Abstract
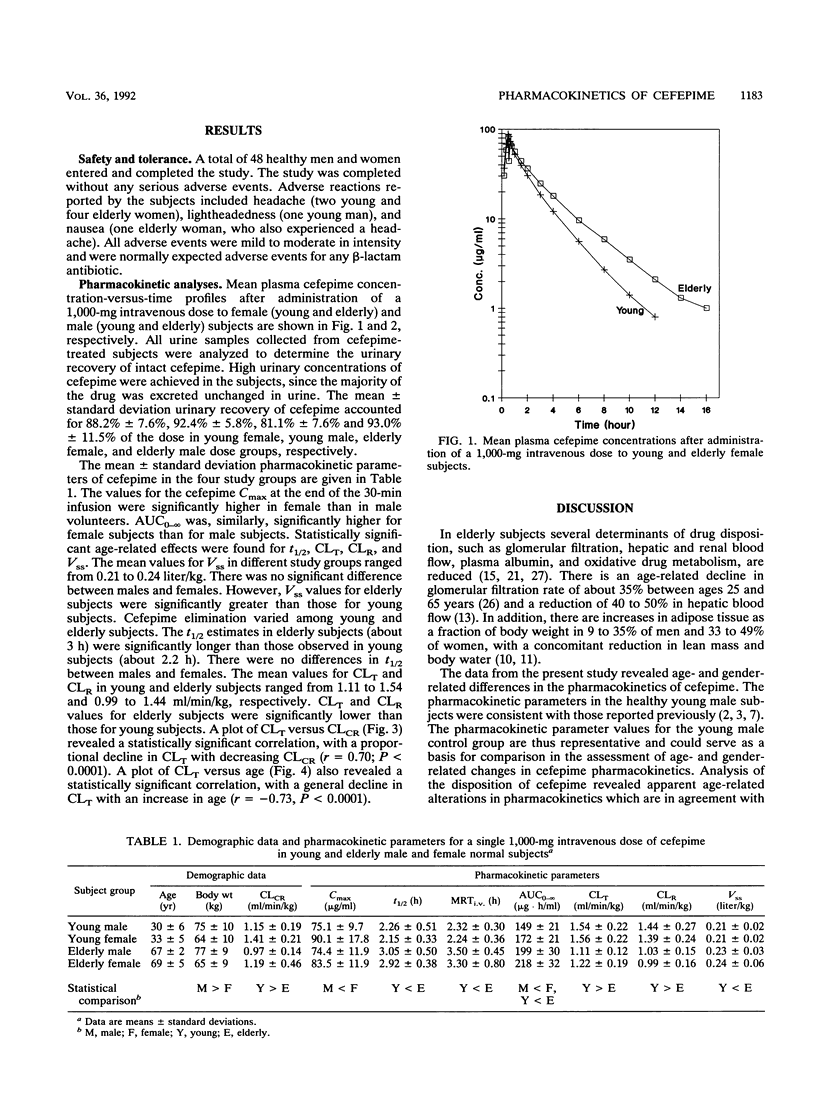
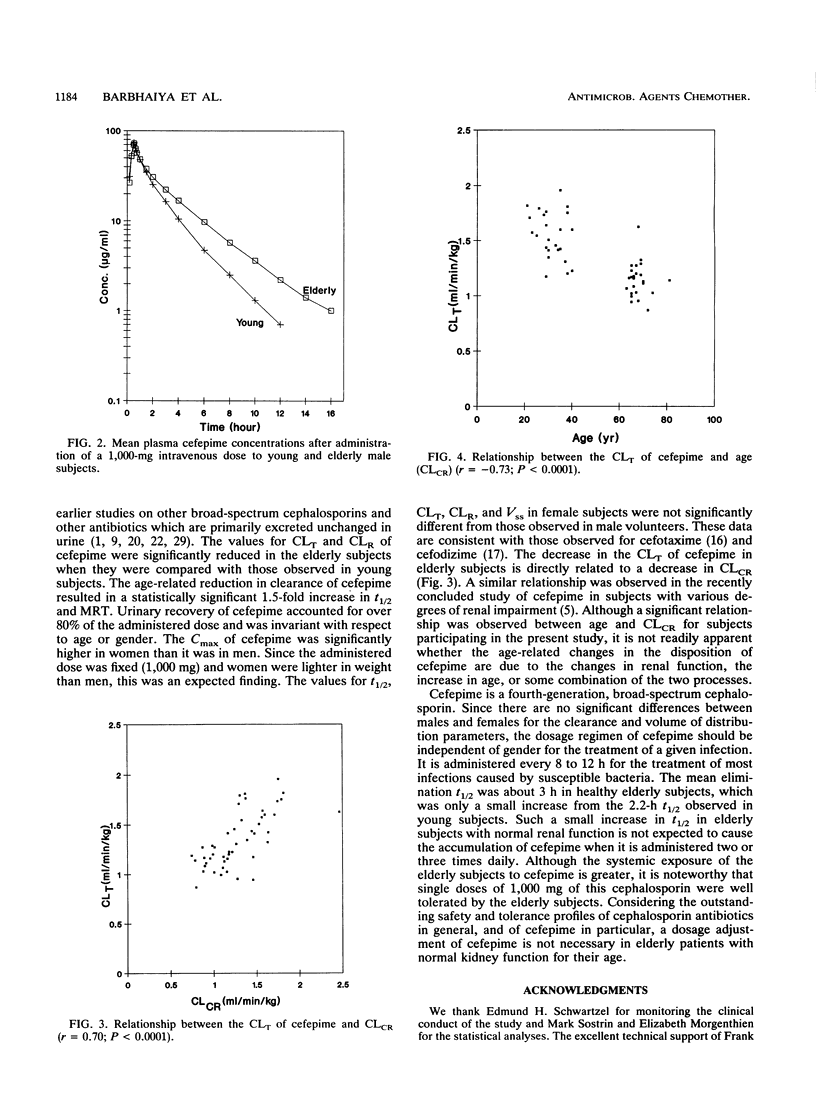
The effects of age and gender on the pharmacokinetics of cefepime were examined in 48 volunteers following administration of a single 1,000-mg intravenous dose. Male and female subjects were divided into four groups, each consisting of 12 subjects, according to their age and gender. The young subjects were between 20 and 40 years of age and elderly subjects were between 65 and 81 years of age. Serial blood and urine samples were collected from each subject and were analyzed for cefepime by validated high-pressure liquid chromatographic assays with UV detection. Key pharmacokinetic parameters were calculated by noncompartmental methods. There were no gender-related differences in elimination half-life (t1/2) and weight-normalized total body clearance (CLT), renal clearance (CLR), and steady-state volume of distribution (Vss). Statistically significant age-related effects were found for t1/2, CLT, CLR, and Vss parameters. In different study groups, Vss ranged from 0.21 to 0.24 liter/kg. The values for Vss were significant greater for elderly subjects than they were for young subjects. The cefepime t1/2 was significantly longer in elderly subjects (about 3 h) than that observed in young subjects (about 2.2 h). The mean values for CLT and CLR in the four study groups ranged from 1.11 to 1.56 and 0.99 to 1.44 ml/min/kg, respectively. In elderly subjects, the estimates for CLT and CLR were significantly lower than those observed in young subjects. Linear regression revealed good correlations between clearance values of cefepime and creatinine. The magnitude of age-related changes in the pharmacokinetics of cefepime is not significant enough to recommend dosage adjustment in elderly patients with kidney functions normal for their age.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balant L., Dayer P., Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clin Pharmacokinet. 1985 Mar-Apr;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Gleason C. R., Knupp C. A., Pittman K. A., Weidler D. J., Martin R. R. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990 Jun;34(6):1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Gleason C. R., Knupp C. A., Pittman K. A., Weidler D. J., Movahhed H., Tenney J., Martin R. R. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother. 1992 Mar;36(3):552–557. doi: 10.1128/aac.36.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Forgue S. T., Matzke G. R., Guay D. R., Pittman K. A. Pharmacokinetics of cefepime in subjects with renal insufficiency. Clin Pharmacol Ther. 1990 Sep;48(3):268–276. doi: 10.1038/clpt.1990.149. [DOI] [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Forgue S. T., Matzke G. R., Halstenson C. E., Opsahl J. A., Pittman K. A. Disposition of the cephalosporin cefepime in normal and renally impaired subjects. Drug Metab Dispos. 1991 Jan-Feb;19(1):68–73. [PubMed] [Google Scholar]
