Abstract
Leupeptin, a nontoxic thiol protease inhibitor, has been proposed to have therapeutic use in hereditary muscular dystrophies. The purpose of this study was to characterize the in vivo changes in proteolytic activity of skeletal muscles induced by the repeated administration of leupeptin. Further, whether the modulation of proteolytic capacity by leupeptin affects the repair process of muscle injuries caused by heavy exercise was studied. Leupeptin was administered in mice intraperitoneally at a dose level of 15.5 mg/kg twice a day for 9 days. Leupeptin, known to be an inhibitor of cathepsin B both in vitro and after a single injection in vivo, paradoxically induced an increase of cathepsin B activity in mouse skeletal muscles after repeated administration. In addition, leupeptin administration for 9 days increased the activities of cathepsins C and D, as well as the rate of acid autolysis. The activity of beta-glucuronidase also increased, while those of arylsulfatase, ribonuclease, and alkaline protease were unaffected. No histopathologic changes were observed. At the low dosage used, leupeptin had no effect on the repair process of skeletal muscle after exercise injuries, although several proteolytic processes occur during the regeneration. It is suggested that the increase of acid protease activities in skeletal muscles is an adaptive response to the administration of the proteolytic inhibitor leupeptin and that leupeptin can be administered without prevention or delay of regenerative processes after the onset of myopathic changes.
Full text
PDF

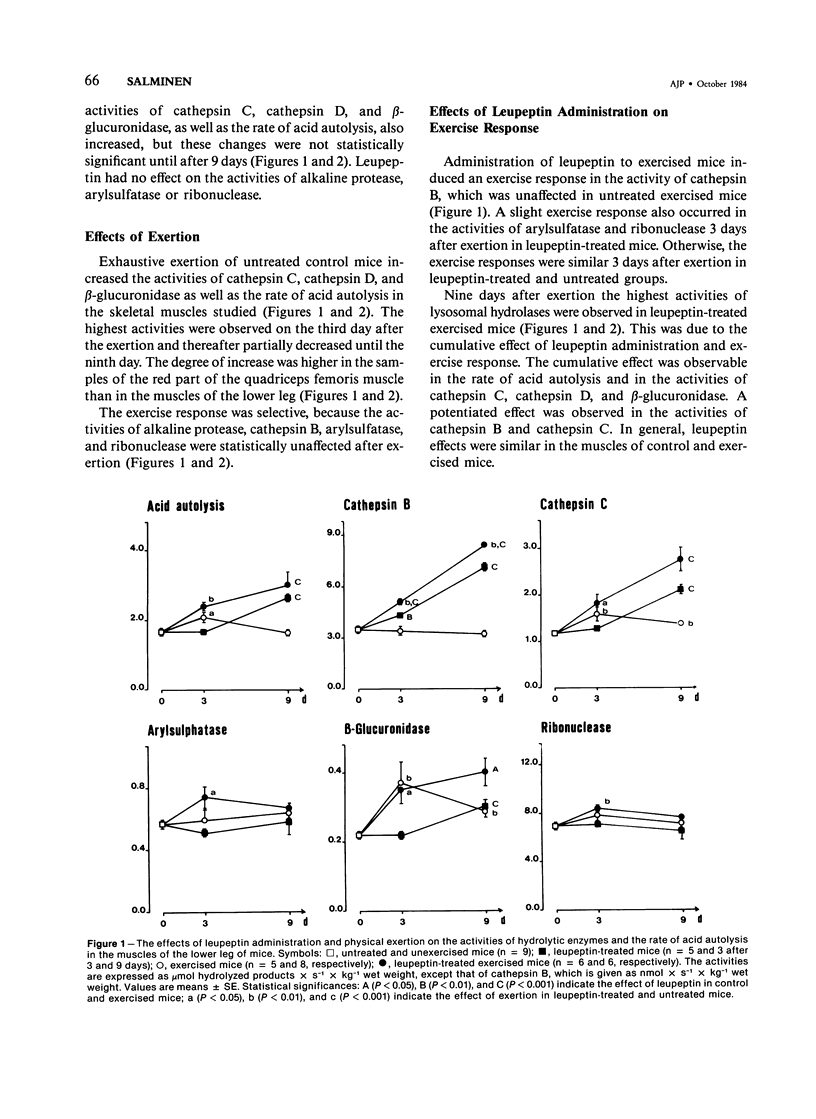
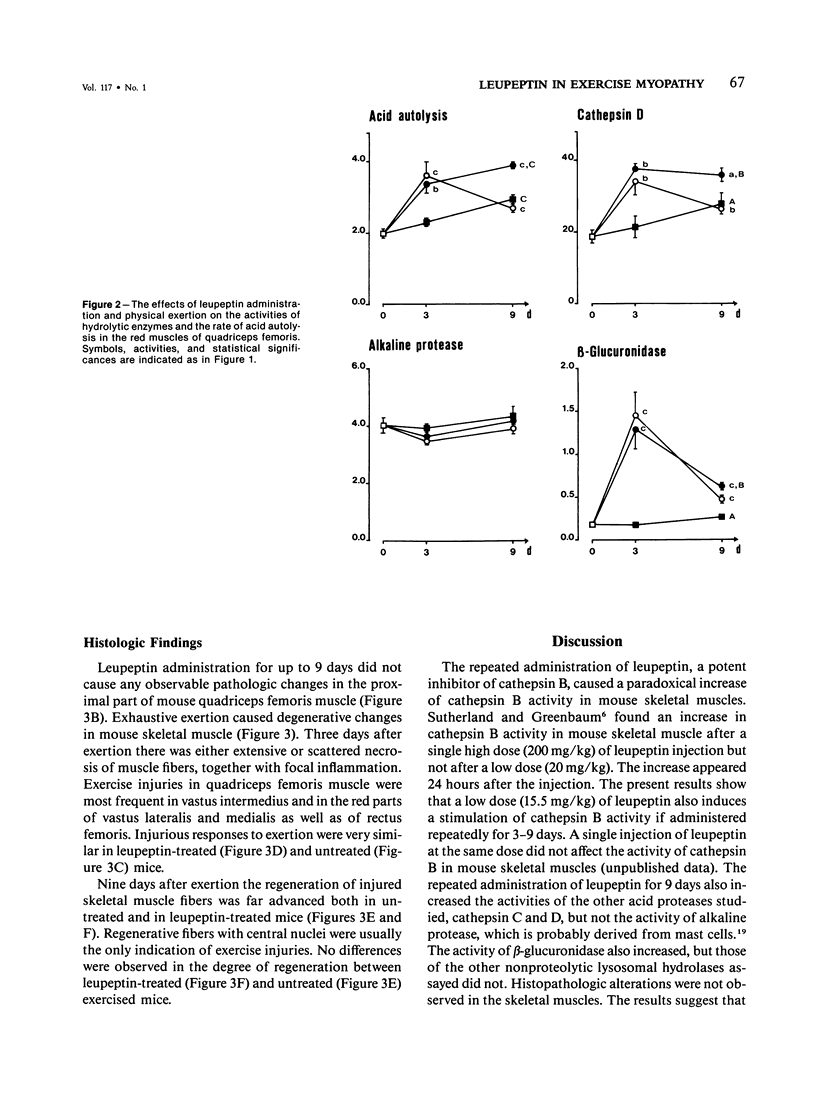
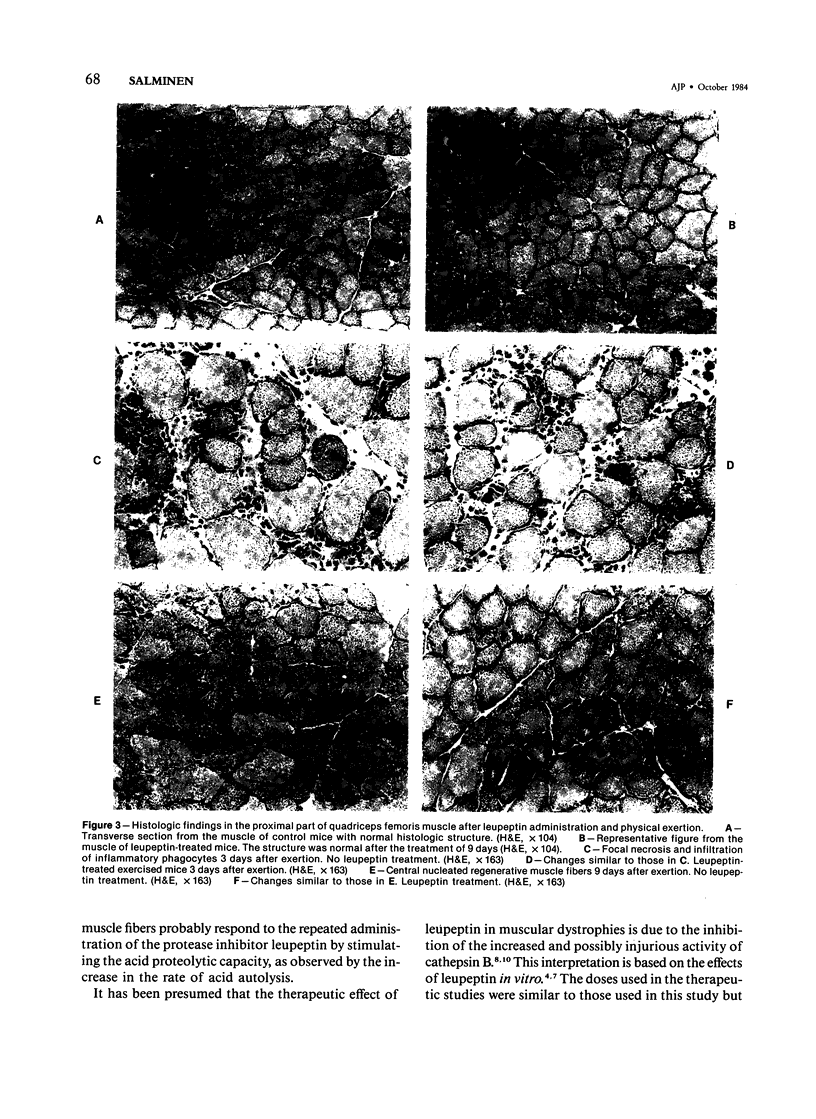


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Brown C. P., Butler P. E. The inactivation of streptomyces-derived proteinase inhibitors by mammalian tissue preparations. Acta Biol Med Ger. 1981;40(10-11):1539–1546. [PubMed] [Google Scholar]
