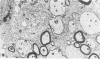Abstract
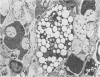
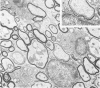
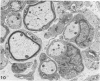
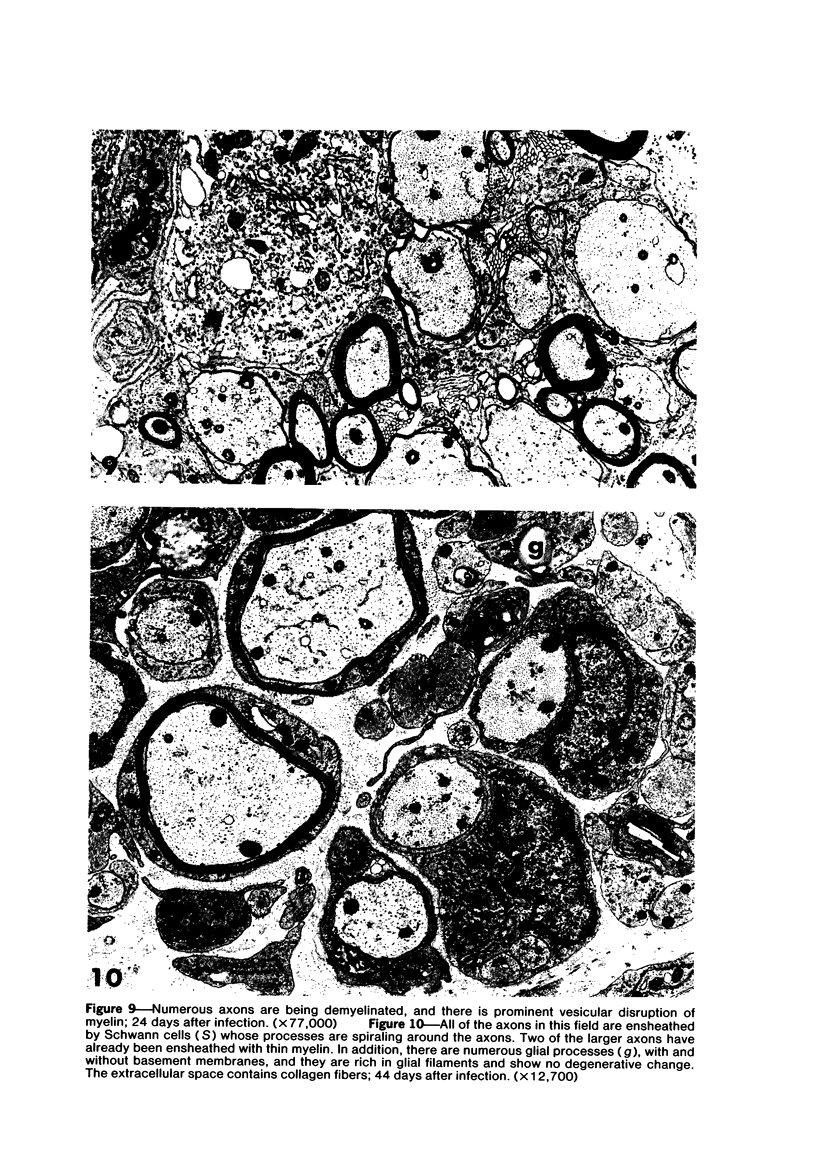
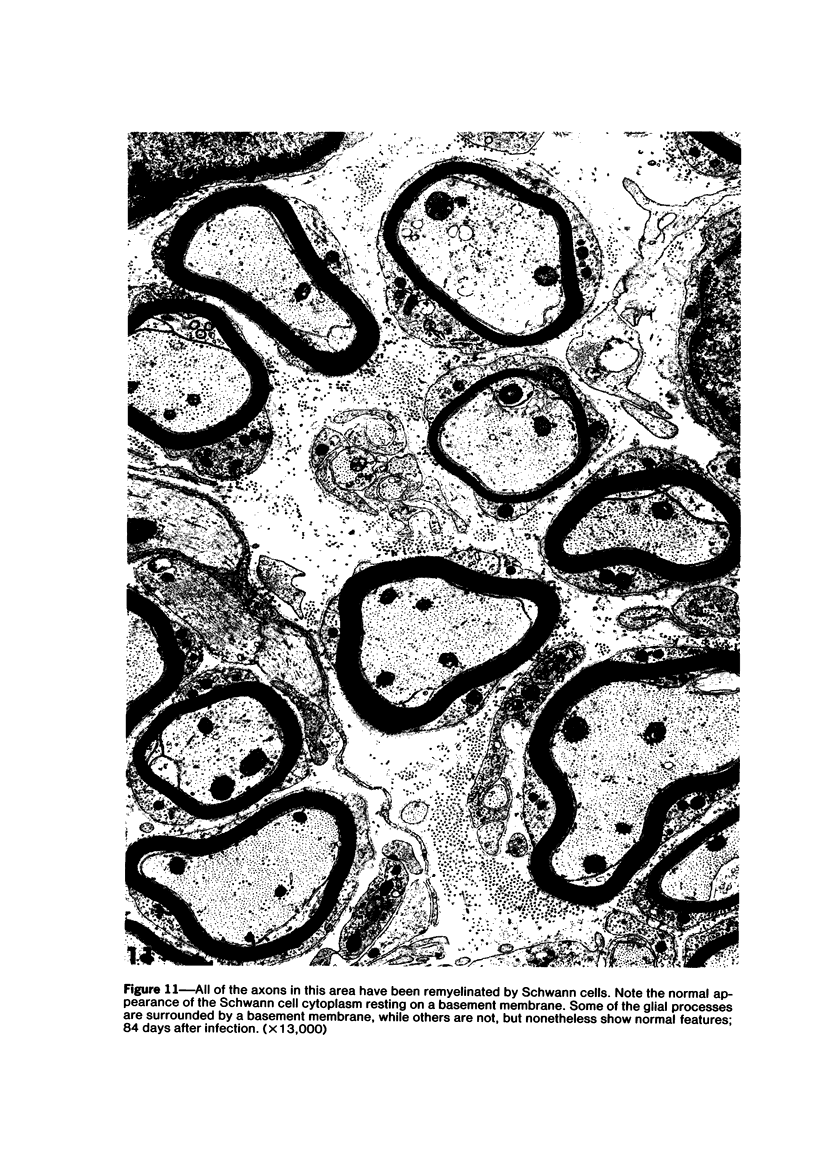
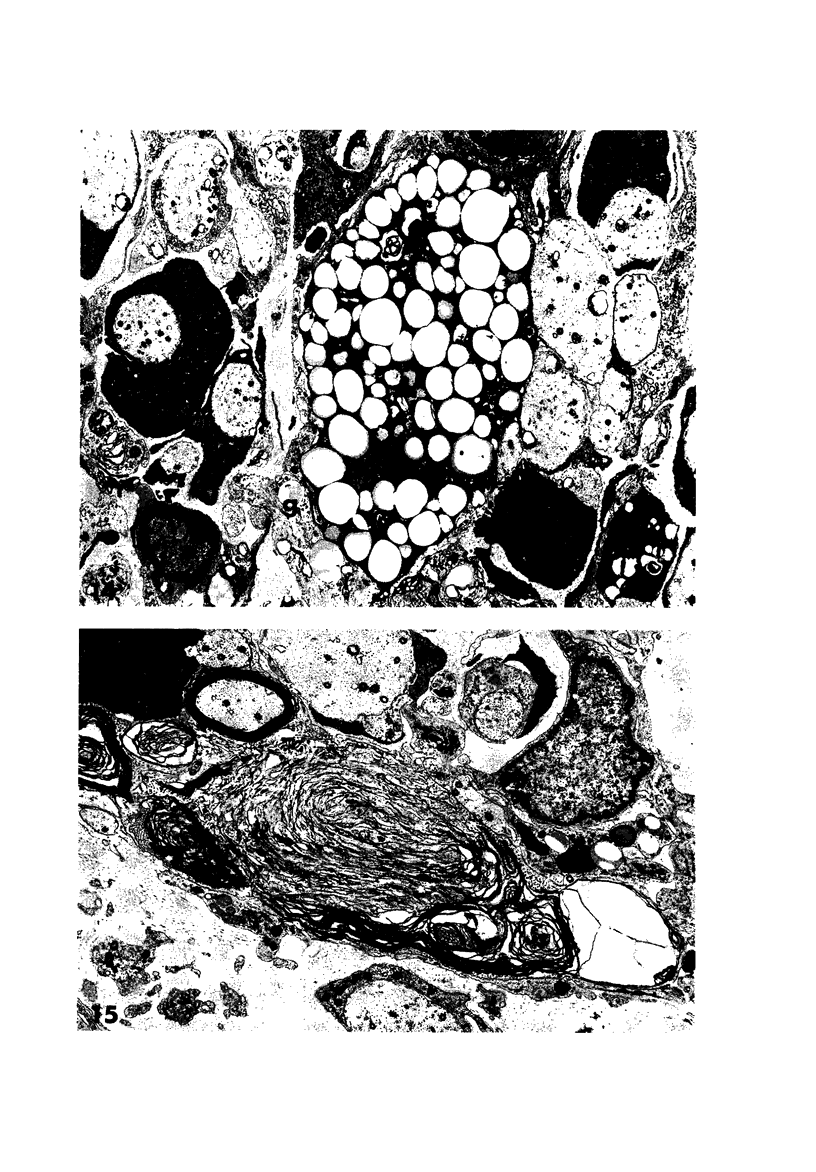
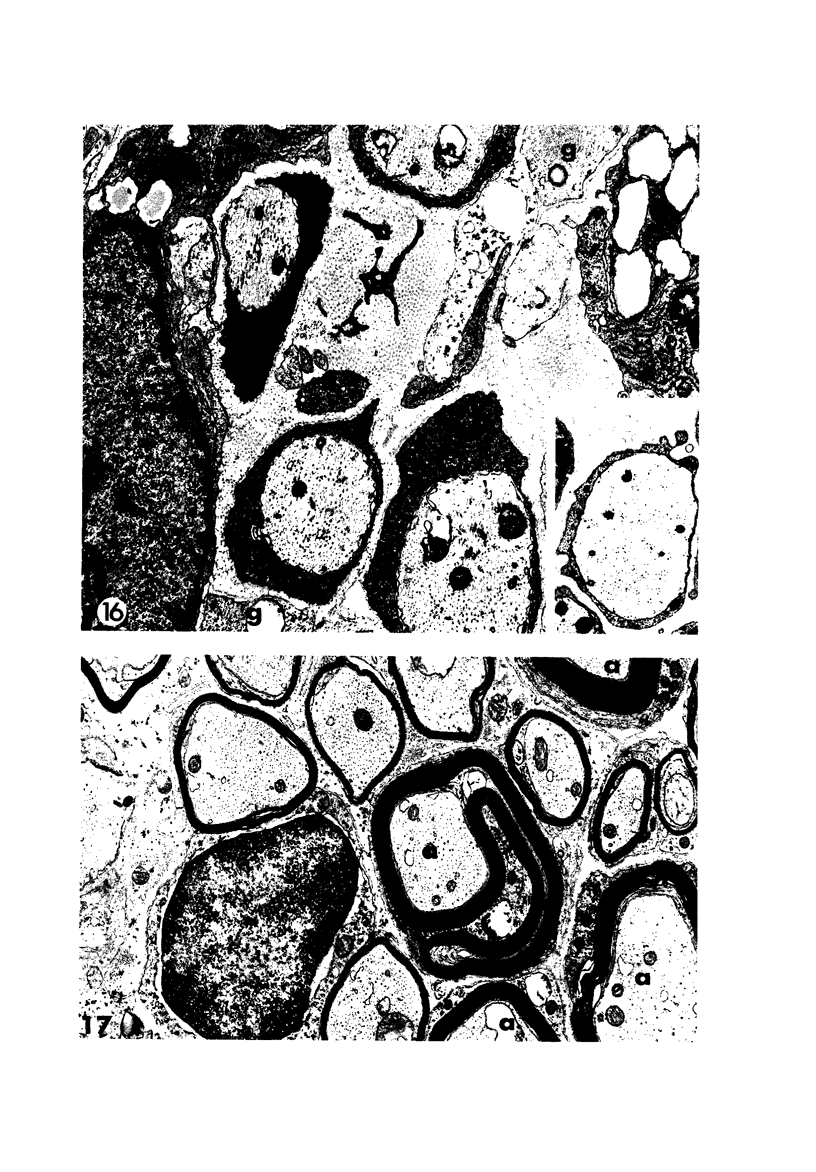
Theiler's murine encephalomyelitis virus (TMEV) infection produces a chronic demyelinating disease in mice, and myelin breakdown appears to be immune-mediated. By using an attenuated TMEC strain, WW virus, to infect mice, the course of the disease was slowed and the severity of the inflammatory and glial responses were reduced. In this circumstance, most of the demyelinating lesions showed extensive remyelination, predominantly by Schwann cells. In addition, it was demonstrated that there was recurrent demyelinating activity in the central nervous system (CNS) of infected animals. It is suggested that the rapidity and intensity of demyelinating lesions may influence the potential for remyelination and that Schwann cell participation may be a more important mechanism of myelin repair than it is now thought to be. The fact that there is a recurrent demyelination in TMEV infection increases its relevance as an experimental animal model for multiple sclerosis.