Abstract
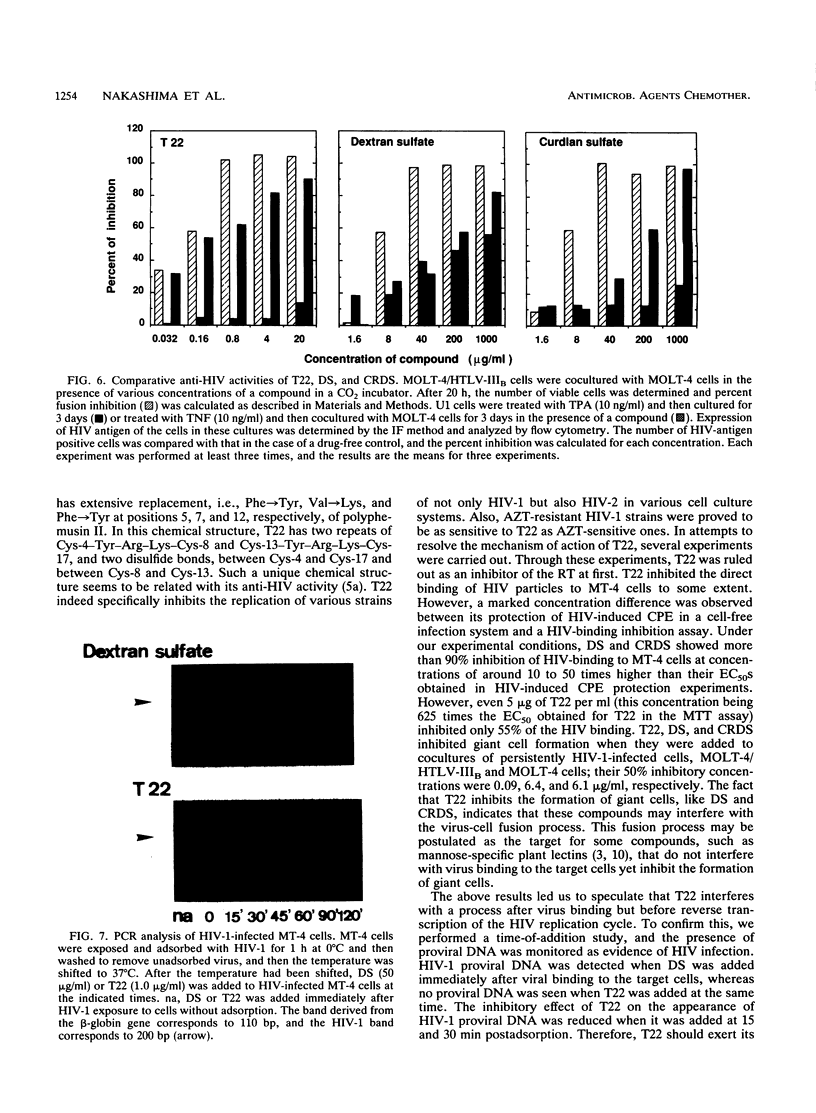
More than 40 peptides associated with tachyplesin and polyphemusin, which are highly abundant in hemocyte debris of the horseshoe crabs Tachypleus tridentatus and Limulus polyphemus, were synthesized. Among these peptides, we found that a novel compound, which was called T22 ([Tyr-5,12, Lys-7]polyphemusin II), strongly inhibited the human immunodeficiency virus type 1 (HIV-1)-induced cytopathic effect and viral antigen expression. Its 50% effective concentration was 0.008 micrograms/ml, while its 50% cytotoxic concentration was 54 micrograms/ml. The anti-HIV activity of T22 was observed with several strains of HIV-1, including zidovudine-resistant strains, and with HIV-2 within the concentration range of 0.006 to 0.071 microgram/ml. T22 efficiently inhibited giant cell formation on the cocultivation of MOLT-4/HIV and MOLT-4 cells but modestly inhibited direct HIV binding. T22 did not inhibit reverse transcriptase activity. A time-of-addition study, which involved monitoring of the appearance of proviral DNA by using the polymerase chain reaction technique, found that T22 exerted its effect on a process, most probably virus-cell fusion or uncoating, immediately after virus adsorption.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Schols D., Neyts J., Van Damme E., Peumans W., De Clercq E. Alpha-(1-3)- and alpha-(1-6)-D-mannose-specific plant lectins are markedly inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob Agents Chemother. 1991 Mar;35(3):410–416. doi: 10.1128/aac.35.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folks T. M., Justement J., Kinter A., Dinarello C. A., Fauci A. S. Cytokine-induced expression of HIV-1 in a chronically infected promonocyte cell line. Science. 1987 Nov 6;238(4828):800–802. doi: 10.1126/science.3313729. [DOI] [PubMed] [Google Scholar]
- Furman P. A., Fyfe J. A., St Clair M. H., Weinhold K., Rideout J. L., Freeman G. A., Lehrman S. N., Bolognesi D. P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-deoxythymidine and selective interaction of the 5'-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Huang P., Farquhar D., Plunkett W. Selective action of 3'-azido-3'-deoxythymidine 5'-triphosphate on viral reverse transcriptases and human DNA polymerases. J Biol Chem. 1990 Jul 15;265(20):11914–11918. [PubMed] [Google Scholar]
- Kaneko Y., Yoshida O., Nakagawa R., Yoshida T., Date M., Ogihara S., Shioya S., Matsuzawa Y., Nagashima N., Irie Y. Inhibition of HIV-1 infectivity with curdlan sulfate in vitro. Biochem Pharmacol. 1990 Feb 15;39(4):793–797. doi: 10.1016/0006-2952(90)90161-d. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Matsui T., Kobayashi S., Yoshida O., Ishii S., Abe Y., Yamamoto N. Effects of succinylated concanavalin A on infectivity and syncytial formation of human immunodeficiency virus. Med Microbiol Immunol. 1990;179(5):225–235. doi: 10.1007/BF00192460. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata T., Tokunaga F., Yoneya T., Yoshikawa K., Iwanaga S., Niwa M., Takao T., Shimonishi Y. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J Biochem. 1989 Oct;106(4):663–668. doi: 10.1093/oxfordjournals.jbchem.a122913. [DOI] [PubMed] [Google Scholar]
