Abstract
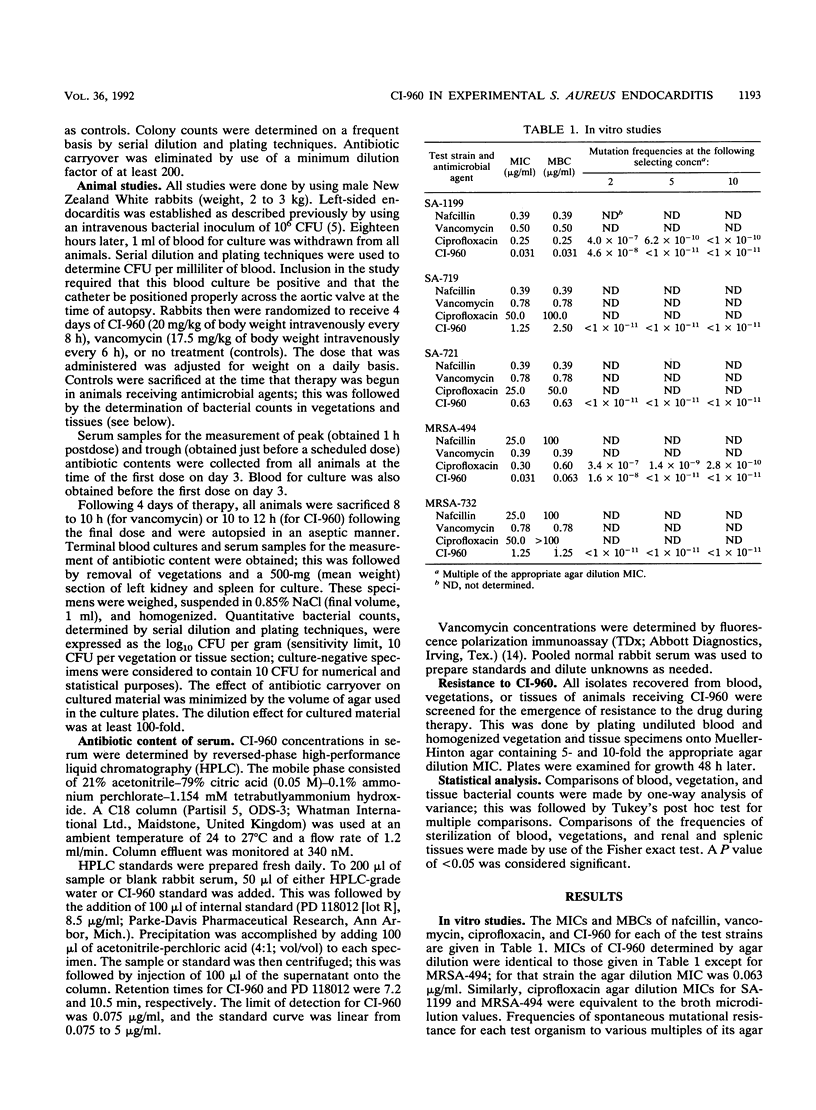
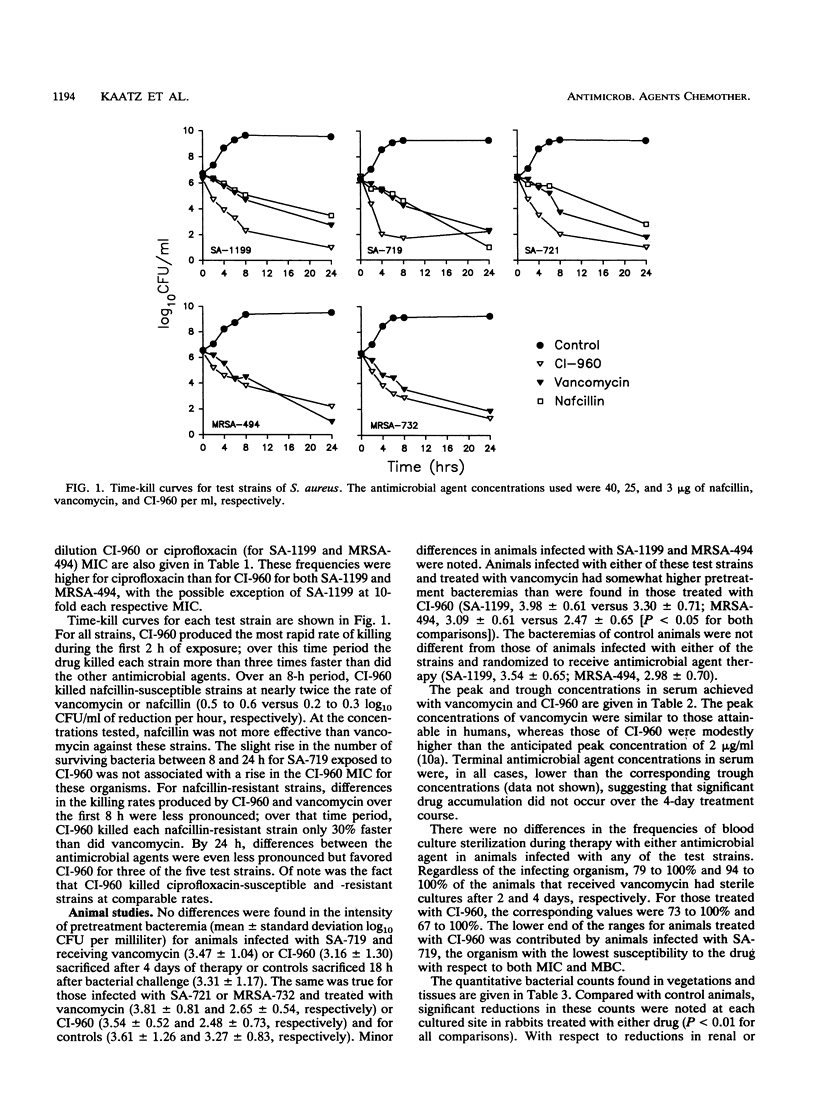
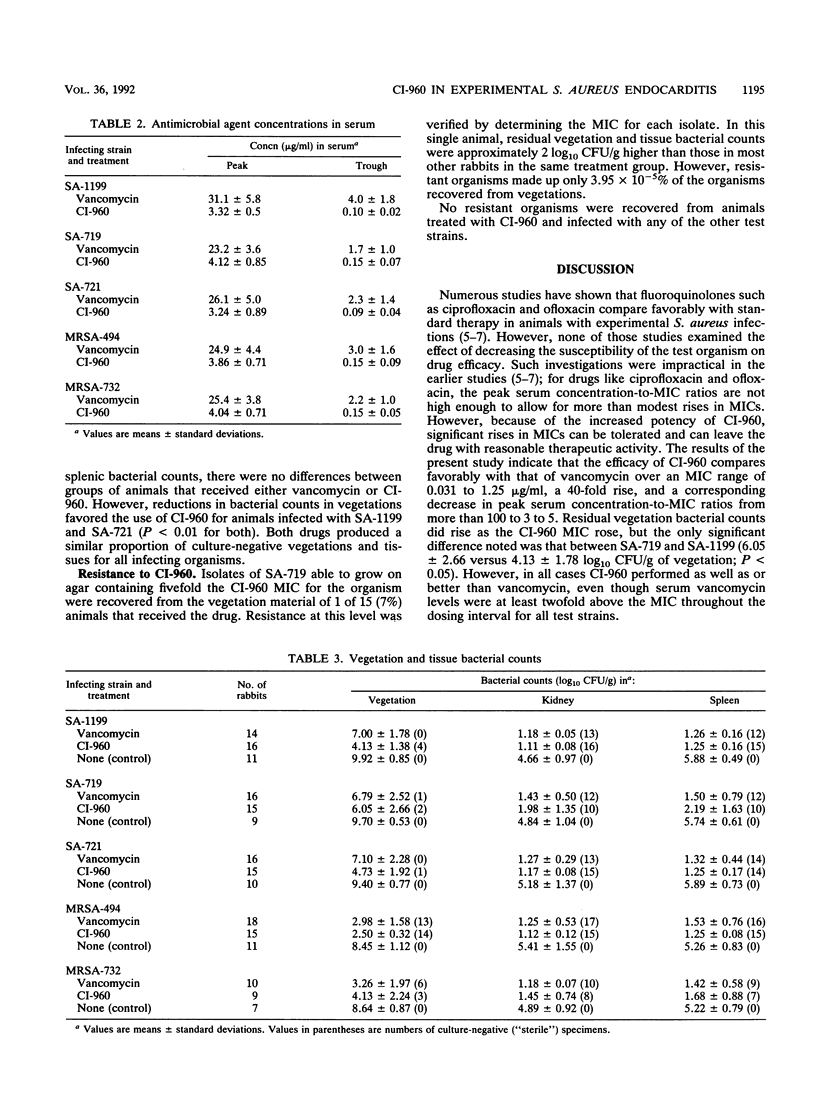
CI-960 is a new fluoroquinolone with enhanced in vitro activity against gram-positive pathogens. The efficacy of the drug was compared with that of vancomycin by using the rabbit model of nafcillin- and ciprofloxacin-susceptible and -resistant Staphylococcus aureus endocarditis. Animals received intravenous therapy with CI-960, 20 mg/kg of body weight every 8 h, or vancomycin, 17.5 mg/kg every 6 h, for 4 days. In a comparison with the effects on untreated controls, both antimicrobial agents effectively cleared bacteremia and significantly reduced bacterial counts in vegetations and tissues of animals infected with any of the test strains. In some cases, the efficacy of CI-960 was superior to that of vancomycin. The therapeutic activity of CI-960 was reduced, but still very good, against ciprofloxacin-resistant strains. One rabbit infected with such a strain and treated with CI-960 was found to harbor a small number of vegetation-associated organisms resistant to the drug at fivefold its original MIC; this was associated with a microbiological, but not a clinical, failure of therapy. We conclude that CI-960 is as effective as vancomycin is in this model of a serious systemic S. aureus infection, including that caused by strains resistant to ciprofloxacin. Increases in CI-960 MICs may develop during therapy of infections caused by strains highly resistant to ciprofloxacin, but they appear unlikely to occur in ciprofloxacin-susceptible strains.