Abstract
Meditation refers to a family of mental training practices that are designed to familiarize the practitioner with specific types of mental processes. One of the most basic forms of meditation is concentration meditation, in which sustained attention is focused on an object such as a small visual stimulus or the breath. In age-matched participants, using functional MRI, we found that activation in a network of brain regions typically involved in sustained attention showed an inverted u-shaped curve in which expert meditators (EMs) with an average of 19,000 h of practice had more activation than novices, but EMs with an average of 44,000 h had less activation. In response to distracter sounds used to probe the meditation, EMs vs. novices had less brain activation in regions related to discursive thoughts and emotions and more activation in regions related to response inhibition and attention. Correlation with hours of practice suggests possible plasticity in these mechanisms.
Keywords: attention, frontal, parietal, response inhibition
In recent years interest has been growing regarding the neural and psychological effects of meditation. The present experiment examined the neural basis of “one-pointed concentration,” which is practiced to strengthen attentional focus and achieve a tranquil state in which preoccupation with thoughts and emotions is gradually reduced (1, 2). In this meditation one sustains concentration on a small object or the breath without succumbing to distractions (3). In addition, one engages in a process of self-monitoring, in which one notes mental states contrary to concentration, such as sleepiness or “mental chatter.”
Studies have shown expertise-related changes in those proficient in meditation and other skills. Concentration meditation has been reported to improve performance on multiple components of attention (4), decrease attentional blink (5), and improve the ability to control perceptual rivalry (6). In addition, changes in electroencephalogram and cortical thickness have been reported in long-term meditation practitioners of compassion (7) and insight meditation (8). For other types of expertise, functional MRI findings vary depending on training. For example, a study of short-term object discrimination training showed increased activation in the working-memory network (9), whereas studies of long-term experts showed either increased [musicians (10)] or decreased [golfers (11)] activation. Other studies showed an inverted u-shaped curve in which those learning a skill initially had increased activation yet eventually showed less activation (12, 13).
We studied expert meditators (EMs) with 10,000–54,000 h of practice in two similar schools of the Tibetan Buddhist tradition. EMs were compared with age-matched novice meditators (NMs) with an interest in meditation but no prior experience except in the week before the scanning session, in which they were given instructions. To control for motivation, a second NM group, the incentive NMs (INMs), were offered a financial bonus if they were among the best activators of attention regions. Participants alternated a state of concentration meditation (Med.) with a focus on a small fixation dot on a screen, with a neutral resting state (Rest) in a standard block paradigm. To probe the meditation, we presented distracting external stimuli (positive, neutral, or negative sounds) during parts of the Med. and Rest blocks in an event-related design.
Because concentration involves focusing attention, our first hypothesis was that Med. vs. Rest would result in activation of attention-related networks and visual cortex to maintain focus on the fixation dot (14–17). We further hypothesized that activation would vary among participants according to a skill-related inverted u-shaped function in which NMs would have less activation than EMs with moderate levels of practice, but those EMs with the most practice would show less sustained activation because of less required effort (12, 13). Next, we predicted that, in Med., EMs would be less perturbed by external stimuli (sounds in Med.) and show less activation compared with NMs and INMs in brain regions that are associated with task-unrelated thoughts (18), daydreams (19), and emotional processing (20). Similarly we predicted that a decrease in distraction-related regions would correlate with EMs' hours of practice.
Results
Concentration Meditation Block Data.
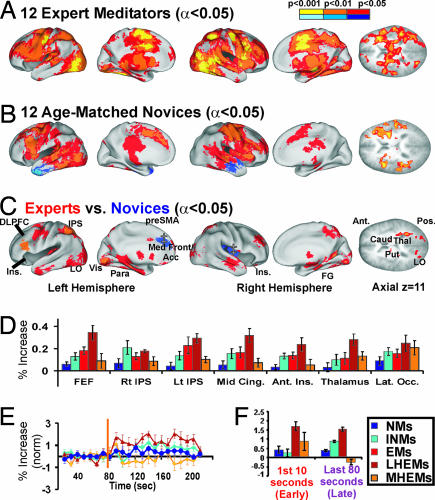
In the Med. block paradigm, participants performed concentration meditation, focusing on a simple visual stimulus, alternating with a specific form of a neutral, resting state while brain function was recorded with functional MRI. The patterns of significant activation for the Med. blocks vs. the Rest blocks are shown for EMs (see Fig. 1A) and NMs (Fig. 1B) on cortical surface models (21). EMs and NMs activated a large overlapping network of attention-related brain regions, including frontal parietal regions, lateral occipital (LO), insula (Ins), multiple thalamic nuclei, basal ganglia, and cerebellar regions (Tables 1 and 2). Only NMs showed negative activation (Rest > Med.) in anterior temporal lobe bilaterally (blue hues in Fig. 1B).
Fig. 1.