- Furuno K., Ishikawa T., Kato K. Appearance of autolysosomes in rat liver after leupeptin treatment. J Biochem. 1982 May;91(5):1485–1494. doi: 10.1093/oxfordjournals.jbchem.a133840. [DOI] [PubMed] [Google Scholar]
- Huisman W., Lanting L., Doddema H. J., Bouma J. M., Gruber M. Role of individual cathepsins in lysosomal protein digestion as tested by specific inhibitors. Biochim Biophys Acta. 1974 Nov 25;370(1):297–307. doi: 10.1016/0005-2744(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Kato T., Okada S., Yutaka T., Yabuuchi H. Induction of beta-galactosidase in beta-galactosidase-alpha-neuraminidase deficiency: effects of leupeptin and sucrose. Biochem Int. 1983 Feb;6(2):267–273. [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Libby P., Ingwall J. S., Goldberg A. L. Reduction of protein degradation and atrophy in cultured fetal mouse hearts by leupeptin. Am J Physiol. 1979 Jul;237(1):E35–E39. doi: 10.1152/ajpendo.1979.237.1.E35. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Shafiq S. A., Stracher A. Delayed degeneration of dystrophic and normal muscle cell cultures treated with pepstatin, leupeptin, and antipain. Exp Neurol. 1976 Mar;50(3):649–657. doi: 10.1016/0014-4886(76)90034-0. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Shuto K., Tsurufuji S. An improved method for determining the activity of benzoyl-arginine p-nitroanilide-hydrolyzing enzyme in crude tissue extracts. Anal Biochem. 1978 Jun 15;87(1):257–262. doi: 10.1016/0003-2697(78)90592-4. [DOI] [PubMed] [Google Scholar]
- Pontremoli S., Melloni E., Salamino F., Sparatore B., Michetti M., Horecker B. L. Endogenous inhibitors of lysosomal proteinases. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1261–1264. doi: 10.1073/pnas.80.5.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A., Vihko V. Autophagic response to strenuous exercise in mouse skeletal muscle fibers. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45(1):97–106. doi: 10.1007/BF02889856. [DOI] [PubMed] [Google Scholar]