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALVORD E. C., Jr, MAGEE K. R., KIES M. W., GOLDSTEIN N. P. Clinico-pathologic correlations in experimental allergic encephalomyelitis. I. Observations on the early lesion. J Neuropathol Exp Neurol. 1959 Jul;18(3):442–446. doi: 10.1097/00005072-195907000-00006. [DOI] [PubMed] [Google Scholar]
- Aguayo A. J., Peyronnard J. M., Terry L. C., Romine J. S., Bray G. M. Neonatal neuronal loss in rat superior cervical ganglia: retrograde effects on developing preganglionic axons and Schwann cells. J Neurocytol. 1976 Apr;5(2):137–155. doi: 10.1007/BF01181653. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F., Patterson R. C. Observations on the interactions of Schwann cells and astrocytes following X-irradiation of neonatal rat spinal cord. J Neurocytol. 1975 Oct;4(5):573–585. doi: 10.1007/BF01351538. [DOI] [PubMed] [Google Scholar]
- Blakemore W. F. Remyelination by Schwann cells of axons demyelinated by intraspinal injection of 6-aminonicotinamide in the rat. J Neurocytol. 1975 Dec;4(6):745–757. doi: 10.1007/BF01181634. [DOI] [PubMed] [Google Scholar]
- Cammer W., Bloom B. R., Norton W. T., Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Multiple sclerosis. Animal model:Theiler's virus infection in mice. Am J Pathol. 