- Morimoto M., Mori H., Otake T., Ueba N., Kunita N., Niwa M., Murakami T., Iwanaga S. Inhibitory effect of tachyplesin I on the proliferation of human immunodeficiency virus in vitro. Chemotherapy. 1991;37(3):206–211. doi: 10.1159/000238855. [DOI] [PubMed] [Google Scholar]
- Nakamura T., Furunaka H., Miyata T., Tokunaga F., Muta T., Iwanaga S., Niwa M., Takao T., Shimonishi Y. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem. 1988 Nov 15;263(32):16709–16713. [PubMed] [Google Scholar]
- Nakashima H., Kido Y., Kobayashi N., Motoki Y., Neushul M., Yamamoto N. Purification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob Agents Chemother. 1987 Oct;31(10):1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Matsui T., Harada S., Kobayashi N., Matsuda A., Ueda T., Yamamoto N. Inhibition of replication and cytopathic effect of human T cell lymphotropic virus type III/lymphadenopathy-associated virus by 3'-azido-3'-deoxythymidine in vitro. Antimicrob Agents Chemother. 1986 Dec;30(6):933–937. doi: 10.1128/aac.30.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima H., Pauwels R., Baba M., Schols D., Desmyter J., De Clercq E. Tetrazolium-based plaque assay for HIV-1 and HIV-2, and its use in the evaluation of antiviral compounds. J Virol Methods. 1989 Dec;26(3):319–329. doi: 10.1016/0166-0934(89)90114-6. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Yoshida O., Baba M., De Clercq E., Yamamoto N. Anti-HIV activity of dextran sulphate as determined under different experimental conditions. Antiviral Res. 1989 Jun-Jul;11(5-6):233–246. doi: 10.1016/0166-3542(89)90033-8. [DOI] [PubMed] [Google Scholar]
- Nakashima H., Yoshida O., Tochikura T. S., Yoshida T., Mimura T., Kido Y., Motoki Y., Kaneko Y., Uryu T., Yamamoto N. Sulfation of polysaccharides generates potent and selective inhibitors of human immunodeficiency virus infection and replication in vitro. Jpn J Cancer Res. 1987 Nov;78(11):1164–1168. [PubMed] [Google Scholar]
- Pang S., Koyanagi Y., Miles S., Wiley C., Vinters H. V., Chen I. S. High levels of unintegrated HIV-1 DNA in brain tissue of AIDS dementia patients. Nature. 1990 Jan 4;343(6253):85–89. doi: 10.1038/343085a0. [DOI] [PubMed] [Google Scholar]
- Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988 Aug;20(4):309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Schols D., Baba M., Pauwels R., De Clercq E. Flow cytometric method to demonstrate whether anti-HIV-1 agents inhibit virion binding to T4+ cells. J Acquir Immune Defic Syndr. 1989;2(1):10–15. [PubMed] [Google Scholar]
- Shibahara S., Mukai S., Morisawa H., Nakashima H., Kobayashi S., Yamamoto N. Inhibition of human immunodeficiency virus (HIV-1) replication by synthetic oligo-RNA derivatives. Nucleic Acids Res. 1989 Jan 11;17(1):239–252. doi: 10.1093/nar/17.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochikura T. S., Nakashima H., Tanabe A., Yamamoto N. Human immunodeficiency virus (HIV)-induced cell fusion: quantification and its application for the simple and rapid screening of anti-HIV substances in vitro. Virology. 1988 Jun;164(2):542–546. doi: 10.1016/0042-6822(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]