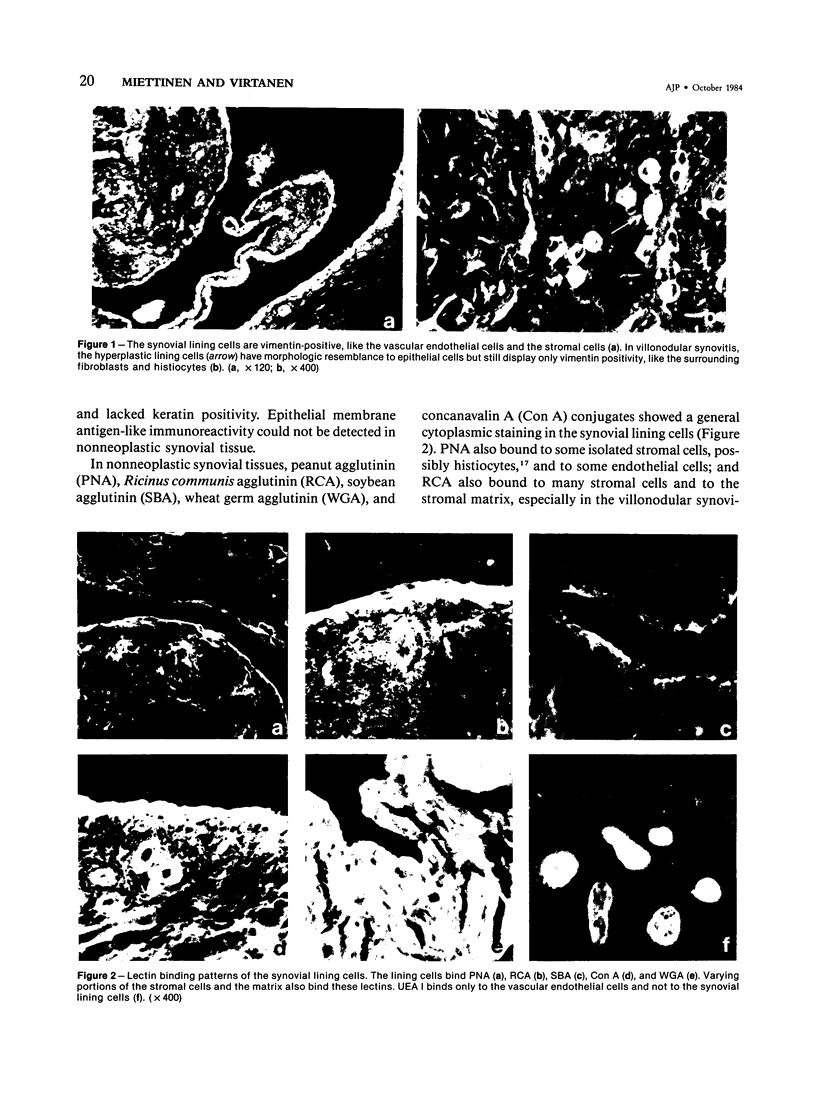
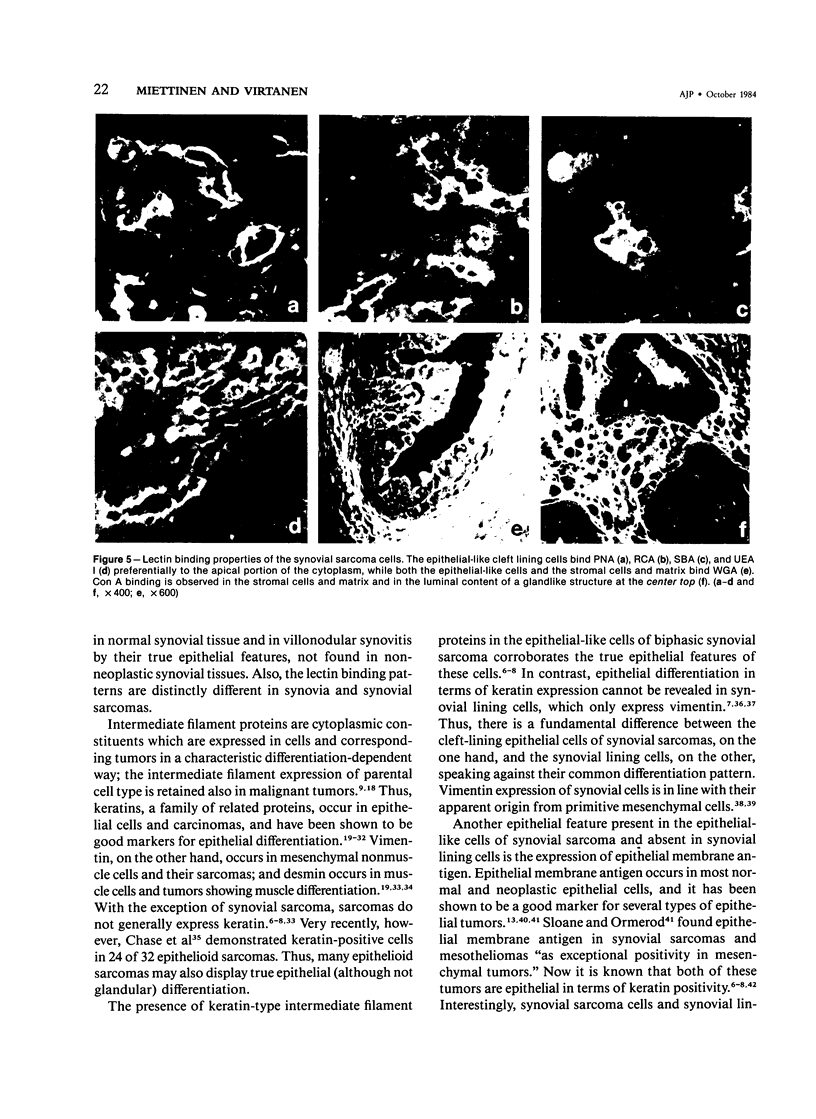
For an evaluation of the putative histogenetic relationship of synovia and synovial sarcomas, normal synovia, villonodular synovitis, and synovial sarcomas were compared for their patterns of expression of intermediate filaments of keratin and vimentin type and epithelial membrane antigen and for lectin binding sites. The lining cells in both normal synovia and villonodular synovitis reacted with anti-vimentin antibodies, but not with antibodies to different types of keratins or epithelial membrane antigen. The cleft-lining cells in synovial sarcoma, on the other hand, showed only keratin positivity, and epithelial membrane antigen could also be detected in these cells. Nonneoplastic synovial lining cells bound peanut agglutinin (PNA), Ricinus communis agglutinin (RCA), soybean agglutinin (SBA), concanavalin A (Con A), and wheat germ agglutinin (WGA) conjugates, but not Ulex europaeus I lectin (UEA I). In contrast, the epithelial-like cleft lining cells in synovial sarcomas showed an apical cytoplasmic binding of PNA, UEA I, RCA, and SBA, and binding of WGA to the whole cytoplasm but did not bind Con A. The distinct differences between synovial lining cells and synovial sarcoma cells speak against synovial cell features in synovial sarcoma. These results indicate that synovial sarcoma is a carcinosarcomalike tumor with true epithelial differentiation, and the term "synovial sarcoma" apparently is a misnomer that should be abandoned.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmannsberger M., Osborn M., Hölscher A., Schauer A., Weber K. The distribution of keratin type intermediate filaments in human breast cancer. An immunohistological study. Virchows Arch B Cell Pathol Incl Mol Pathol. 1981;37(3):277–284. doi: 10.1007/BF02892576. [DOI] [PubMed] [Google Scholar]
- Altmannsberger M., Weber K., Hölscher A., Schauer A., Osborn M. Antibodies to intermediate filaments as diagnostic tools: human gastrointestinal carcinomas express prekeratin. Lab Invest. 1982 May;46(5):520–526. [PubMed] [Google Scholar]
- Balsaver A. M., Gibley C. W., Jr, Tessmer C. F. Ultrastructural studies in Wilms's tumor. Cancer. 1968 Aug;22(2):417–427. doi: 10.1002/1097-0142(196808)22:2<417::aid-cncr2820220220>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Barsky S. H., Siegal G. P., Jannotta F., Liotta L. A. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983 Aug;49(2):140–147. [PubMed] [Google Scholar]
