Abstract
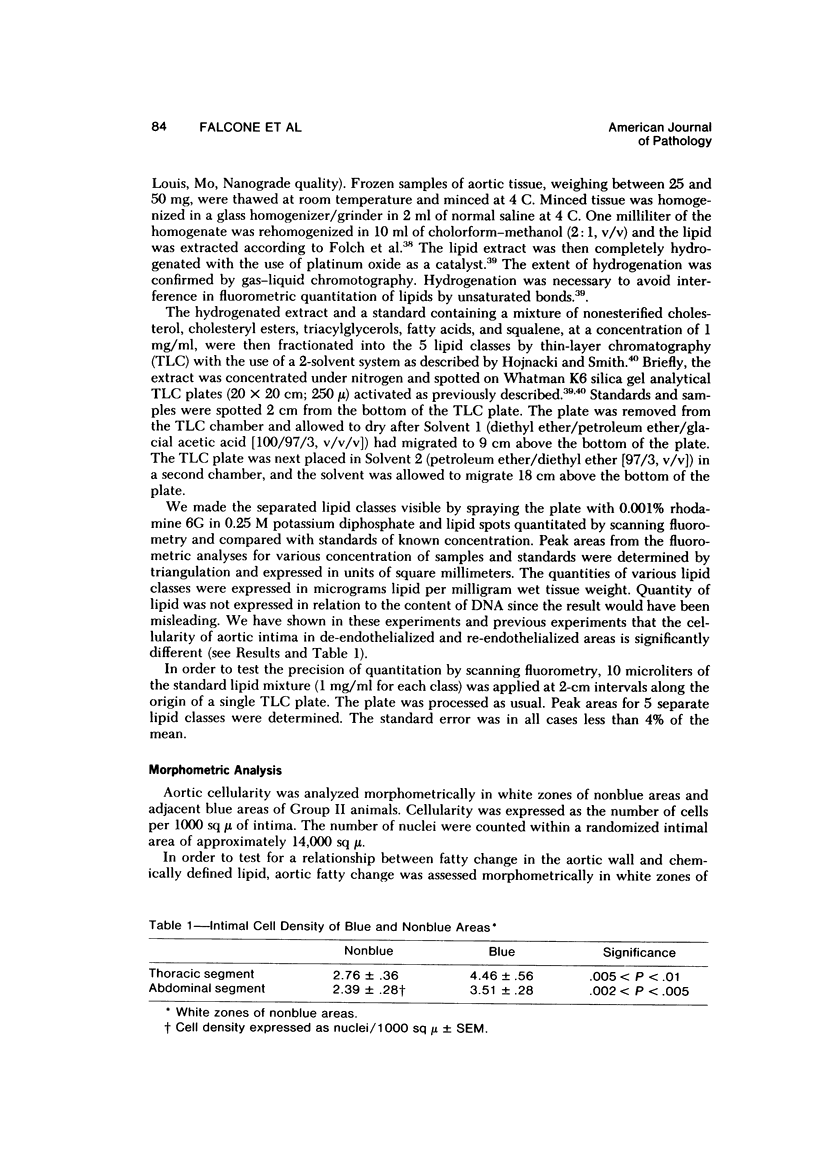
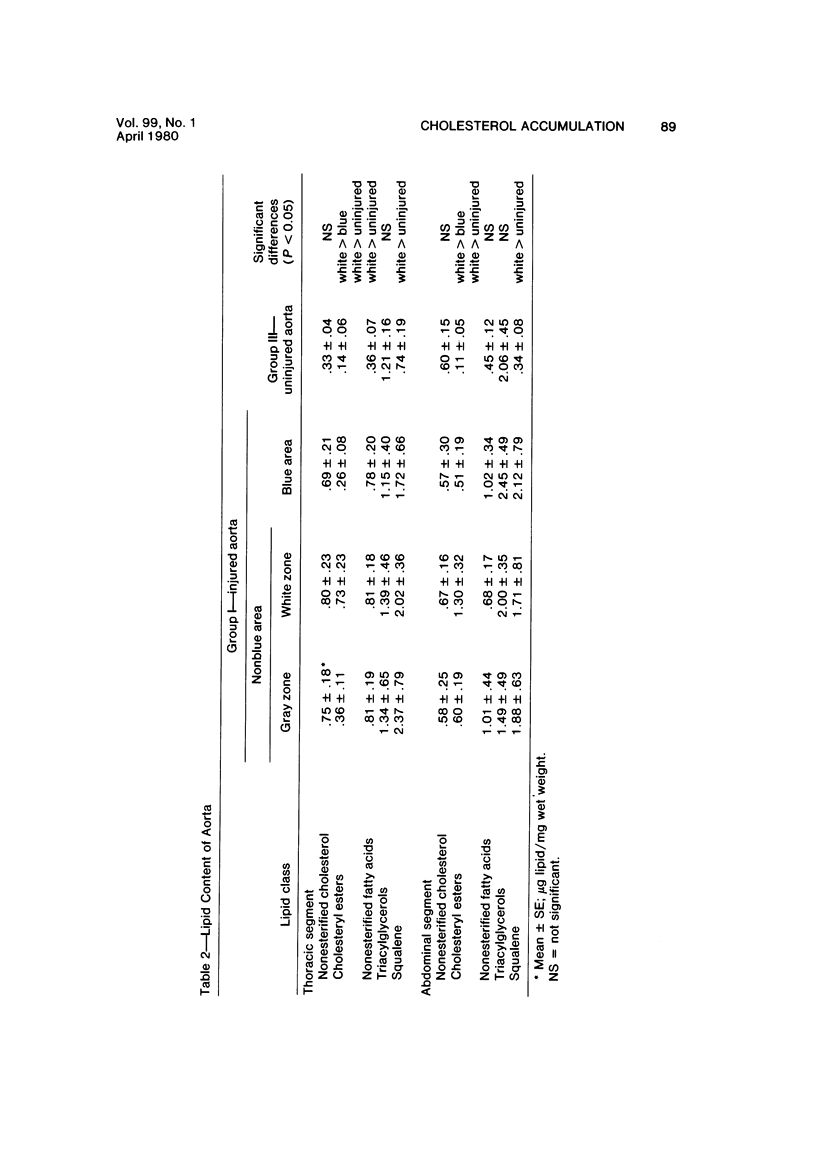
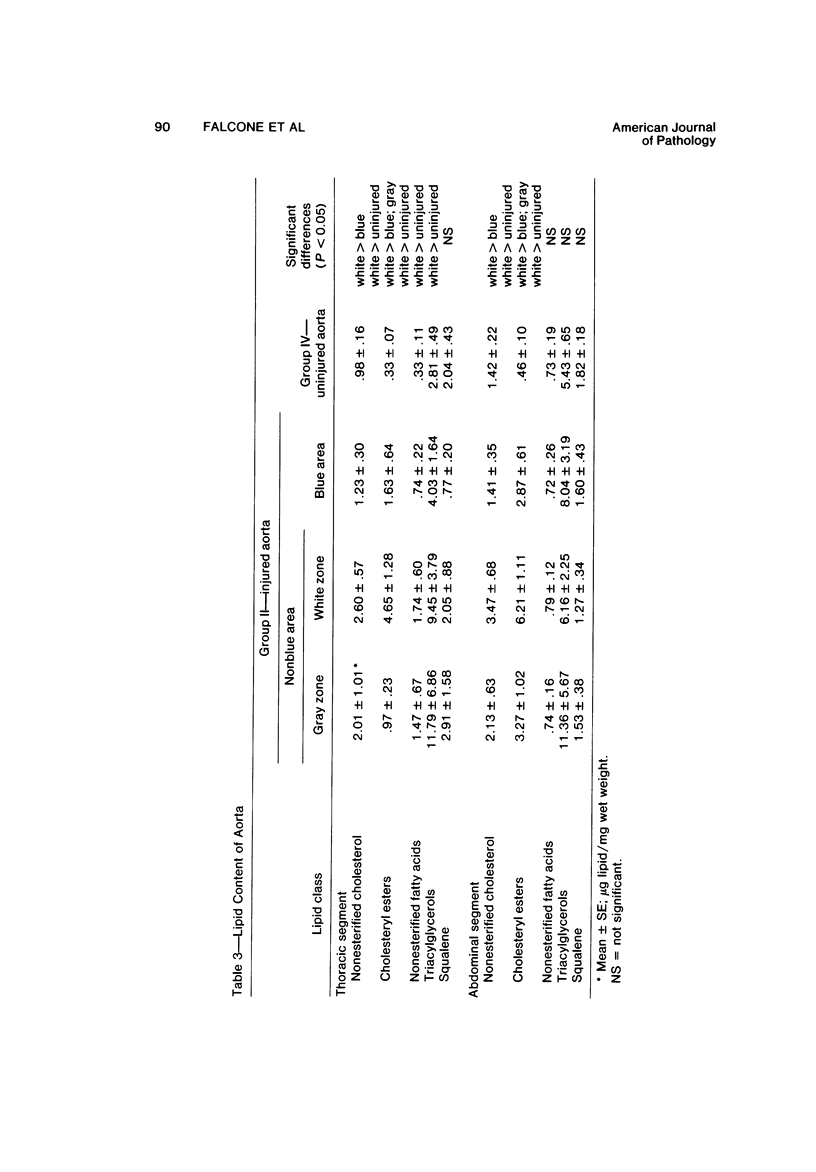
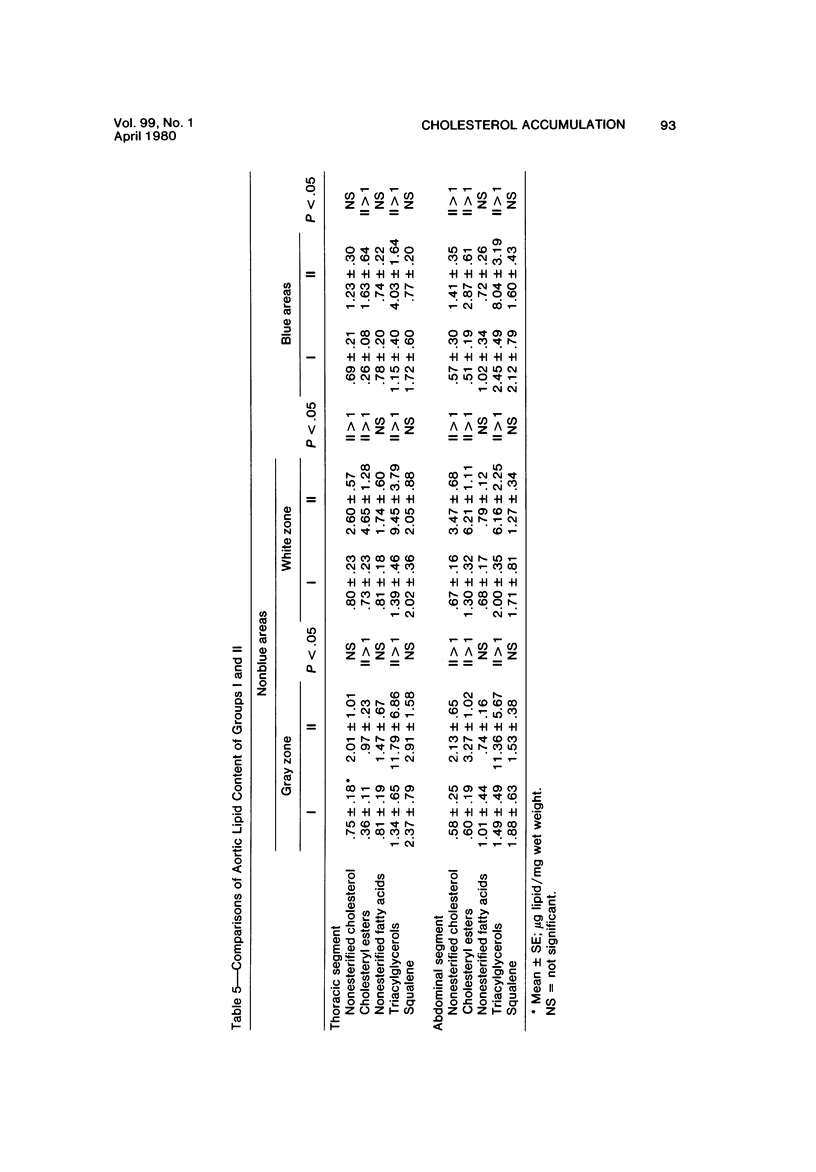
The purpose of the experiments reported here was to determine chemically the character and quantity of lipid in re-endothelialized and de-endothelialized areas of rabbit aortas. The aortas of 22 rabbits, Groups I and II, were de-endothelialized with a balloon catheter, and the rabbits were maintained on a lipid-poor diet for 4 weeks. Thirteen rabbits, Group II, were then fed an egg-supplemented diet for 6 weeks. Nine rabbits, Group I, were continued on the lipid-poor diet for an additional 6 weeks. Control rabbits with uninjured aortas were fed a lipid-poor diet for 10 weeks (Group III) or an egg-supplemented diet for 6 weeks (Group IV). Nonesterified cholesterol and fatty acids, cholesteryl esters, triacylglycerols, and squalene were quantitated in re-endothelialized and de-endothelialized aorta by thin-layer chromatography and fluorometric analysis. The results indicate 1) that there was approximately three times as much nonesterified cholesterol and cholesteryl ester in re-endothelialized aorta of Groups I and II as compared with adjacent de-endothelialized aorta and 2) that in re-endothelialized aorta of Group II the amount of total cholesterol correlated with serum cholesterol concentration in contrast to adjacent de-endothelialized aorta, with no correlation over a range of nearly 900 mg/100 ml. These studies indicate that the presence of endothelium favors accumulation of aortic cholesteryl esters. The results suggest that arterial lipid accumulation is not simply a result of passive filtration but may result from metabolic differences in the re-endothelialized neointima.
Full text
PDF


















