Abstract
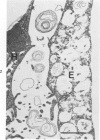
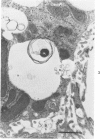
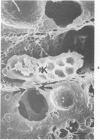
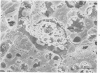
Scanning and transmission electron-microscopic study of the livers of 2 kittens suffering from GM2 gangliosidosis revealed discontinuities in the plasma membrane of hepatocytes and Kupffer cells, indicating an attempt by the cells to free themselves of excess lysosomal residues by means of exocytosis. Inclusions morphologically similar to those seen in the hepatocytes were observed in the perisinusoidal space. Openings were seen on all surfaces of the hepatocytes. Such extrusion of lysosomal residues is an example of a mechanism rarely observed in viable metazoan cells.
Full text
PDF





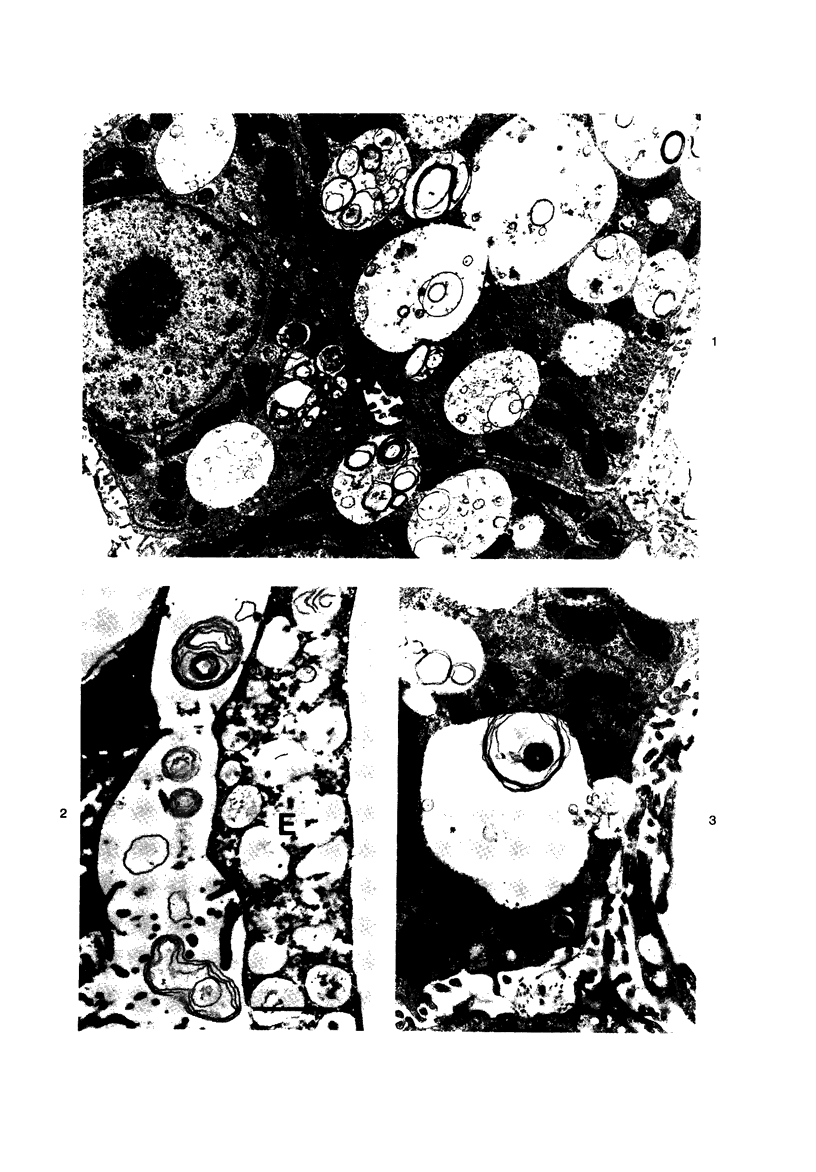
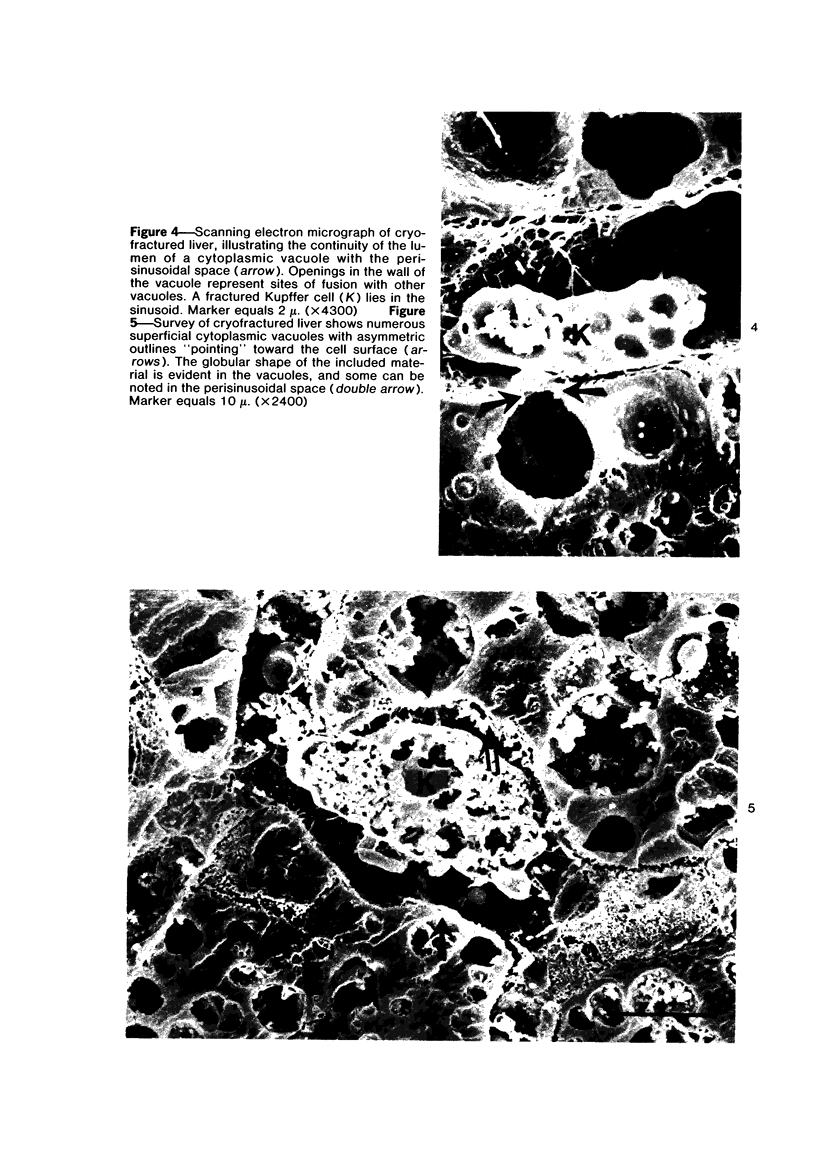
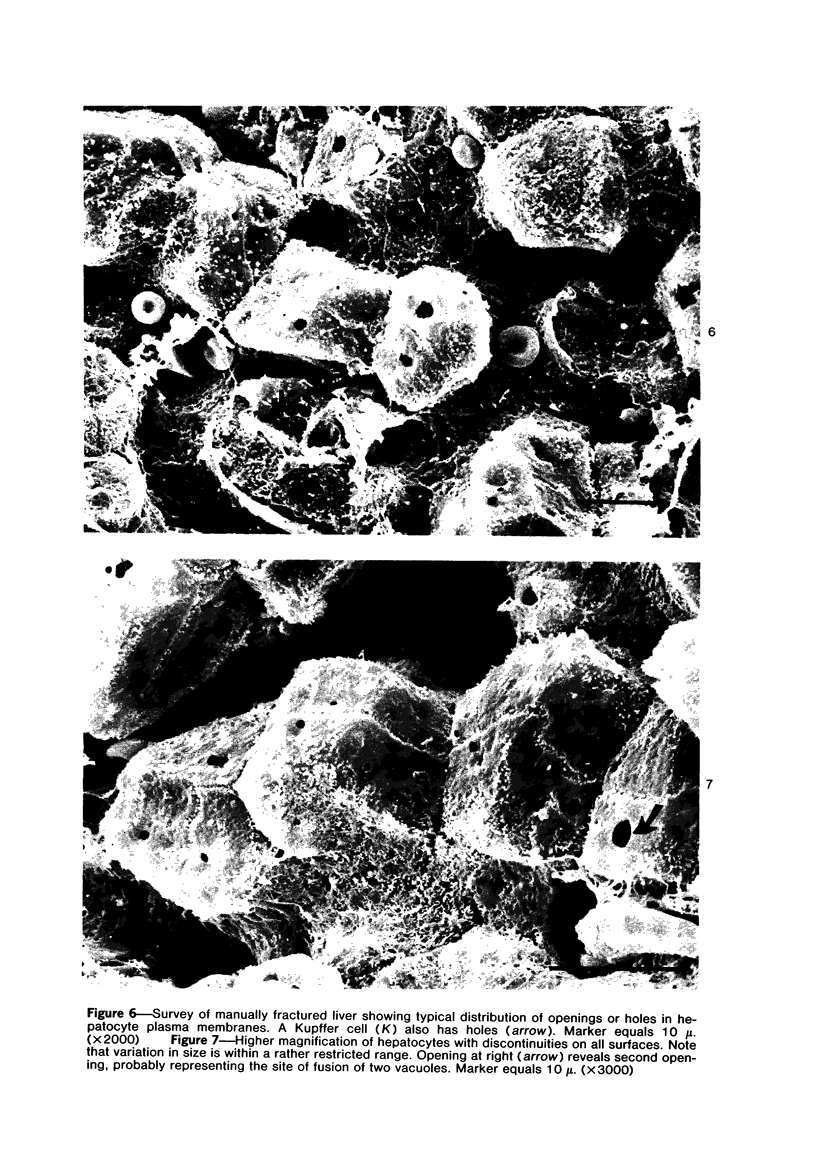

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford W. D., Elchlepp J. G., Arstila A. U., Trump B. F., Kinney T. D. Iron metabolism and cell membranes. I. Relation between ferritin and hemosiderin in bile and biliary excretion of lysosome contents. Am J Pathol. 1969 Aug;56(2):201–228. [PMC free article] [PubMed] [Google Scholar]