Meditation block data. Activation in concentration meditation block (Med.) vs. resting state block (Rest) for 12 EMs (A), 12 age-matched NMs (B), and t test subtraction of EMs (C) (red hues reflect greater activation in EMs vs. NMs) vs. regular NMs (blue hues reflect greater activation in NMs vs. EMs). Alpha maps ranging from P < 0.001 (orange, positive activation; medium blue, negative activation) to P < 0.01 (orange/medium blue) to P < 0.05, corrected (red, positive activation; dark blue, negative activation) are overlaid on inflated population-average, landmark- and surface-based atlas cortical model brains and an axial slice at z = 11 to show midbrain regions. ‡, smaller than corrected for multiple comparisons. (D) Activation in attention-shifting metaanalysis ROIs. Color scale is the same for all panels (see key). (E) Response over time (seconds) for left DLPFC. Start of the meditation block is indicated by an orange line at 80 sec. Standard error bars are shown for every 10 sec. (F) Bar graphs for amplitude of activation in DLPFC in the “early” part of the meditation block (the first 10 sec, excluding the first 2 sec because of hemodynamic delay) and the “late” part of the meditation block (120 sec to 200 sec).
Table 1.
Meditation block data: Brain regions differentially activated for EMs vs. NMs
| ROI | Volume, mm3 | Talairach coordinates, x, y, and z | EM t value | NM t value | EM vs. NM t value | EM hours partial r value |
|---|---|---|---|---|---|---|
| EMs > NMs | ||||||
| Frontal | ||||||
| Left MFG/IFG, BA45, 46 | 1,355 | −49, 29, 19 | 4.4** | −0.77 | 3.2*** | −0.72** |
| Right SFG, BA9 | 1,009 | 31, 42, 31 | 2.9** | 0.02 | 2.4* | −0.47 |
| Left supplementary motor area, MFG, DLPFC, BA9, BA32 | 924 | −21, 6, 50 | 3.3** | 1.0 | 2.5* | −0.63* |
| Left rectal gyrus, BA11 | 811 | −0.5, 43, −26 | 3.8*** | −1.9 | 3.4*** | −0.32 |
| Left precentral, DLPFC, BA6 | 1,535 | −34, −2, 36 | 4.2*** | 1.3 | 3.0** | −0.72** |
| Parietal/posterior | ||||||
| Left IPS, superior parietal, supramarginal gyrus, BA7 | 7,400 | −24, −61, 46 | 3.6*** | -.46 | 3.2*** | −0.71** |
| Right superior parietal, BA7 | 1,359 | 14, −62, 54 | 4.8*** | −1.3 | 3.8*** | −0.62* |
| Occipital/temporal | ||||||
| Right cuneus, BA17 | 1,792 | 22, −85, 11 | 3.7*** | −1.6 | 4*** | −0.52 |
| Left middle temporal gyrus, IFG, BA20, BA21 | 1,938 | −38, −7, −26 | 4*** | −3.2*** | 5.1*** | −0.53 |
| Right middle temporal gyrus, BA21, BA22 | 786 | 54, −12, −8 | 1.7 | −2.7* | 3.2*** | −0.63* |
| Fusiform, BA37 | 3,272 | −42, −55, −16 | 4.5*** | 0.16 | 3.5*** | −0.61* |
| Noncortical | ||||||
| Left putamen | 808 | −30, −20, 3 | 3.9*** | 0.83 | 2.8** | −0.61* |
| Right lentiform, parahippocampus, BA28 | 2,989 | 29, −42, 11 | 4.0*** | −0.41 | 2.9** | −0.60 |
| Cerebellum, declive, culmen | 22,082 | −4, −56, −14 | 4.4*** | 0.27 | 3.3*** | −0.68* |
| Left cerebellar tonsil | 1,944 | −22, −39, −40 | 4.0*** | −0.33 | 3.3*** | −0.67* |
| NM > EM | ||||||
| Left medial front/Acc, BA6, BA32 | 941 | −10, 39, 26 | −1.4 | 2.2* | −2.5* | −0.32 |
| Right Ins, BA13 | 851 | 39, −13, 15 | −2.2* | 2.1 | −3.0** | −0.21 |
Data are from a t test subtraction (significantly different between groups at P < 0.05 corrected).
*, P < 0.05;
**, P < 0.01;
***, P < 0.005.
As predicted in our hypothesis, in Med. vs. Rest, EMs showed greater activation than NMs in multiple attentional and other regions including frontoparietal regions, cerebellar, temporal, parahippocampal, and posterior (P.) occipital cortex, likely including the foveal visual cortex of the attended dot (red in Fig. 1C and Tables 1 and 2). NMs showed more activation than EMs in medial frontal gyrus (MeFG)/anterior cingulate (Acc) and in the right mid-Ins to P. Ins (Fig. 1C and Table 3), regions that have been shown to negatively correlate with performance in a sustained attention task (16, 22).
Table 2.
Meditation block data: Regions differentially activated for EMs vs. INMs
| ROI | Volume, mm3 | Talairach coordinates, x, y, and z | EM t value | INM t value | EM vs. INM t value | EM hours partial r value |
|---|---|---|---|---|---|---|
| EM > INM | ||||||
| Left anterior MFG | 854 | −26, 43, 7 | 2.85* | −1.94 | −3.17** | −0.20 |
| INM > EM | ||||||
| Left IFG/anterior superior temporal gyrus | 1,135 | −36, 12, −16 | −1.70 | 2.91* | 3.40** | −0.21 |
| Superior P. central/BA4† | 495 | 34, −26, 59 | −1.72 | 4.59** | 4.56*** | 0.05 |
| LO/medial occipital† | 464 | −39, −62, −3 | 4.04** | 6.32*** | 3.41** | −0.27 |
| Right P. Ins† | 406 | 40, −33, 18 | 0.84 | 6.80*** | 3.83** | −0.15 |
Data are from a t test subtraction (significantly different between groups at P < 0.05 corrected; smaller clusters marked with †).