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anseth A. Influence of corneal epithelium on the incorporation of 35SO4 into stromal glycosaminoglycans. Exp Eye Res. 1971 Mar;11(2):251–254. doi: 10.1016/s0014-4835(71)80029-5. [DOI] [PubMed] [Google Scholar]
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L., Convit J. Physicochemical characteristics of the glycosaminoglycan-lysosomal enzyme interaction in vitro. A model of control of leucocytic lysosomal activity. Biochem J. 1976 Nov 15;160(2):129–136. doi: 10.1042/bj1600129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila J. L. The influence of the type of sulphate bond and degree of sulphation of glycosaminoglycans on their interaction with lysosomal enzymes. Biochem J. 1978 May 1;171(2):489–491. doi: 10.1042/bj1710489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner H. R., Stemerman M. B., Spaet T. H. Adhesion of blood platelets to subendothelial surface: distinct from adhesion to collagen. Experientia. 1971 Mar 15;27(3):283–285. doi: 10.1007/BF02138148. [DOI] [PubMed] [Google Scholar]
- Bell F. P., Adamson I. L., Schwartz C. J. Aortic endothelial permeability to albumin: focal and regional patterns of uptake and transmural distribution of 131I-albumin in the young pig. Exp Mol Pathol. 1974 Feb;20(1):57–68. doi: 10.1016/0014-4800(74)90043-4. [DOI] [PubMed] [Google Scholar]
- Bell F. P., Gallus A. S., Schwartz C. J. Focal and regional patterns of uptake and the transmural distribution of 131-I-fibrinogen in the pig aorta in vivo. Exp Mol Pathol. 1974 Apr;20(2):281–292. doi: 10.1016/0014-4800(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Bratzler R. L., Chisolm G. M., Colton C. K., Smith K. A., Lees R. S. The distribution of labeled low-density lipoproteins across the rabbit thoracic aorta in vivo. Atherosclerosis. 1977 Nov;28(3):289–307. doi: 10.1016/0021-9150(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Chen R. M., Getz G. S., Fischer-Dzoga K., Wissler R. W. The role of hyperlipidemic serum on the proliferation and necrosis of aortic medial cells in vitro. Exp Mol Pathol. 1977 Jun;26(3):359–374. doi: 10.1016/0014-4800(77)90039-9. [DOI] [PubMed] [Google Scholar]