- Bruni C., Porter K. R. The Fine Structure of the Parenchymal Cell of the Normal Rat Liver: I. General Observations. Am J Pathol. 1965 May;46(5):691–755. [PMC free article] [PubMed] [Google Scholar]
- CONFER D. B., STENGER R. J. THE EVOLUTION OF LYSOSOMES IN HYPOXIC LIVER PARENCHYMA AS SEEN WITH THE ELECTRON MICROSCOPE. Am J Pathol. 1964 Oct;45:533–546. [PMC free article] [PubMed] [Google Scholar]
- Compagno J., Grisham J. W. Scanning electron microscopy of extrahepatic biliary obstruction. Arch Pathol. 1974 Jun;97(6):348–351. [PubMed] [Google Scholar]
- Cork L. C., Munnell J. F., Lorenz M. D., Murphy J. V., Baker H. J., Rattazzi M. C. GM2 ganglioside lysosomal storage disease in cats with beta-hexosaminidase deficiency. Science. 1977 May 27;196(4293):1014–1017. doi: 10.1126/science.404709. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Munnell J. F., Lorenz M. D. The pathology of feline GM2 gangliosidosis. Am J Pathol. 1978 Mar;90(3):723–734. [PMC free article] [PubMed] [Google Scholar]
- Glinsmann W. H., Ericsson J. L. Observations on the subcellular organization of hepatic parenchymal cells. II. Evolution of reversible alterations induced by hypoxia. Lab Invest. 1966 Apr;15(4):762–777. [PubMed] [Google Scholar]