- Birembaut P., Caron Y., van Cauwenberge D., Foidart J. M. Distribution of laminin, a basement membrane glycoprotein in epithelial proliferations. A preliminary study in the breast, the lungs and uterine cervix. Coll Relat Res. 1983;3(1):25–31. [PubMed] [Google Scholar]
- Bonazzi del Poggetto C., Virtanen I., Lehto V. P., Wahlström T., Saksela E. Expression of intermediate filaments in ovarian and uterine tumors. Int J Gynecol Pathol. 1983;1(4):359–366. doi: 10.1097/00004347-198301040-00006. [DOI] [PubMed] [Google Scholar]
- Brozman M. Immunohistochemical analysis of formaldehyde- and trypsin- or pepsin-treated material. Acta Histochem. 1978;63(2):251–260. doi: 10.1016/S0065-1281(78)80032-4. [DOI] [PubMed] [Google Scholar]
- CADMAN N. L., SOULE E. H., KELLY P. J. SYNOVIAL SARCOMA; AN ANALYSIS OF 34 TUMORS. Cancer. 1965 May;18:613–627. doi: 10.1002/1097-0142(196505)18:5<613::aid-cncr2820180510>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Corson J. M., Pinkus G. S. Mesothelioma: profile of keratin proteins and carcinoembryonic antigen: an immunoperoxidase study of 20 cases and comparison with pulmonary adenocarcinomas. Am J Pathol. 1982 Jul;108(1):80–88. [PMC free article] [PubMed] [Google Scholar]
- Corson J. M., Weiss L. M., Banks-Schlegel S. P., Pinkus G. S. Keratin proteins in synovial sarcoma. Am J Surg Pathol. 1983 Jan;7(1):107–109. doi: 10.1097/00000478-198301000-00014. [DOI] [PubMed] [Google Scholar]
- Denk H., Krepler R., Artlieb U., Gabbiani G., Rungger-Brändle E., Leoncini P., Franke W. W. Proteins of intermediate filaments. An immunohistochemical and biochemical approach to the classification of soft tissue tumors. Am J Pathol. 1983 Feb;110(2):193–208. [PMC free article] [PubMed] [Google Scholar]
- Dische F. E., Darby A. J., Howard E. R. Malignant synovioma: electron microscopical findings in three patients and review of the literature. J Pathol. 1978 Mar;124(3):149–155. doi: 10.1002/path.1711240304. [DOI] [PubMed] [Google Scholar]
- Espinoza C. G., Azar H. A. Immunohistochemical localization of keratin-type proteins in epithelial neoplasms. Correlation with electron microscopic findings. Am J Clin Pathol. 1982 Oct;78(4):500–507. doi: 10.1093/ajcp/78.4.500. [DOI] [PubMed] [Google Scholar]
- Evans H. L. Synovial sarcoma. A study of 23 biphasic and 17 probable monophasic examples. Pathol Annu. 1980;15(Pt 2):309–331. [PubMed] [Google Scholar]
- Farris K. B., Reed R. J. Monophasic, glandular, synovial sarcomas and carcinomas of the soft tissues. Arch Pathol Lab Med. 1982 Mar;106(3):129–132. [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franklin W. A. Tissue binding of lectins in disorders of the breast. Cancer. 1983 Jan 15;51(2):295–300. doi: 10.1002/1097-0142(19830115)51:2<295::aid-cncr2820510222>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- GRAY D. J., GARDNER E. Prenatal development of the human knee and superior tibiofibular joints. Am J Anat. 1950 Mar;86(2):235–287. doi: 10.1002/aja.1000860204. [DOI] [PubMed] [Google Scholar]