1977 Aug;88(2):497–500. [PMC free article] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- Dalcanto M. C., Wiśniewski H. M., Johnson A. B., Brostoff S. W., Raine C. S. Vesicular disruption of myelin in autoimmune demyelination. J Neurol Sci. 1975 Mar;24(3):313–319. doi: 10.1016/0022-510x(75)90251-8. [DOI] [PubMed] [Google Scholar]
- Feigin I., Ogata J. Schwann cells and peripheral myelin within human central nervous tissues: the mesenchymal character of Schwann cells. J Neuropathol Exp Neurol. 1971 Oct;30(4):603–612. doi: 10.1097/00005072-197110000-00005. [DOI] [PubMed] [Google Scholar]
- Ghatak N. R., Hirano A., Doron Y., Zimmerman H. M. Remyelination in multiple sclerosis with peripheral type myelin. Arch Neurol. 1973 Oct;29(4):262–267. doi: 10.1001/archneur.1973.00490280074011. [DOI] [PubMed] [Google Scholar]
- Hirano A., Zimmerman H. M., Levine S. Electron microscopic observations of peripheral myelin in a central nervous system lesion. Acta Neuropathol. 1969;12(4):348–365. doi: 10.1007/BF00809131. [DOI] [PubMed] [Google Scholar]
- LAMPERT P., CRESSMAN M. AXONAL REGENERATION IN THE DORSAL COLUMNS OF THE SPINAL CORD OF ADULT RATS. AN ELECTRON MICROSCOPIC STUDY. Lab Invest. 1964 Aug;13:825–839. [PubMed] [Google Scholar]
- Levine S., Sowinski R., Shaw C. M., Alvord E. C., Jr Do neurological signs occur in experimental allergic encephalomyelitis in the absence of inflammatory lesions of the central nervous system?. J Neuropathol Exp Neurol. 