Abstract
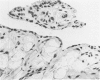
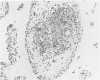
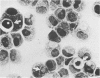
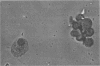
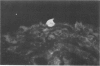
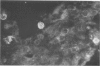
Experiments were designed to correlate morphologic lesions with the presence of caprine arthritis-encephalitis virus (CAEV). Twenty-one cesarean-derived goat kids were infected with 10(6) to 10(7) TCID50 of virus, killed sequentially, and examined for viral antigens by immunofluorescence, viral infectivity by isolation and titration, and morphologic changes by light microscopy. Fluorescent viral antigens were detected from 1 to 10 days postinoculation (DPI) and only in synovial cells. Virus was reisolated from several joints and from brain 0.5 to 79 DPI. Increases in synovial fluid cell counts were noted by 1 DPI, and morphologic changes in synovial membranes were present from 3 to 45 DPI. Joint lesions progressed from mild synovial cell hyperplasia and perivascular mononuclear cell infiltration to severe synovial cell hyperplasia and mononuclear cell infiltration with villous hypertrophy. Lesions elsewhere were mild, consisting only of perivascular mononuclear cell infiltrates. Eleven cesarean-derived control goats were negative for viral antigens, virus, and morphologic lesions.
Full text
PDF




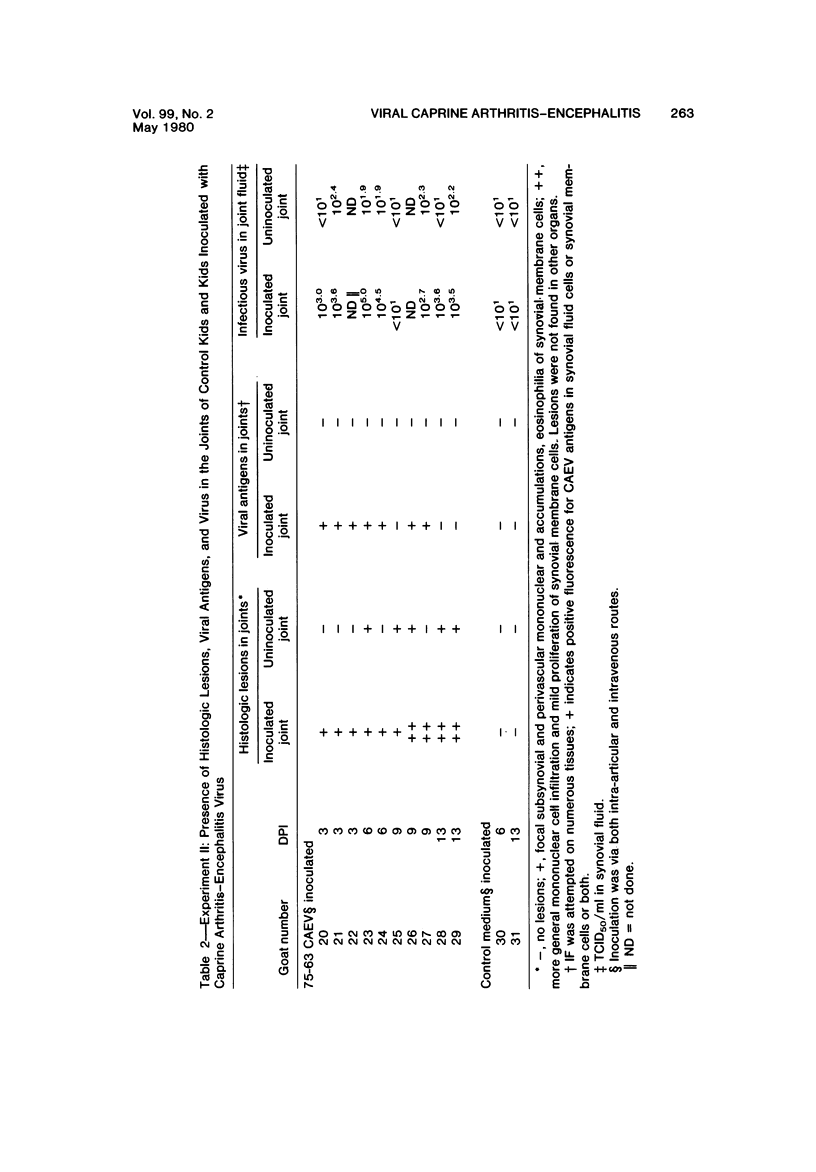