*, P < 0.05;
**, P < 0.01;
***, P < 0.005.
Table 3.
Distracting sound data
| ROI | Volume, mm3 | Talairach coordinates, x, y, and z | EM t value | INM t value | EM vs. INM t value |
|---|---|---|---|---|---|
| INM > EM | |||||
| Left MFG | 4,087 | −26, 22, 38 | −1.09 | 2.54* | −2.75* |
| Right anterior cingulate | 2,659 | 15, 31, 26 | −0.18 | 2.57* | −2.26 |
| Right culmen | 919 | 1, −47, −7 | 1.18 | 4.39** | −2.47* |
| Left pulvinar† | 553 | −6, −32, 9 | 1.58 | 5.01*** | −2.46* |
| Right caudate† | 487 | 16, 7, 11 | 1.00 | 3.38** | −2.42* |
| Right cerebellum† | 373 | 9, −52, −31 | 1.74 | 4.03** | −2.19 |
| Left P. Cing† | 373 | −2, −63, 25 | 1.50 | 3.24* | −2.42* |
| EM > INM | |||||
| Left central sulcus/parietal | 1,583 | −53, −13, 30 | 6.45*** | 1.09 | 2.80* |
| Right central sulcus/parietal | 1,217 | 45, −20, 48 | 5.38*** | −1.31 | 4.45*** |
| Right SFG | 1,008 | 19, −15, 68 | 3.48** | −1.81 | 3.80** |
| Right central sulcus | 995 | 53, −7, 29 | 4.05** | 0.65 | 2.07 |
| Left visual cortex† | 679 | −8, −87, 16 | 2.11 | −0.21 | 1.60 |
| Left IFG† | 428 | −48, 24, 2 | 2.82** | −0.19 | 2.50* |
| Right superior temporal gyrus† | 401 | 25, 6, −34 | 1.92 | −3.19* | 3.75*** |
State-by-group results from ANOVA. Areas with significant differences for event-related distracter sounds (vs. silence) in Med. vs. Rest (state) and EMs vs. INMs (group) (P < 0.02 uncorrected; cluster sizes >680 mm3 are P < 0.02 corrected, and smaller clusters are marked with †).
*, P < 0.05;
**, P < 0.01;
***, P < 0.005. t values for sounds in Med. are shown.
We were concerned that these differences may have resulted in part from structural differences between participant-group brains, because seven of 12 EMs were Asian (five Caucasian), and all NMs were Caucasian. Therefore, we performed a separate analysis in which structural differences were taken to account by using probability of gray matter maps as voxel-wise covariates in a t test comparison between groups (23). All significant regions remained significant in this analysis, and several regions just below threshold became larger and thus survived multiple correction [supporting information (SI) Fig. 3A]. In addition, we were concerned with possible motivation differences between groups. Therefore, to better match motivational arousal, we collected data from a set of 10 INMs who were told they would receive a monetary award ($50) if they were in the top one-third of the INMs in activating attention-related regions.
We examined all participant groups, including the INMs, using a priori regions of interest (ROIs) from a metaanalysis of 31 studies involving attention-shifting paradigms (24). The EMs showed significantly more activation (two-tailed t test) than NMs in all attention ROIs except the thalamus (red vs. dark blue in Fig. 1D). However, the INMs (light blue) showed more activation than the NMs and were not significantly different from the EMs in these ROIs. In the t test of EMs vs. INMs, EMs had more activation in the left superior frontal gyrus (SFG)/middle frontal gyrus (MFG), and INMs had more activation in left P. Ins, left inferior frontal gyrus (IFG), and LO (SI Fig. 3 B and C and Table 2).
Next, because we predicted that these results would correlate with hours of practice, we split the EM group into those with the most hours of practice (top four MHEMs, mean hours = 44,000, range 37,000–52,000, mean age 52.3 years) and those with the least hours of practice (lower four LHEMs, mean hours = 19,000, range 10,000–24,000, mean age 48.8 years, youngest participant not included to ensure age-matching). Two Asians and two Caucasians were in each group. Consistent with an inverted u-shaped function, we found that the LHEMs (brown) had the strongest activation, significantly higher than both sets of NMs in all attention ROIs except left intraparietal sulcus (IPS) and LO (SI Fig. 3D) and significantly higher than MHEMs (orange) in all ROIs except LO. Results were not significantly different when the top five MHEMs were used (rather than the top one-third; data not shown) nor when the youngest LHEM was used (making mean age 42.3 years), except in thalamus ROI, in which LHEMs were not significantly different (same trend) from INMs or MHEMs (the thalamus ROI was more posterior than the thalamus cluster activated in our study).