- Salminen A., Vihko V. Effects of age and prolonged running on proteolytic capacity in mouse cardiac and skeletal muscles. Acta Physiol Scand. 1981 May;112(1):89–95. doi: 10.1111/j.1748-1716.1981.tb06787.x. [DOI] [PubMed] [Google Scholar]
- Sher J. H., Stracher A., Shafiq S. A., Hardy-Stashin J. Successful treatment of murine muscular dystrophy with the proteinase inhibitor leupeptin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7742–7744. doi: 10.1073/pnas.78.12.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracher A., McGowan E. B., Shafiq S. A. Muscular dystrophy: inhibition of degeneration in vivo with protease inhibitors. Science. 1978 Apr 7;200(4337):50–51. doi: 10.1126/science.635570. [DOI] [PubMed] [Google Scholar]
- Sutherland J. H., Greenbaum L. M. Paradoxical effect of leupeptin in vivo on cathepsin B activity. Biochem Biophys Res Commun. 1983 Jan 14;110(1):332–338. doi: 10.1016/0006-291x(83)91300-1. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Ikegaki N., Ichihara A. Effects of leupeptin and pepstatin on protein turnover in adult rat hepatocytes in primary culture. Arch Biochem Biophys. 1981 Apr 15;208(1):296–304. doi: 10.1016/0003-9861(81)90152-1. [DOI] [PubMed] [Google Scholar]
- Vihko V., Rantamäki J., Salminen A. Exhaustive physical exercise and acid hydrolase activity in mouse skeletal muscle. A histochemical study. Histochemistry. 1978 Sep 15;57(3):237–249. doi: 10.1007/BF00492083. [DOI] [PubMed] [Google Scholar]
- Vihko V., Salminen A., Rantamäki J. Acid hydrolase activity in red and white skeletal muscle of mice during a two-week period following exhausting exercise. Pflugers Arch. 1978 Dec 28;378(2):99–106. doi: 10.1007/BF00584441. [DOI] [PubMed] [Google Scholar]