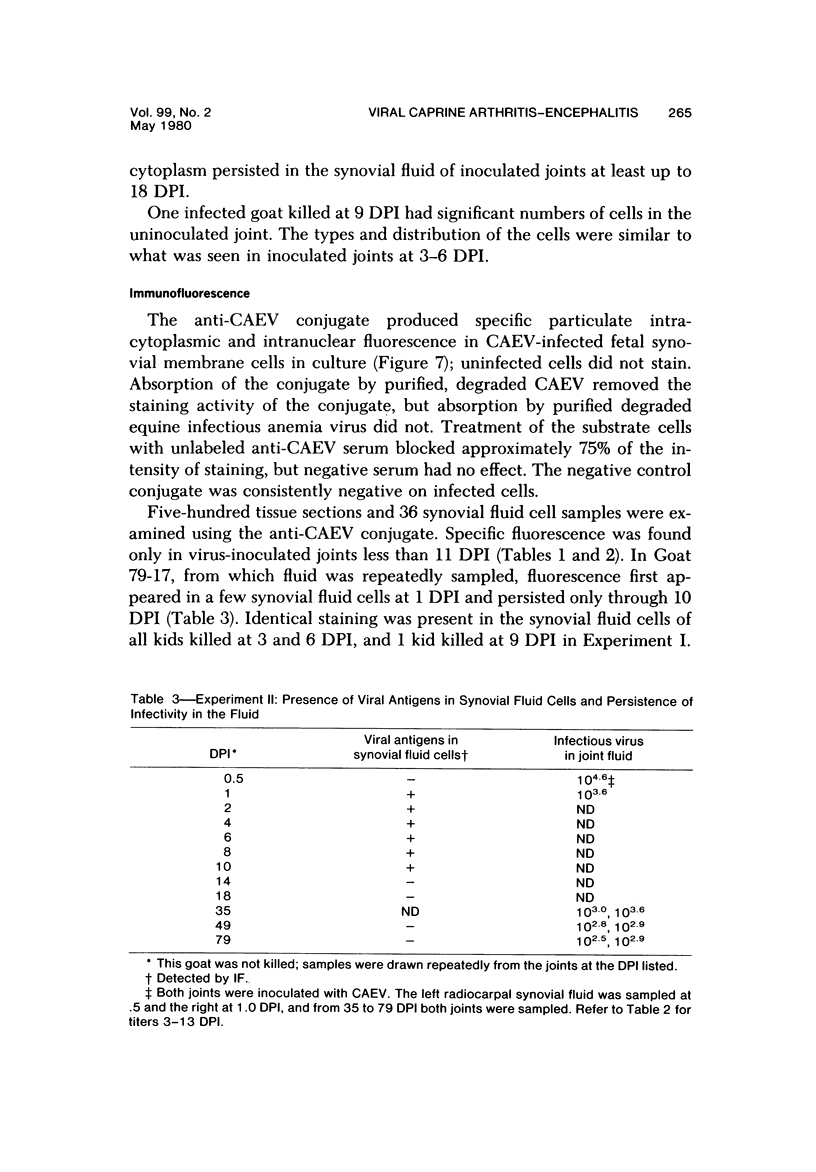







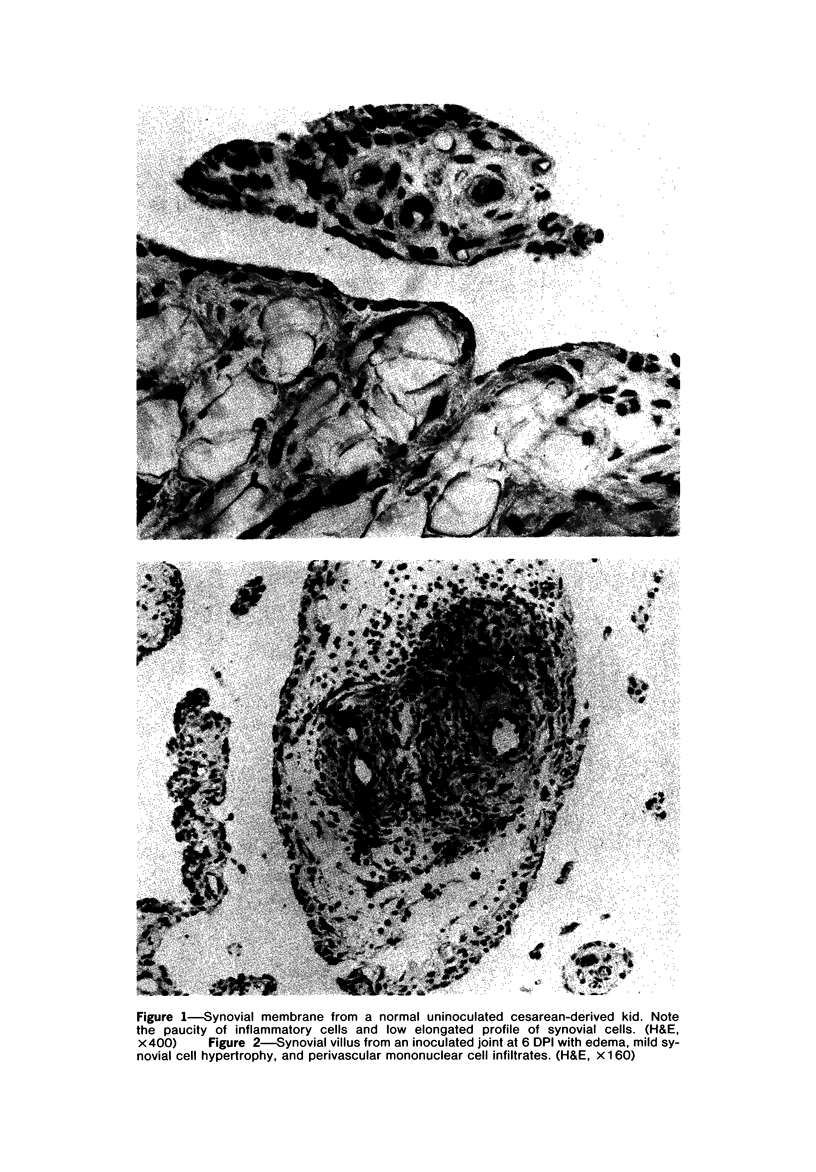
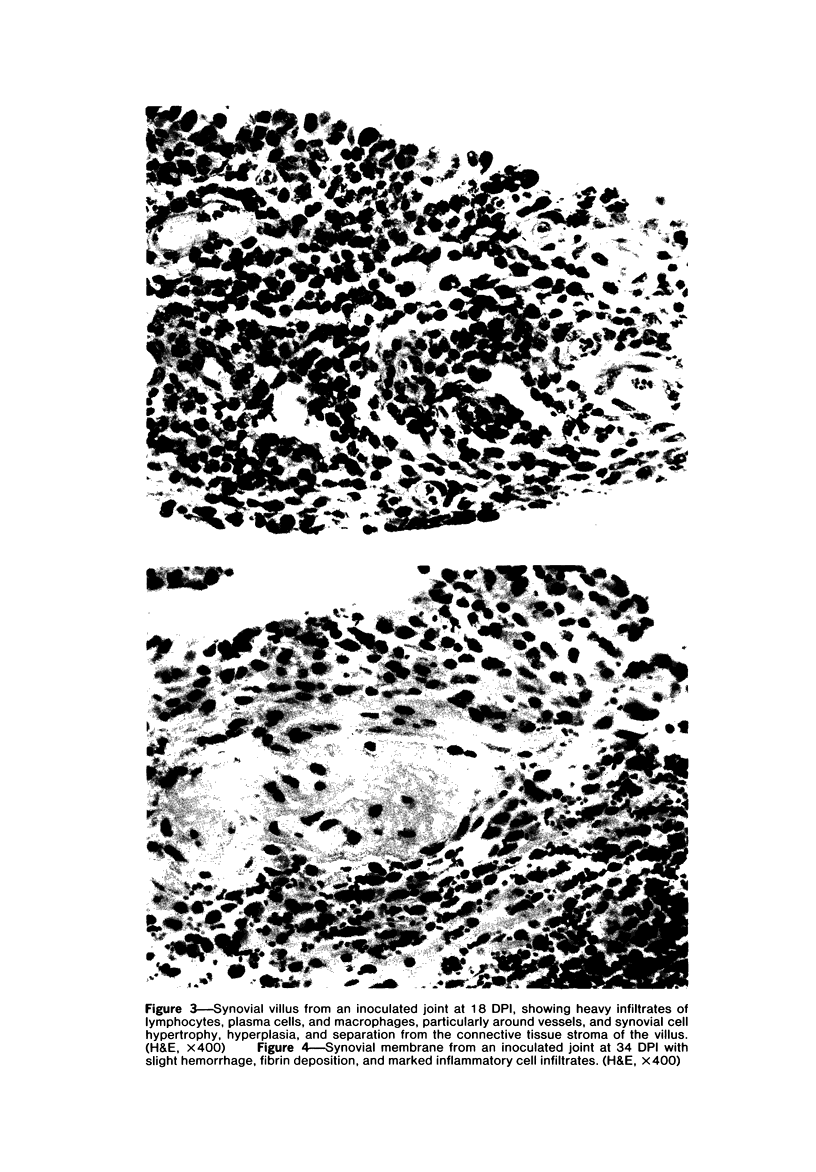
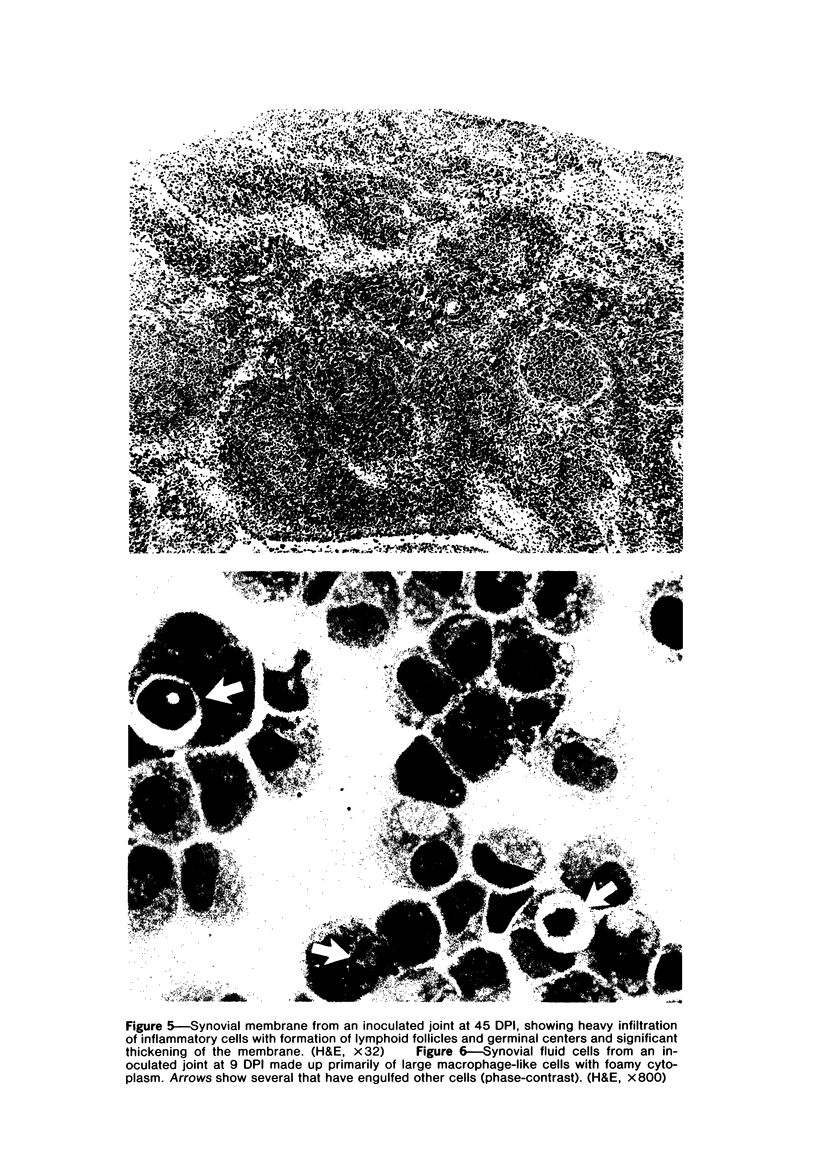
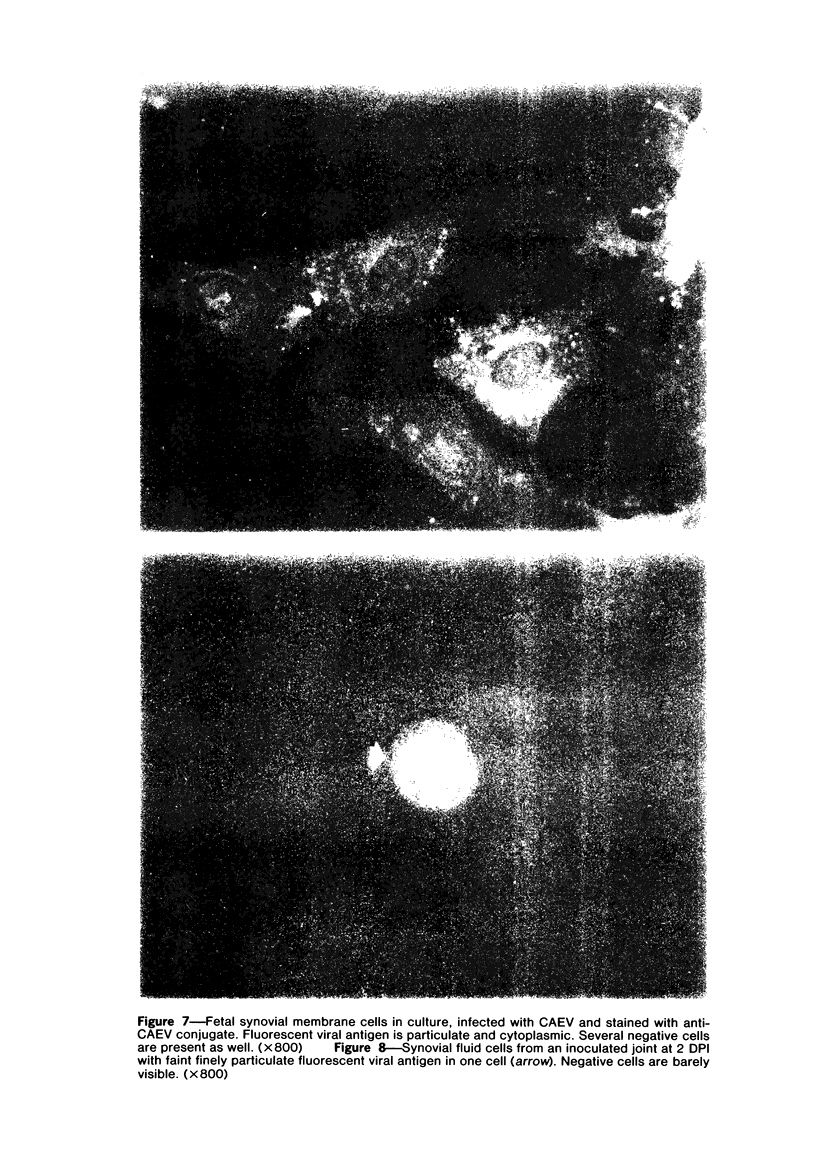
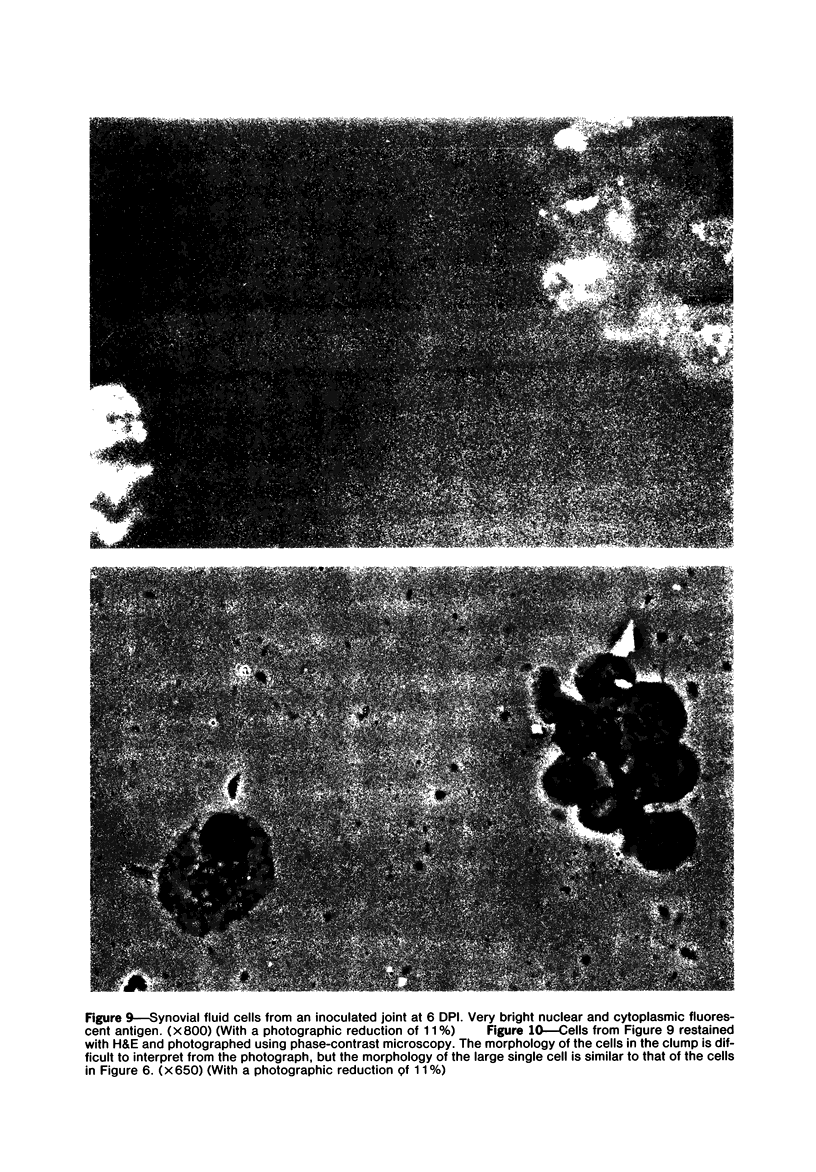
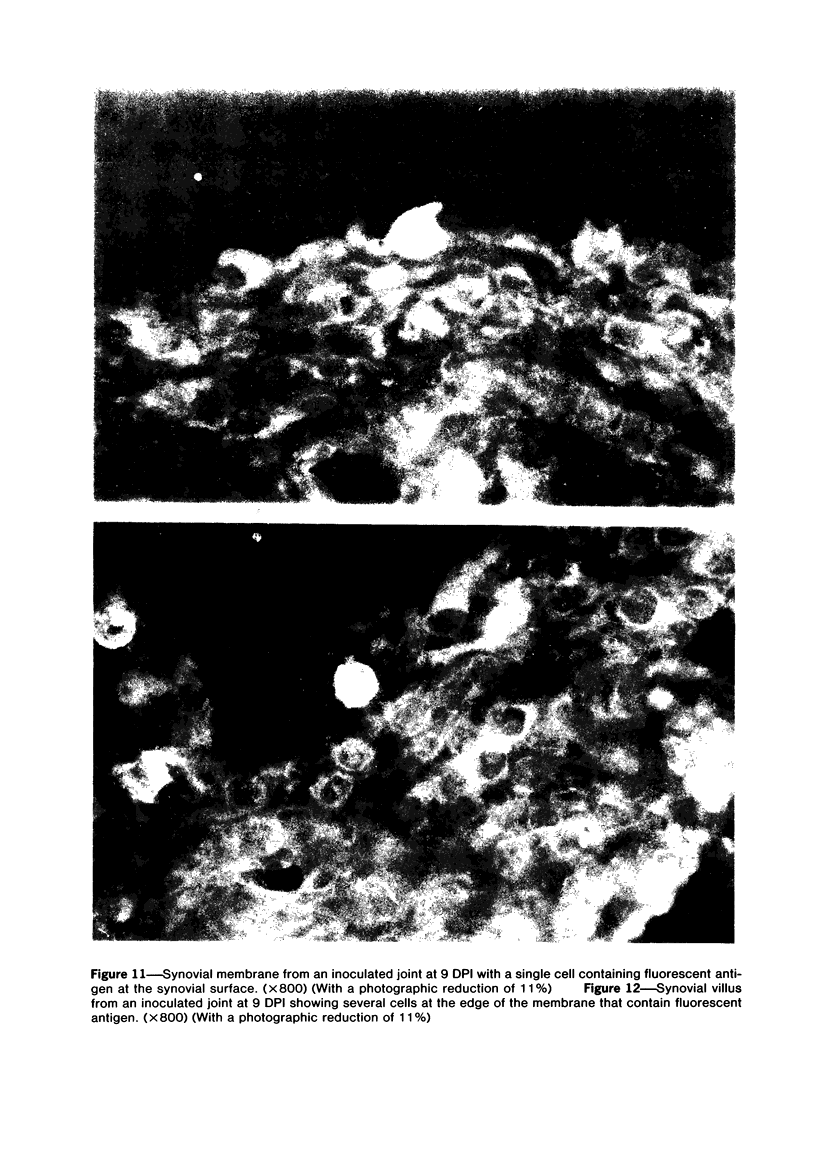
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett E. V., Balduzzi P., Vaughan J. H., Morgan H. R. Search for infectious agents in rheumatoid arthritis. Arthritis Rheum. 1966 Oct;9(5):720–724. doi: 10.1002/art.1780090509. [DOI] [PubMed] [Google Scholar]
- Cheevers W. P., Archer B. G., Crawford T. B. Characterization of RNA from equine infectious anemia virus. J Virol. 1977 Nov;24(2):489–497. doi: 10.1128/jvi.24.2.489-497.