In addition, we performed correlations with hours of practice within the EM group. Because age was a potential confound, we calculated the correlation between a participant's age and hours of practice. This was not significant (r = 0.22, P < 0.44), but it had a positive trend of older participants having more hours. Thus, we list partial r values for activation vs. hours of practice, accounting for age. Many regions, including those in the attention network, showed significant negative correlation with hours, whereas no regions showed positive correlation with hours (see last columns of Tables 1 and 2, SI Table 4, and SI Fig. 4A), consistent with the view that expertise may lead to decreased activation, possibly because of increased processing efficiency. The notion of increased processing efficiency in long-term practitioners is consistent with recent evidence from our laboratory using another task, the attentional blink task, where we found that a 3-month period of intensive meditation led to decreased amplitude of the late component of the event-related potential to an initial target, a marker of increased processing efficiency that predicted improved behavioral performance on a subsequent target (5).
We reasoned that if these results could be explained by differences in the amount of effort required to maintain attentional focus with expertise, one should see differences in the time courses of the hemodynamic response. In the left dorsal lateral prefrontal cortex (DLPFC) ROI, MHEMs had only a short activation period at the beginning of the Med. block (P < 0.02) that returned to baseline within the first 10–20 sec (significantly less than the LHEMs; P < 0.001). In contrast, LHEMs had a larger, sustained response over the duration of the block (Fig. 1E). This short vs. sustained response contributed in part to the decreased activation for MHEMs vs. LHEMs in the attention-related ROIs (Fig. 1D) because the hemodynamic response function we used in our analysis modeled a continuous response over the entire block. “Meditation startup” increases occurred in most attention ROIs except for the thalamus and left anterior Ins and were also seen in right fusiform gyrus and bilateral caudate. Several other types of responses were seen in MHEMs, including suppression in regions like MeFG/Acc and P. Ins and more sustained responses in IPS, LO, inferior occipital, SFG, and MFG (regions with activation in last 80 sec of Med.) [SI Fig. 3E (P < 0.05 uncorrected); also see representative time course plots in SI Fig. 4B]. The left SFG/MFG region overlapped with the only region that was significantly greater in the 12 EMs vs. INMs (compare SI Fig. 3 C and E; see also Table 2).
If the hemodynamic time course is influenced by effort, one should also see a more sustained response in the highly motivated INMs compared with the regular NMs. Indeed, INMs had a greater sustained response than the NMs in which activation at times fell within baseline levels. However, both NM groups had reduced sustained activation over time compared with LHEMs and also showed a delay in the amount of time it took to reach maximum activation in these regions, typically 10–20 sec longer. These results are presented for the DLPFC ROI in Fig. 1F. All groups had significant (NMs and LHEMs) or near significant (INM and MHEMs; P < 0.06) activation in the first 10 sec of the meditation block (LHEMs significantly greater than all other groups). However, for the last 80 sec of the block, there was an inverted u-shaped curve in which activation for NM < INM < LHEM > MHEM (all groups significantly different from each other; P < 0.001). However, whether these activation differences are due to skill learning or strategy and task performance differences cannot be definitely resolved in this study.
Because MHEMs may have been able to reach a less effortful tranquil meditation state within these short blocks, it is possible that regions that remained active in the latter part (last 80 sec) of the meditation block for the MHEMs may be the minimal brain regions necessary to sustain attention on a visual object.
Distracting Sound Data.
In addition to looking at the brain regions involved in generating and sustaining the meditation state, we examined event-related neural responses during presentation of distracting sounds, presented at 2-sec intervals during the last two-thirds of the Med. and Rest blocks. These sounds could be neutral (restaurant ambiance), positive (baby cooing), or negative (woman screaming) and were contrasted with randomly presented silent, null events with the same timing. In this paradigm, 13 EMs, 13 NMs, and 10 INMs were included (see Methods). General auditory processing pathways (temporal cortex and Ins) were commonly activated for all participant groups in response to distracting sounds during both states (data not shown). A state ANOVA (sounds in Med. vs. Rest) revealed that participants showed an overall “active response” (no suppressed regions) in response to the sounds in Med., involving regions such as right intraparietal lobule/temporal parietal junction, bilateral pre- and post-central sulci, DLPFC, Ins, and anterior SFG (see SI Table 5 for state effects for all three groups; also see SI Fig. 5 A–C).
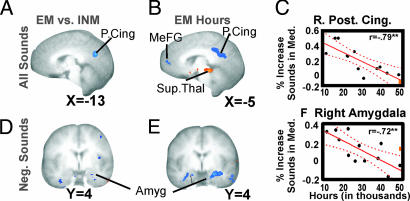
Next we looked for differences between the groups. Our hypothesis predicted that NMs would be more distracted by the sounds and thus would show more activation in default-mode regions related to task-irrelevant thoughts and in emotion regions. First, NMs did not have any regions that were more active than either the EMs or INMs [SI Fig. 5C vs. A and B; see also state-by-group (EM vs. NM) ANOVA in SI Table 6]. These reduced differences for NMs may have been due to the greater similarity between Med. and Rest states for these participants, as we saw in the Med. block data. Therefore, we viewed the better motivated INMs as the more appropriate control group who would more accurately demonstrate the full potential of novices. As predicted, EMs had less involvement than INMs in medial “default-mode network” regions such as P. cingulate (P. Cing)/precuneus and MeFG/Acc [Fig. 2A, SI Fig. 4C (state by group, EM vs. INM), and Table 3]. EMs also had less activation in left DLPFC, caudate, and pulvinar (Table 3). In contrast, EMs showed more activation than INMs in bilateral dorsal IPS extending into post-central sulcus, visual cortex, and left, IFG (area 47) (Table 3).