- Kerr J. F. Liver cell defaecation: an electron-microscope study of the discharge of lysosomal residual bodies into the intercellular space. J Pathol. 1970 Feb;100(2):99–103. doi: 10.1002/path.1711000204. [DOI] [PubMed] [Google Scholar]
- MALET P., JOYON L., TURCHINI J. P. EXPULSION ENDOCANALICULAIRE DE MAT'ERIEL FIGUR'E (FRAGMENTS CYTOPLASMIQUES, MITOCHONDRIES) ET CORPS LYSOSOMIQUES G'EANTS DANS LE FOIE N'EO-NATAL. OBSERVATIONS INFRAMICROSCOPIQUES. C R Hebd Seances Acad Sci. 1964 May 20;258:5067–5069. [PubMed] [Google Scholar]
- Maunsbach A. B. Observations on the ultrastructure and acid phosphatase activity of the cytoplasmic bodies in rat kidney proximal tubule cells. With a comment on their classification. J Ultrastruct Res. 1966 Oct;16(3):197–238. doi: 10.1016/s0022-5320(66)80059-x. [DOI] [PubMed] [Google Scholar]
- Schellens J. P. Ageing of mouse liver lysosomes. An experimental study using indigestible marker substances. Cell Tissue Res. 1974;155(4):455–473. doi: 10.1007/BF00227009. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Jr, Brady R. O., Suzuki K. Enzymic activities associated with membranous cytoplasmic bodies and isolated brain lysosomes. J Neurochem. 1971 Sep;18(9):1775–1777. doi: 10.1111/j.1471-4159.1971.tb03754.x. [DOI] [PubMed] [Google Scholar]