- Cremer-Bartels G., Grundmann E., Mielonen P., Buddecke E. Light-induced variation of glucose metabolism in bovine cornea. Exp Eye Res. 1972 Mar;13(2):172–177. doi: 10.1016/0014-4835(72)90030-9. [DOI] [PubMed] [Google Scholar]
- Day A. J., Bell F. P., Moore S., Friedman R. Lipid composition and metabolism of thromboatherosclerotic lesions produced by continued endothelial damage in normal rabbits. Circ Res. 1974 Apr;34(4):467–476. doi: 10.1161/01.res.34.4.467. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fielding C. J. Metabolism of cholesterol-rich chylomicroms. Mechanism of binding and uptake of cholesteryl esters by the vascular bed of the perfused rat heart. J Clin Invest. 1978 Jul;62(1):141–151. doi: 10.1172/JCI109099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Dzoga K., Fraser R., Wissler R. W. Stimulation of proliferation in stationary primary cultures of monkey and rabbit aortic smooth muscle cells. I. Effects of lipoprotein fractions of hyperlipemic serum and lymph. Exp Mol Pathol. 1976 Jun;24(3):346–359. doi: 10.1016/0014-4800(76)90070-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. J., Moore S., Singal D. P., Gent M. Regression of injury-induced atheromatous lesions in rabbits. Arch Pathol Lab Med. 1976 Apr;100(4):189–195. [PubMed] [Google Scholar]
- Friedman R. J., Moore S., Singal D. P. Repeated endothelial injury and induction of atherosclerosis in normolipemic rabbits by human serum. Lab Invest. 1975 Mar;32(3):404–415. [PubMed] [Google Scholar]