Fig. 2.
Expertise-related differences in response to distractor sounds. (A) State (all sounds in Med. vs. Rest) by group (EM vs. INM) ANOVA results (left) showing cluster in P. Cing that is more active for the INMs. (B) Voxel-wise regression of sounds in Med. with hours of practice in the EMs showing negative (blue) correlation and positive (orange) correlation (P < 0.02 uncorrected). (C) Example of negative correlation in right P. Cing. (D) State by group ANOVA for negative sounds showing small focus of greater activation in Amyg in INMs vs. EMs. (E) Voxel-wise regression of response to negative sounds in Med. with hours in EMs showing bilateral Amyg (P < 0.02 uncorrected). (F) Correlation within EMs in right Amyg ROI. One outlier (orange) was not included in correlation.
According to our hypothesis, areas that showed differential effects for EMs vs. NMs should show similar trends when comparing MHEMs vs. LHEMs. A voxel-wise analysis identified multiple regions in which activation in response to sounds correlated with hours of practice (see Fig. 2 B and C, SI Table 7, and SI Fig. 4 D and E). When all sounds were included together (positive, negative, and neutral), the voxel-wise regression identified negative correlation with hours of practice in multiple regions including right amygdala (Amyg), MeFG/Acc, and P. Cing (19, 25) (see Fig. 2 B and C and SI Table 7). This P. Cing cluster partially overlapped the P. Cing region more active in INM vs. EMs (compare A and B in Fig. 2). In addition, there was negative correlation with hours of practice in intraparietal lobule, fusiform, and P. temporal regions. There were also several regions with positive correlation with hours of practice, including Ins, subthalamic, left IFG, supplementary motor area, and others; however, slopes of these correlations were usually less steep than areas showing negative correlation (see Fig. 2B, SI Table 7, and examples in SI Fig. 4 D and E). Partial correlations are reported here because the participants included in these analyses showed a substantial but nonsignificant positive association between age and hours of practice (r = 0.53, P < 0.08).
Voxel-wise regression of brain responses of each sound valence separately vs. hours of practice identified similar regions (compared with all sounds together) for positive and neutral sounds (data not shown). In response to negative sounds in the EMs, there was a significant inverse correlation between MR signal change in the Amyg and MeFG/Acc and hours; a greater number of hours was associated with less activation to negative sounds in these brain regions (SI Table 8). These regions overlapped with results from a state by group (EMs vs. INMs) ANOVA for negative sounds, in which INMs also showed more activation than EMs in default network regions (compare F and G in SI Fig. 4) and in right Amyg (compare D and E in Fig. 2). The correlation with hours for negative sounds within the EMs was significantly greater than the correlation for positive (happy baby) sounds in the Amyg (negative sounds, partial r = −0.64; positive sounds, partial r = −0.13; difference, Steiger's Z = 2.6 and P < 0.04) and in MeFG/Acc (left MeFG, negative sounds, partial r = −0.86, positive sounds, partial r = 0.33, Steiger's Z = 3.3, P < 0.01; right MeFG, negative sounds, partial r = −0.81, positive sounds, partial r = 0.41, Steiger's Z = 2.4, P < 0.05). Differences between zero order r values (without age statistically removed) are also significant (data not shown).
The only positive correlations between response to the negative sounds in Med. and hours of practice were seen in left cerebellar tonsil and subthalamic regions (SI Fig. 4 G and H and SI Table 8).
Pupil Diameter Data.
In this study we did not include a behavioral task because practitioners reported that a task would disrupt their ongoing meditation. However, we did measure pupil diameter to obtain an independent index of autonomic arousal (eyes open and loosely fixated on dot in both Rest and Med. blocks). Ongoing measurements of pupil diameter changes during Med. and Rest were collected for 10 EMs, 10 NMs, and 10 INMs. For pupil response to distracting sounds, we performed a state (Med. vs. Rest) by group (EM, NM, and INM) ANOVA. We found a main effect of state [F(1,24) = 5.778, P = 0.024] in which peak pupil diameter in response to sounds increased in Med. vs. Rest (SI Fig. 6). There was no significant state by group interaction [F(2,24) = 0.087, P = 0.917] and baseline pupil responses (1 sec before sound) did not differ between groups (P = 0.65). (Note that we could not measure absolute pupil diameters, only relative changes within individuals.) This suggests that all participants were engaged in the Med. task. The similarities and differences in brain regions activated in response to sounds in Med. vs. Rest between groups suggest that the types of processes eliciting the increased pupil diameters for EMs vs. NMs and INMs overlapped but also had important differences (see Discussion).
Discussion
Meditation State Effects.