- Gabbiani G., Kaye G. I., Lattes R., Majno G. Synovial sarcoma. Electron microscopic study of a typical case. Cancer. 1971 Oct;28(4):1031–1039. doi: 10.1002/1097-0142(1971)28:4<1031::aid-cncr2820280429>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Roy S. Experimentally produced synovial sarcomas. Cancer. 1966 Dec;19(12):1901–1908. doi: 10.1002/1097-0142(196612)19:12<1901::aid-cncr2820191218>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hayes C. E. The lectins: carbohydrate-binding proteins of plants and animals. Adv Carbohydr Chem Biochem. 1978;35:127–340. doi: 10.1016/s0065-2318(08)60220-6. [DOI] [PubMed] [Google Scholar]
- Heyderman E., Steele K., Ormerod M. G. A new antigen on the epithelial membrane: its immunoperoxidase localisation in normal and neoplastic tissue. J Clin Pathol. 1979 Jan;32(1):35–39. doi: 10.1136/jcp.32.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthöfer H., Miettinen A., Paasivuo R., Lehto V. P., Linder E., Alfthan O., Virtanen I. Cellular origin and differentiation of renal carcinomas. A fluorescence microscopic study with kidney-specific antibodies, antiintermediate filament antibodies, and lectins. Lab Invest. 1983 Sep;49(3):317–326. [PubMed] [Google Scholar]
- Holthöfer H., Virtanen I., Pettersson E., Törnroth T., Alfthan O., Linder E., Miettinen A. Lectins as fluorescence microscopic markers for saccharides in the human kidney. Lab Invest. 1981 Nov;45(5):391–399. [PubMed] [Google Scholar]
- Howard D. R., Batsakis J. G. Peanut agglutinin: a new marker for tissue histiocytes. Am J Clin Pathol. 1982 Apr;77(4):401–408. doi: 10.1093/ajcp/77.4.401. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Ingber D. E., Muresan V., Hull B. E., Sarras M. P., Jr, Maylié-Pfenninger M. F., Iwanij V. Cell surface properties of normal, differentiating, and neoplastic pancreatic acinar cells. Cancer. 1981 Mar 15;47(6 Suppl):1516–1527. doi: 10.1002/1097-0142(19810315)47:6+<1516::aid-cncr2820471413>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Kahn H. J., Yeger H., Baumal R., Thom H., Phillips J. M. Categorization of pediatric neoplasms by immunostaining with antiprekeratin and antivimentin antisera. Cancer. 1983 Feb 15;51(4):645–653. doi: 10.1002/1097-0142(19830215)51:4<645::aid-cncr2820510417>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Katenkamp D., Stiller D. Synovial sarcoma of the abdominal wall. Light microscopic, histochemical and electron microscopic investigations. Virchows Arch A Pathol Anat Histol. 1980;388(3):349–360. doi: 10.1007/BF00430864. [DOI] [PubMed] [Google Scholar]