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins L., Norcross N. L. Immunodiffusion reaction in equine infectious anemia. Cornell Vet. 1970 Apr;60(2):330–335. [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Crawford T. B., Gorham J. R., Piper R. C. Infectious leukoencephalomyelitis of young goats. J Infect Dis. 1974 Feb;129(2):134–141. doi: 10.1093/infdis/129.2.134. [DOI] [PubMed] [Google Scholar]
- Cork L. C., Hadlow W. J., Gorham J. R., Piper R. C., Crawford T. B. Pathology of viral leukoencephalomyelitis of goats. Acta Neuropathol. 1974;29(4):281–292. doi: 10.1007/BF00685482. [DOI] [PubMed] [Google Scholar]
- Crawford T. B., McGuire T. C., Henson J. B. Detection of equine infectious anemia virus in vitro by immunofluorescence. Arch Gesamte Virusforsch. 1971;34(4):332–339. doi: 10.1007/BF01242979. [DOI] [PubMed] [Google Scholar]
- Essex M., Klein G., Snyder S. P., Harrold J. B. Correlation between humoral antibody and regression of tumours induced by feline sarcoma virus. Nature. 1971 Sep 17;233(5316):195–196. doi: 10.1038/233195a0. [DOI] [PubMed] [Google Scholar]
- Ford D. K., Oh J. O. Use of "synovial" cell cultures in the search for virus in rheumatoid arthritis. Arthritis Rheum. 1965 Dec;8(6):1047–1052. doi: 10.1002/art.1780080604. [DOI] [PubMed] [Google Scholar]
- Georgsson G., Pétursson G., Miller A., Nathanson N., Pálsson P. A. Experimental visna in foetal Icelandic sheep. J Comp Pathol. 1978 Oct;88(4):597–605. doi: 10.1016/0021-9975(78)90013-0. [DOI] [PubMed] [Google Scholar]