Concentration meditation in contrast with a Rest condition resulted in activation in attention-related regions (14, 24) in all participant groups including NMs. However, between the groups of NMs and EMs, there was variation in both the strength and time course of activation. Our findings of activation in attention regions and visual cortex (14, 16, 24, 26), are consistent with classical descriptions of this meditation that emphasize a cognitive component called concentration, which includes aiming and sustaining attention to keep the object in mind and making adjustments to the meditation when necessary (3).
Meditation texts describe concentration meditation as initially requiring greater levels of effortful concentration but later becoming less effortful, such that late stages of this meditation are said to require minimal effort, with the practitioner being “settled” in a state of decreased mental effort but alert focus. LHEMs showed significantly more activation, applied on a faster timescale compared even with the INMs, who were highly motivated to try their best. This difference, combined with the decrease of activation in MHEMS (who had, on average, more than twice as much meditation experience than LHEMS), fits an inverted u-shaped function associated with skill acquisition in others domains of expertise (12, 13). As with these studies, differences compared with NMs may be due to differences in strategy, technique, and the types of mental processes involved, rather than plasticity per se. However, the differences between LHEMS and MHEMS who were age-matched, culture-matched, and more similarly trained are more likely to be explained by some level of skill learning or plasticity. Larger groups of such practitioners, as well as longitudinal studies, are needed to further elucidate these findings.
Activation differences may also result from differences in the allocation of cognitive resources. The decrease in activation for the MHEMs was in accord with a recent attentional blink study from our laboratory, in which practitioners fresh out of a 3-month intensive meditation retreat showed a decrease in attentional resources (measured via event-related potential) to the first presentation of the visual target stimulus (5). This decrease in resource utilization to the initial target in the visual stream strongly predicted more accurate detection of the closely adjacent subsequent target with no loss of accuracy in detecting the first target. These findings, taken together, suggest that, at the highest levels of expertise, concentration meditation may result in a less cognitively active (quieter) mental state, such that other tasks performed in its wake may become less effortful (decreased resources allocated without any compromise in performance), perhaps resulting from fewer cognitive processes competing for resources (5).
Distracting Sound Data.
The distracting sounds were intended to serve as probes to test the distractibility of the meditators. Decreased activation in affective and default-mode regions was in accord with our hypothesis that EMs, especially those with the most practice, would have less reaction to the sounds (19, 20, 25, 27, 28). In contrast, the active response to the sounds in other brain regions, coupled with the increased pupil dilation during meditation, was unexpected. These active regions may have been related to “monitoring” (3), a form of metacognition (29) that is said to evaluate the quality of the meditation, monitor and signal when attention leaves the object of meditation, and detect and signal present and future problems with concentration such as being too distracted or drowsy. Activation in anterior Ins may have mediated monitoring one's internal state (30), whereas ventral attention network regions such as ventral prefrontal cortex and intraparietal lobule (31) may have signaled distraction. Prevention of habitual discursive or emotional reactions may have been mediated in part by prefrontal regions, basal ganglia, and subthalamic nuclei, which have been shown to be involved in inhibiting habitual physical (32, 33) and mental processes (34–36). These activations, combined with decreased activation in P. Cing and Amyg in EMs vs. NMs, suggest that the increased pupil diameter for the sounds was not due to cognitive and emotional reactions in EMs but rather the monitoring and adjustment of concentration after a potentially disturbing stimulus.
Potential Caveats.
In a cross-sectional study of this kind that involves a comparison between two rather disparate groups of individuals, it is not possible to definitively attribute the differences we observed exclusively to the meditation training that characterizes the EMs. The correlations we found with hours of practice are more plausibly due to skill learning and plasticity; however, it will be necessary to conduct longitudinal studies within individuals to make stronger inferences about the impact of training per se. Consistent with the conclusions we suggest here, our recent study (5) showed longitudinal changes in both brain and behavior after only 3 months of meditation.
In addition, the hemodynamic response differences may have been influenced by differences in vasculature and hematocrit level (37), as seen in previous studies on aging (38) and in an attention study where older participants showed larger blood oxygenation level-dependent responses (39). However, we statistically controlled for age, the most likely correlate of basic hemodynamic differences, and MHEMs and LHEMs were culturally matched. Nevertheless, other control groups matched for culture, diet, and lifestyle will be important to include in future research.
Although we did not have a behavioral task to demonstrate that subjects were meditating effectively, pupil dilation evidence at least suggests that all participant groups were engaged in the task. Future research is required that includes behavioral tests of attention subcomponents to delineate with more precision those networks modulated by meditation. Finally, in the future it will be important to have multiple control periods against which to compare the meditation blocks because NMs may have had more variation in how they carried out their resting state. Having said that, it is difficult to propose alternative control conditions that would not act as experimental confounds in other ways. Therefore, using a variety of baselines such as reading, or other attention-demanding tasks in addition to a resting baseline, may be necessary in future studies to isolate different cognitive aspects of the meditation.
Practical Implications.