- Hardin N. J., Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. 3. The role of earlier acquired fibromuscular intimal thickening in the pathogenesis of later developing atherosclerosis. Am J Pathol. 1973 Nov;73(2):301–326. [PMC free article] [PubMed] [Google Scholar]
- Harker L. A., Slichter S. J., Scott C. R., Ross R. Homocystinemia. Vascular injury and arterial thrombosis. N Engl J Med. 1974 Sep 12;291(11):537–543. doi: 10.1056/NEJM197409122911101. [DOI] [PubMed] [Google Scholar]
- Hojnacki J. L., Smith S. C. Separation of six lipid classes on one thin-layer chromatogram. J Chromatogr. 1974 Apr 10;90(2):364–367. doi: 10.1016/s0021-9673(00)92542-1. [DOI] [PubMed] [Google Scholar]
- Iverius P. H. The interaction between human plasma lipoproteins and connective tissue glycosaminoglycans. J Biol Chem. 1972 Apr 25;247(8):2607–2613. [PubMed] [Google Scholar]
- Kint J. A., Dacremont G., Carton D., Orye E., Hooft C. Mucopolysaccharidosis: secondarily induced abnormal distribution of lysosomal isoenzymes. Science. 1973 Jul 27;181(4097):352–354. doi: 10.1126/science.181.4097.352. [DOI] [PubMed] [Google Scholar]
- Klintworth G. K., Smith C. F. A comparative study of extracellular sulfated glycosaminoglycans synthesized by rabbit corneal fibroblasts in organ and confluent cultures. Lab Invest. 1976 Sep;35(3):258–263. [PubMed] [Google Scholar]
- LEVINE J. B., ZAK B. AUTOMATED DETERMINATION OF SERUM TOTAL CHOLESTEROL. Clin Chim Acta. 1964 Oct;10:381–384. doi: 10.1016/0009-8981(64)90073-7. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Endothelium 1977: a review. Adv Exp Med Biol. 1978;104:169-225, 481-526. doi: 10.1007/978-1-4684-7787-0_9. [DOI] [PubMed] [Google Scholar]