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Fuchs P. C. Antistaphylococcal activity of the fluoroquinolones CI-960, PD 131628, sparfloxacin, ofloxacin and ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1991 Mar;10(3):168–171. doi: 10.1007/BF01964451. [DOI] [PubMed] [Google Scholar]
- Bryson V., Szybalski W. Microbial Selection. Science. 1952 Jul 18;116(3003):45–51. doi: 10.1126/science.116.3003.45. [DOI] [PubMed] [Google Scholar]
- Forstall G. J., Knapp C. C., Washington J. A. Activity of new quinolones against ciprofloxacin-resistant staphylococci. Antimicrob Agents Chemother. 1991 Aug;35(8):1679–1681. doi: 10.1128/aac.35.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett N., Brown S., Krishnan C. Emergence of quinolone resistance among clinical isolates of methicillin-resistant Staphylococcus aureus in Ontario, Canada. Antimicrob Agents Chemother. 1991 Sep;35(9):1911–1913. doi: 10.1128/aac.35.9.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Barriere S. L., Schaberg D. R., Fekety R. Ciprofloxacin versus vancomycin in the therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1987 Apr;31(4):527–530. doi: 10.1128/aac.31.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Barriere S. L., Schaberg D. R., Fekety R. The emergence of resistance to ciprofloxacin during treatment of experimental Staphylococcus aureus endocarditis. J Antimicrob Chemother. 1987 Nov;20(5):753–758. doi: 10.1093/jac/20.5.753. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Barriere S. L., Albrecht L. M., Rybak M. J. Efficacy of ofloxacin in experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990 Feb;34(2):257–260. doi: 10.1128/aac.34.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Dorman N. J., Lerner S. A. Emergence of teicoplanin resistance during therapy of Staphylococcus aureus endocarditis. J Infect Dis. 1990 Jul;162(1):103–108. doi: 10.1093/infdis/162.1.103. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Novelli A., Chin N. X. Comparative in vitro activity of a new quinolone, AM-1091. Antimicrob Agents Chemother. 1989 Jul;33(7):1036–1041. doi: 10.1128/aac.33.7.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Quick J. N., Jensen B., Homann S., Johnson S., Tenquist J., Shanholtzer C., Petzel R. A., Sinn L., Gerding D. N. Emergence of ciprofloxacin resistance in nosocomial methicillin-resistant Staphylococcus aureus isolates. Resistance during ciprofloxacin plus rifampin therapy for methicillin-resistant S aureus colonization. Arch Intern Med. 1990 Oct;150(10):2151–2155. [PubMed] [Google Scholar]
- Schaefler S. Methicillin-resistant strains of Staphylococcus aureus resistant to quinolones. J Clin Microbiol. 1989 Feb;27(2):335–336. doi: 10.1128/jcm.27.2.335-336.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Schwenzer K. S., Wang C. H., Anhalt J. P. Automated fluorescence polarization immunoassay for monitoring vancomycin. Ther Drug Monit. 1983;5(3):341–345. doi: 10.1097/00007691-198309000-00017. [DOI] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]