Regions in this study showing differences between groups and correlations with hours of practice overlapped with regions showing abnormal structural and functional variation in persons with attention deficit disorders. For example, compared with normal controls, individuals with attention deficit disorders have shown activation differences in the sustained attention network (40), regions involved in response inhibition (22, 40–42), and reduction in size of prefrontal cortex and cerebellum (43). In addition, it is plausible from our results that meditation may strengthen the ability to inhibit cognitive and emotional mental processes such as rumination that can lead to or exacerbate stress, anxiety, or depression (44). Thus, our data encourage the examination of meditation as a potential form of attentional training in both disordered and normal populations and may provide an answer to William James's question posed >100 years ago when he asked how we might educate attention because such education would be “the education par excellence” (original italics; ref. 45).
Methods
Participants.
Participants included 14 long-term Buddhist practitioners whom we classified as EMs (mean age = 46.8 years, ages 29–64 years, SD 12.1 years), 16 age-matched healthy NMs (mean age = 46.6 years, ages 23–56 years, SD 10.8 years), and 11 INMs who were told they would receive a $50 bonus if among the top one-third in activating attention-related regions (mean age = 39 years, ages 31–51 years, SD 7.1 years). (For more details, see SI Methods.) One week before the actual functional MRI scan session, NMs were given written instructions on how to perform the meditation practices, written by M. Ricard, and practiced concentration and two other meditations for 1 h per day for 1 week, 20 min per meditation (also see SI Methods).
Task and Protocol.
The technical term for this meditation in Tibetan literally means one-pointed concentration. As described in M. Ricard's instructions for the NMs: “this is a state in which one tries to focus all one's attention on one object, keep it on that object, and bring it back to that object when one finds that one has been distracted (by outer perceptions or inner thoughts).” Two incorrect tendencies would be sinking into dullness or sleepiness, or being carried away by mental agitation and internal thought “chatter.” All NMs were informed of these tendencies and instructed to simply return to the object of meditation with a sense of sharp focus. The technical term for the Rest state was, in Tibetan, neutral mind, in which the eyes remained open and fixated. In the instructions for NMs, the neutral state was explained as one in which “your emotional state is neither pleasant nor unpleasant and that you remain relaxed. Try to be in the most ordinary state without being engaged into an active mental state (like voluntarily remembering or planning something or actively looking at an object).”
We used a block design with blocks of varying length (more ideal for deconvolution analysis), alternating an average of 2.7 min (range 146–170 sec) of the state of meditation (object of meditation was a small dot on a gray screen) with an average of 1.6 min (range 84–106 sec) of Rest (four cycles plus one extra 128-sec Rest state of ≈20 min). A total of 25 2-sec auditory sounds from the International Affective Digitized Sounds (46) were presented in random order for each valence (positive, neutral, and negative). These sounds were presented every 6–10 sec after the first 40 sec of the meditative blocks and after 15 sec of the resting blocks. Null trials (silent events) were randomly presented between the auditory stimuli (47, 48). Participants were instructed to maintain their practice during the presentation of the sounds.
Standard data collection and analysis processing procedures were followed and are described in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Matthieu Ricard for assistance with task design, participant recruitment, and written meditation instructions; Dr. Larry Greischar for assistance with pupil diameter assessment; Dr. Tom Johnstone and Dr. Doug Ward with processing assistance; Dr. Robin Kornman for help with results interpretation; Dr. John Dunne for Tibetan translation; Drs. James Lewis and Aina Puce for reviewing earlier versions of the manuscript; undergraduate students A. Francis, W. A. Phillips, A. Shah, S. Harkness, and S. P. Simhan for assistance in data collection and data processing; and the Mind and Life Institute for help in recruiting practitioners and laying the foundation for this work. Support was provided by National Institute of Mental Health Grant P50-MH069315 (to R.J.D.), National Center for Complementary and Alternative Medicine Grant U01AT002114-01A1 (to A.L.), and gifts from Adrianne and Edwin Cook-Ryder, Bryant Wangard, Keith and Arlene Bronstein, and the John W. Kluge Foundation.
Abbreviations
- Amyg
amygdala
- DLPFC
dorsal lateral prefrontal cortex
- EM
expert meditator
- NM
novice meditator
- INM
incentive NM
- IFG
inferior frontal gyrus
- Ins
insula
- IPS
intraparietal sulcus
- LO
lateral occipital
- LHEMs
EMs with the least hours of practice
- MHEM
EMs with the least hours of practice
- MeFG
medial frontal gyrus
- Acc
anterior cingulate
- Med.
meditation
- P.
posterior
- P. Cing
P. cingulate
- ROI
region of interest
- SFG
superior frontal gyrus
- MFG
middle frontal gyrus.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0606552104/DC1.