- Barbhaiya R. H., Knupp C. A., Tenney J., Martin R. R., Weidler D. J., Pittman K. A. Safety, tolerance, and pharmacokinetics of cefepime administered intramuscularly to healthy subjects. J Clin Pharmacol. 1990 Oct;30(10):900–910. doi: 10.1002/j.1552-4604.1990.tb03569.x. [DOI] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Blouin R. A., Kneer J., Stoeckel K. Pharmacokinetics of intravenous cefetamet (Ro 15-8074) and oral cefetamet pivoxil (Ro 15-8075) in young and elderly subjects. Antimicrob Agents Chemother. 1989 Mar;33(3):291–296. doi: 10.1128/aac.33.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce A., Andersson M., Arvidsson B., Isaksson B. Body composition. Prediction of normal body potassium, body water and body fat in adults on the basis of body height, body weight and age. Scand J Clin Lab Invest. 1980 Sep;40(5):461–473. doi: 10.3109/00365518009101869. [DOI] [PubMed] [Google Scholar]
- Forbes G. B., Reina J. C. Adult lean body mass declines with age: some longitudinal observations. Metabolism. 1970 Sep;19(9):653–663. doi: 10.1016/0026-0495(70)90062-4. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Barry A. L., Thornsberry C. Evaluation of the in vitro activity of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 May;27(5):679–682. doi: 10.1128/aac.27.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geokas M. C., Haverback B. J. The aging gastrointestinal tract. Am J Surg. 1969 Jun;117(6):881–892. doi: 10.1016/0002-9610(69)90078-6. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Sellers E. M., Shader R. I. Drug therapy: drug disposition in old age. N Engl J Med. 1982 May 6;306(18):1081–1088. doi: 10.1056/NEJM198205063061804. [DOI] [PubMed] [Google Scholar]
- Guay D. R., Matzke G. R., Heim K. L., Halstenson C. E., Abraham P. A., Keane W. F. Influence of gender on the disposition of cefotaxime and desacetylcefotaxime. Ther Drug Monit. 1987 Sep;9(3):259–262. doi: 10.1097/00007691-198709000-00002. [DOI] [PubMed] [Google Scholar]
- Jonkman J. H., Reinberg A., Oosterhuis B., de Noord O. E., Kerkhof F. A., Motohashi Y., Levi F., Dammacco F., Carandente F. Dosing time and sex-related differences in the pharmacokinetics of cefodizime and in the circadian cortisol rhythm. Chronobiologia. 1988 Jan-Jun;15(1-2):89–102. [PubMed] [Google Scholar]