- Mawhinney T. P., Augustyn J. M., Fritz K. E. Glycosaminoglycan-lipoprotein complexes from aortas of hypercholesterolemic rabbits. Part 1. Isolation and characterization. Atherosclerosis. 1978 Oct;31(2):155–167. doi: 10.1016/0021-9150(78)90161-2. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Litrenta M. M., Alonso D. R., Silane M. F., Stemerman M. B. Further studies on the effect of regenerated endothelium on intimal lipid accumulation. Prog Biochem Pharmacol. 1977;13:115–122. [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E., Campbell W. G., Jr Experimental induction of athero-arteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. I. Effect of repeated injections of horse serum in rabbits fed a dietary cholesterol supplement. J Exp Med. 1966 Oct 1;124(4):635–652. doi: 10.1084/jem.124.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Murphy G. E. Experimental induction of atheroarteriosclerosis by the synergy of allergic injury to arteries and lipid-rich diet. II. Effect of repeatedly injected foreign protein in rabbits fed a lipid-rich, cholesterol-poor diet. Am J Pathol. 1973 Nov;73(2):265–300. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. B., Insull W., Jr Role of endothelium and hypercholesterolemia in intimal thickening and lipid accumulation. Am J Pathol. 1979 Apr;95(1):131–158. [PMC free article] [PubMed] [Google Scholar]
- Minick C. R., Stemerman M. G., Insull W., Jr Effect of regenerated endothelium on lipid accumulation in the arterial wall. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1724–1728. doi: 10.1073/pnas.74.4.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S., Ihnatowycz T. O. Vessel injury and atherosclerosis. Adv Exp Med Biol. 1978;102:145–161. doi: 10.1007/978-1-4757-1217-9_9. [DOI] [PubMed] [Google Scholar]
- Moore S. Thromboatherosclerosis in normolipemic rabbits. A result of continued endothelial damage. Lab Invest. 1973 Nov;29(5):478–487. [PubMed] [Google Scholar]