- Krall R. A., Kostianovsky M., Patchefsky A. S. Synovial sarcoma: a clinical, pathological, and ultrastructural study of 26 cases supporting the recognition of a monophasic variant. Am J Surg Pathol. 1981 Mar;5(2):137–151. [PubMed] [Google Scholar]
- Krepler R., Denk H., Weirich E., Schmid E., Franke W. W. Keratin-like proteins in normal and neoplastic cells of human and rat mammary gland as revealed by immunofluorescence microscopy. Differentiation. 1981;20(3):242–252. doi: 10.1111/j.1432-0436.1981.tb01179.x. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Stenman S., Miettinen M., Dahl D., Virtanen I. Expression of a neural type of intermediate filament as a distinguishing feature between oat cell carcinoma and other lung cancers. Am J Pathol. 1983 Feb;110(2):113–118. [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Nicolson G. L. Purification of cell membrane glycoproteins by lectin affinity chromatography. Biochim Biophys Acta. 1979 Dec 20;559(4):329–376. doi: 10.1016/0304-4157(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Mackenzie D. H. Synovial sarcoma. A review of 58 cases. Cancer. 1966 Feb;19(2):169–180. doi: 10.1002/1097-0142(196602)19:2<169::aid-cncr2820190205>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Barwick K. W. An immunohistochemical study of nasopharyngeal neoplasms using keratin antibodies: epithelial versus nonepithelial neoplasms. Am J Surg Pathol. 1982 Mar;6(2):143–149. doi: 10.1097/00000478-198203000-00006. [DOI] [PubMed] [Google Scholar]
- Mickelson M. R., Brown G. A., Maynard J. A., Cooper R. R., Bonfiglio M. Synovial sarcoma: an electron microscopic study of monophasic and biphasic forms. Cancer. 1980 Apr 15;45(8):2109–2118. doi: 10.1002/1097-0142(19800415)45:8<2109::aid-cncr2820450819>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Foidart J. M., Ekblom P. Immunohistochemical demonstration of laminin, the major glycoprotein of basement membranes, as an aid in the diagnosis of soft tissue tumors. Am J Clin Pathol. 1983 Mar;79(3):306–311. doi: 10.1093/ajcp/79.3.306. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Alveolar rhabdomyosarcoma. Demonstration of the muscle type of intermediate filament protein, desmin, as a diagnostic aid. Am J Pathol. 1982 Aug;108(2):246–251. [PMC free article] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Badley R. A., Virtanen I. Expression of intermediate filaments in soft-tissue sarcomas. Int J Cancer. 