1975 Nov;34(6):501–506. doi: 10.1097/00005072-197511000-00004. [DOI] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. The TO strains of Theiler's viruses cause "slow virus-like" infections in mice. Ann Neurol. 1979 Jul;6(1):25–28. doi: 10.1002/ana.410060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Dal Canto M. C. Theiler's virus-induced demyelination: prevention by immunosuppression. Science. 1976 Apr 2;192(4234):62–64. doi: 10.1126/science.176726. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin D. E., Blank S. E., Kibler R. F. Recurrent experimental allergic encephalomyelitis in the Lewis rat. J Immunol. 1974 Aug;113(2):712–715. [PubMed] [Google Scholar]
- Paterson P. Y. Joseph E. Smadel Memorial Lecture: neuroimmunologic diseases of animals and humans. Rev Infect Dis. 1979 May-Jun;1(3):469–482. [PubMed] [Google Scholar]
- Prineas J. W., Connell F. Remyelination in multiple sclerosis. Ann Neurol. 1979 Jan;5(1):22–31. doi: 10.1002/ana.410050105. [DOI] [PubMed] [Google Scholar]
- Raine C. S. On the occurrence of Schwann cells within the normal central nervous system. J Neurocytol. 1976 Jun;5(3):371–380. doi: 10.1007/BF01175122. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Traugott U., Stone S. H. Chronic relapsing experimental allergic encephalomyelitis: CNS plaque development in unsuppressed and suppressed animals. Acta Neuropathol. 1978 Aug 7;43(1-2):43–53. doi: 10.1007/BF00684997. [DOI] [PubMed] [Google Scholar]