- Kessler R. E., Bies M., Buck R. E., Chisholm D. R., Pursiano T. A., Tsai Y. H., Misiek M., Price K. E., Leitner F. Comparison of a new cephalosporin, BMY 28142, with other broad-spectrum beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Feb;27(2):207–216. doi: 10.1128/aac.27.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Bayer A. S. Efficacy of BMY-28142 in experimental bacteremia and meningitis caused by Escherichia coli and group B streptococci. Antimicrob Agents Chemother. 1985 Jul;28(1):51–54. doi: 10.1128/aac.28.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B., Nilsson-Ehle I. Influence of age on the pharmacokinetics of ceftazidime in acutely ill, adult patients. Eur J Clin Pharmacol. 1988;34(2):173–178. doi: 10.1007/BF00614555. [DOI] [PubMed] [Google Scholar]
- MacLennan W. J., Martin P., Mason B. J. Protein intake and serum albumin levels in the elderly. Gerontology. 1977;23(5):360–367. doi: 10.1159/000212209. [DOI] [PubMed] [Google Scholar]
- Matzke G. R., Jameson J. J., Halstenson C. E. Gentamicin disposition in young and elderly patients with various degrees of renal function. J Clin Pharmacol. 1987 Mar;27(3):216–220. doi: 10.1002/j.1552-4604.1987.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Meyers B. R., Wilkinson P. Clinical pharmacokinetics of antibacterial drugs in the elderly. Implications for selection and dosage. Clin Pharmacokinet. 1989 Dec;17(6):385–395. doi: 10.2165/00003088-198917060-00003. [DOI] [PubMed] [Google Scholar]
- Phelps D. J., Carlton D. D., Farrell C. A., Kessler R. E. Affinity of cephalosporins for beta-lactamases as a factor in antibacterial efficacy. Antimicrob Agents Chemother. 1986 May;29(5):845–848. doi: 10.1128/aac.29.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegelman S., Collier P. The application of statistical moment theory to the evaluation of in vivo dissolution time and absorption time. J Pharmacokinet Biopharm. 1980 Oct;8(5):509–534. doi: 10.1007/BF01059549. [DOI] [PubMed] [Google Scholar]
- Rowe J. W., Andres R., Tobin J. D. Letter: Age-adjusted standards for creatinine clearance. Ann Intern Med. 1976 May;84(5):567–569. doi: 10.7326/0003-4819-84-5-567. [DOI] [PubMed] [Google Scholar]
- Shand D. G. Biological determinants of altered pharmacokinetics in the elderly. Gerontology. 1982;28 (Suppl 1):8–17. doi: 10.1159/000212568. [DOI] [PubMed] [Google Scholar]
- Trang J. M., Monson T. P., Ackerman B. H., Underwood F. L., Manning J. T., Kearns G. L. Effect of age and renal function on cefonicid pharmacokinetics. Antimicrob Agents Chemother. 1989 Feb;33(2):142–146. doi: 10.1128/aac.33.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M. G., Hackbarth C. J., Scott K. G., Rusnak M. G., Sande M. A. New cephalosporins cefotaxime, cefpimizole, BMY 28142, and HR 810 in experimental pneumococcal meningitis in rabbits. Antimicrob Agents Chemother. 1985 Mar;27(3):340–342. doi: 10.1128/aac.27.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]