1982 Nov 15;30(5):541–546. doi: 10.1002/ijc.2910300502. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Vartio T., Virtanen I. Epithelioid sarcoma. Ultrastructural and immunohistologic features suggesting a synovial origin. Arch Pathol Lab Med. 1982 Nov;106(12):620–623. [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Expression of intermediate filaments in normal ovaries and ovarian epithelial, sex cord-stromal, and germinal tumors. Int J Gynecol Pathol. 1983;2(1):64–71. doi: 10.1097/00004347-198301000-00006. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Keratin in the epithelial-like cells of classical biphasic synovial sarcoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):157–161. doi: 10.1007/BF02932860. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Monophasic synovial sarcoma of spindle-cell type. Epithelial differentiation as revealed by ultrastructural features, content of prekeratin and binding of peanut agglutinin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1983;44(2):187–199. doi: 10.1007/BF02890169. [DOI] [PubMed] [Google Scholar]
- Miettinen M., Lehto V. P., Virtanen I. Nasopharyngeal lymphoepithelioma. Histological diagnosis as aided by immunohistochemical demonstration of keratin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982 Aug;40(2):163–169. [PubMed] [Google Scholar]
- Nagle R. B., McDaniel K. M., Clark V. A., Payne C. M. The use of antikeratin antibodies in the diagnosis of human neoplasms. Am J Clin Pathol. 1983 Apr;79(4):458–466. doi: 10.1093/ajcp/79.4.458. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tumor diagnosis by intermediate filament typing: a novel tool for surgical pathology. Lab Invest. 1983 Apr;48(4):372–394. [PubMed] [Google Scholar]
- Osung O. A., Chandra M., Holborow E. J. Intermediate filaments in synovial lining cells in rheumatoid arthritis and other arthritides are of vimentin type. Ann Rheum Dis. 1982 Feb;41(1):74–77. doi: 10.1136/ard.41.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisa R., Bonetti F., Chilosi M., Iannucci A., Menestrina F. Synovial sarcoma enzyme histochemistry of a typical case. Virchows Arch A Pathol Anat Histopathol. 1982;398(1):67–73. doi: 10.1007/BF00585614. [DOI] [PubMed] [Google Scholar]
- Ramaekers F., Huysmans A., Moesker O., Kant A., Jap P., Herman C., Vooijs P. Monoclonal antibody to keratin filaments, specific for glandular epithelia and their tumors. Use in surgical pathology. Lab Invest. 1983 Sep;49(3):353–361. [PubMed] [Google Scholar]