- Nicolosi R. J., Smith S. C., Santerre R. F. Simultaneous fluorometric analysis of five lipid classes on thin-layer chromatograms. J Chromatogr. 1971 Aug 5;60(1):111–117. [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Harker L. Hyperlipidemia and atherosclerosis. Science. 1976 Sep 17;193(4258):1094–1100. doi: 10.1126/science.822515. [DOI] [PubMed] [Google Scholar]
- Saito H., Uzman B. G. Uptake of chondroitin sulfate by mammalian cells in culture. II. Kinetics of uptake and autoradiography. Exp Cell Res. 1971 May;66(1):90–96. doi: 10.1016/s0014-4827(71)80015-0. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976 Mar;68(3):705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N., Simionescu M., Palade G. E. Recent studies on vascular endothelium. Ann N Y Acad Sci. 1976;275:64–75. doi: 10.1111/j.1749-6632.1976.tb43338.x. [DOI] [PubMed] [Google Scholar]
- Spaet T. H., Rhee C., Geiger C. Delayed consequences of endothelial removal from rabbit aortae. Adv Exp Med Biol. 1978;102:165–173. doi: 10.1007/978-1-4757-1217-9_10. [DOI] [PubMed] [Google Scholar]
- Srinivasan S. R., Dolan P., Radhakrishnamurthy B., Berenson G. S. Isolation of lipoprotein-acid mucopolysaccharide complexes from fatty streaks of human aortas. Atherosclerosis. 1972 Jul-Aug;16(1):95–104. doi: 10.1016/0021-9150(72)90012-3. [DOI] [PubMed] [Google Scholar]
- Stemerman M. B. Thrombogenesis of the rabbit arterial plaque. An electron microscopic study. Am J Pathol. 1973 Oct;73(1):7–26. [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson G., Robertson A. L., Jr The vascular endothelium-pathobiologic significance. Am J Pathol. 1978 Dec;93(3):803–848. [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fielding P. E., Fielding C. J., Gospodarowicz D. Role of contact inhibition in the regulation of receptor-mediated uptake of low density lipoprotein in cultured vascular endothelial cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):356–360. doi: 10.1073/pnas.75.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]