- Raine C. S., Traugott U., Stone S. H. Glial bridges and Schwann cell migration during chronic demyelination in the C.N.S. J Neurocytol. 1978 Oct;7(5):541–553. doi: 10.1007/BF01260888. [DOI] [PubMed] [Google Scholar]
- STONE S. H., LERNER E. M., 2nd CHRONIC DISSEMINATED ALLERGIC ENCEPHALOMYELITIS IN GUINEA PIGS. Ann N Y Acad Sci. 1965 Mar 31;122:227–241. doi: 10.1111/j.1749-6632.1965.tb20206.x. [DOI] [PubMed] [Google Scholar]
- Snyder D. H., Valsamis M. P., Stone S. H., Raine C. S. Progressive demyelination and reparative phenomena in chronic experimental allergic encephalomyelitis. J Neuropathol Exp Neurol. 1975 May;34(3):209–221. doi: 10.1097/00005072-197505000-00001. [DOI] [PubMed] [Google Scholar]
- Theiler M. SPONTANEOUS ENCEPHALOMYELITIS OF MICE--A NEW VIRUS DISEASE. Science. 1934 Aug 3;80(2066):122–122. doi: 10.1126/science.80.2066.122-a. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Keith A. B. Chronic relapsing experimental allergic encephalomyelitis: an experimental model of multiple sclerosis. Ann Neurol. 1977 Feb;1(2):144–148. doi: 10.1002/ana.410010207. [DOI] [PubMed] [Google Scholar]
- Wood P. M., Bunge R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 1975 Aug 21;256(5519):662–664. doi: 10.1038/256662a0. [DOI] [PubMed] [Google Scholar]