- Ramaekers F., Puts J., Moesker O., Kant A., Jap P., Vooijs P. Demonstration of keratin in human adenocarcinomas. Am J Pathol. 1983 May;111(2):213–223. [PMC free article] [PubMed] [Google Scholar]
- Roth J. A., Enzinger F. M., Tannenbaum M. Synovial sarcoma of the neck: a followup study of 24 cases. Cancer. 1975 Apr;35(4):1243–1253. doi: 10.1002/1097-0142(197504)35:4<1243::aid-cncr2820350432>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Said J. W. Immunohistochemical localization of keratin proteins in tumor diagnosis. Hum Pathol. 1983 Dec;14(12):1017–1019. doi: 10.1016/s0046-8177(83)80255-x. [DOI] [PubMed] [Google Scholar]
- Saxén L., Koskimies O., Lahti A., Miettinen H., Rapola J., Wartiovaara J. Differentiation of kidney mesenchyme in an experimental model system. Adv Morphog. 1968;7:251–293. doi: 10.1016/b978-1-4831-9954-2.50011-2. [DOI] [PubMed] [Google Scholar]
- Schmidt D., Mackay B. Ultrastructure of human tendon sheath and synovium: implications for tumor histogenesis. Ultrastruct Pathol. 1982 Jul-Sep;3(3):269–283. doi: 10.3109/01913128209016653. [DOI] [PubMed] [Google Scholar]
- Sloane J. P., Ormerod M. G. Distribution of epithelial membrane antigen in normal and neoplastic tissues and it value in diagnostic tumor pathology. Cancer. 1981 Apr 1;47(7):1786–1795. doi: 10.1002/1097-0142(19810401)47:7<1786::aid-cncr2820470711>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- To A., Dearnaley D. P., Ormerod M. G., Canti G., Coleman D. V. Epithelial Membrane Antigen. Its use in the cytodiagnosis of malignancy in serous effusions. Am J Clin Pathol. 1982 Aug;78(2):214–219. doi: 10.1093/ajcp/78.2.214. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Lehto V. P., Lehtonen E., Vartio T., Stenman S., Kurki P., Wager O., Small J. V., Dahl D., Badley R. A. Expression of intermediate filaments in cultured cells. J Cell Sci. 1981 Aug;50:45–63. doi: 10.1242/jcs.50.1.45. [DOI] [PubMed] [Google Scholar]
- Virtanen I., von Koskull H., Lehto V. P., Vartio T., Aula P. Cultured human amniotic fluid cells characterized with antibodies against intermediate filaments in indirect immunofluorescence microscopy. J Clin Invest. 1981 Nov;68(5):1348–1355. doi: 10.1172/JCI110382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whillis J. The development of synovial joints. J Anat. 1940 Jan;74(Pt 2):277–283. [PMC free article] [PubMed] [Google Scholar]
- Yonezawa S., Nakamura T., Tanaka S., Sato E. Glycoconjugate with Ulex europaeus agglutinin-I-binding sites in normal mucosa, adenoma, and carcinoma of the human large bowel. J Natl Cancer Inst. 1982 Oct;69(4):777–785. [PubMed] [Google Scholar]