References
- 1.Lutz A, Dunne JD, Davidson RJ. In: The Cambridge Handbook of Consciousness. Zelazo PD, Moscovitch M, Thompson E, editors. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 2.Kyabgon T. The Benevolent Mind, A Manual in Mind Training. Auckland, New Zealand: Zhyisil Chokyi Gyatsal Trust; 2003. [Google Scholar]
- 3.Mipham SJ. 1999 Seminary Transcripts, Teaching from the Sutra Tradition. Nova Scotia, Canada: Vajradhatu; 2000. [Google Scholar]
- 4.Jha A, Klein R, Krompinger J, Baime M. Cogn Affect Behav Neurosci. 2007;7:109–119. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- 5.Slagter HA, Lutz A, Greischar LL, Francis AD, Nieuwenhuis S, Davis JM, Davidson RJ. PLoS Biol. 2007;5:e138. doi: 10.1371/journal.pbio.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter OL, Presti DE, Callistemon C, Ungerer Y, Liu GB, Pettigrew JD. Curr Biol. 2005;15:R412–R413. doi: 10.1016/j.cub.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 7.Lutz A, Greischar LL, Rawlings NB, Ricard M, Davidson RJ. Proc Natl Acad Sci USA. 2004;101:16369–16373. doi: 10.1073/pnas.0407401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, et al. NeuroReport. 2005;16:1893–1897. doi: 10.1097/01.wnr.0000186598.66243.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore CD, Cohen MX, Ranganath C. J Neurosci. 2006;26:11187–11196. doi: 10.1523/JNEUROSCI.1873-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seung Y, Kyong JS, Woo SH, Lee BT, Lee KM. Neurosci Res. 2005;52:323–329. doi: 10.1016/j.neures.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Ross JS, Tkach J, Ruggieri PM, Lieber M, Lapresto E. Am J Neuroradiol. 2003;24:1036–1044. [PMC free article] [PubMed] [Google Scholar]
- 12.Sakai KL. Science. 2005;310:815–819. doi: 10.1126/science.1113530. [DOI] [PubMed] [Google Scholar]
- 13.Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Proc Natl Acad Sci USA. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam MM. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. J Cognit Neurosci. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 17.Wager TD, Smith EE. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 18.Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Rao SM, Cox RW. J Cognit Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 19.Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Science. 2007;315:393–395. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 21.Van Essen DC, Lewis JW, Drury HA, Hadjikhani N, Tootell RBH, Bakircioglu M, Miller MI. Vision Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- 22.Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Am J Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- 23.Oakes TR, Fox AS, Johnstone T, Chung MK, Kalin N, Davidson RJ. NeuroImage. 2007;34:500–508. doi: 10.1016/j.neuroimage.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wager TD, Jonides J, Reading S. NeuroImage. 2004;22:1679–1693. doi: 10.1016/j.neuroimage.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 25.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brefczynski JA, DeYoe EA. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 27.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter MD, Eickhoff SB, Miller TW, Farrow TF, Wilkinson ID, Woodruff PW. Proc Natl Acad Sci USA. 2006;103:189–194. doi: 10.1073/pnas.0506268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Duque D, Baird JA, Posner MI. Conscious Cogn. 2000;9:288–307. doi: 10.1006/ccog.2000.0447. [DOI] [PubMed] [Google Scholar]
- 30.Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 31.Corbetta M, Shulman GL. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 32.Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, Vauss YC, Vaituzis AC, Dickstein DP, Sarfatti SE, Rapoport JL. J Am Acad Child Adolesc Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 33.Aron A, Poldrack RA. J Neurosci. 2006;26:2424–2433. doi: 10.1523/JNEUROSCI.4682-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson MC, Ochsner KN, Kuhl B, Cooper J, Robertson E, Gabrieli SW, Glover GH, Gabrieli JD. Science. 2004;303:232–235. doi: 10.1126/science.1089504. [DOI] [PubMed] [Google Scholar]
- 35.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. J Cognit Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 36.Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, Jackson CA, Frye CJ, Greischar LL, Alexander AL, Davidson RJ. J Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin JM, Frederick BB, Ross MH, Fox JF, von Rosenberg HL, Kaufman MJ, Lange N, Mendelson JH, Cohen BM, Renshaw PF. Magn Reson Imaging. 2001;19:1055–1062. doi: 10.1016/s0730-725x(01)00460-x. [DOI] [PubMed] [Google Scholar]
- 38.Huettel SA, Singerman JD, McCarthy G. NeuroImage. 2001;13:161–175. doi: 10.1006/nimg.2000.0675. [DOI] [PubMed] [Google Scholar]
- 39.Townsend J, Adamo M, Haist F. NeuroImage. 2006;31:1682–1692. doi: 10.1016/j.neuroimage.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 40.Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. J Child Psychol Psychiatry. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 41.Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JD. Am J Psychiatry. 2005;162:1605–1613. doi: 10.1176/appi.ajp.162.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E. Am J Psychiatry. 2005;162:1067–1075. doi: 10.1176/appi.ajp.162.6.1067. [DOI] [PubMed] [Google Scholar]
- 43.Hill DE, Yeo RA, Campbell RA, Hart B, Vigil J, Brooks W. Neuropsychology. 2003;17:496–506. doi: 10.1037/0894-4105.17.3.496. [DOI] [PubMed] [Google Scholar]
- 44.Watkins E, Teasdale JD. J Affect Disord. 2004;82:1–8. doi: 10.1016/j.jad.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 45.James W. The Principles of Psychology. Birmingham, UK: Classics of Psychiatry and Behavioral Sciences Library; 1890. [Google Scholar]
- 46.Bradley MM, Lang PJ. Psychophysiology. 2000;37:204–215. [PubMed] [Google Scholar]
- 47.Buckner RL. Hum Brain Mapp. 1998;6:373–377. doi: 10.1002/(SICI)1097-0193(1998)6:5/6<373::AID-HBM8>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dale AM. Hum Brain Mapp. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.