- Grayzel A. I. Uridine incorporation into the media and RNA of cultured rheumatoid synovial cells. Arthritis Rheum. 1973 May-Jun;16(3):419–421. doi: 10.1002/art.1780160320. [DOI] [PubMed] [Google Scholar]
- Gudnadóttir M. Visna-maedi in sheep. Prog Med Virol. 1974;18(0):336–349. [PubMed] [Google Scholar]
- HARRIS J., VAUGHAN J. H. Transfusion studies in rheumatoid arthritis. Arthritis Rheum. 1961 Feb;4:47–55. doi: 10.1002/art.1780040105. [DOI] [PubMed] [Google Scholar]
- Haase A. T., Stowring L., Narayan P., Griffin D., Price D. Slow persistent infection caused by visna virus: role of host restriction. Science. 1977 Jan 14;195(4274):175–177. doi: 10.1126/science.188133. [DOI] [PubMed] [Google Scholar]
- Helder A. E., Feltkamp-Vroom T. M., Nienhuis R. L. Electron and light microscopical observations and serological findings in rheumatoid arthritis. Ann Rheum Dis. 1973 Nov;32(6):515–523. doi: 10.1136/ard.32.6.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KULKA J. P., BOCKING D., ROPES M. W., BAUER W. Early joint lesions of rheumatoid arthritis; report of eight cases, with knee biopsies of lesions of less than one year's duration. AMA Arch Pathol. 1955 Feb;59(2):129–150. [PubMed] [Google Scholar]
- Kennel S. J., Feldman J. D. Distribution of viral glycoprotein gp 69/71 on cell surfaces of producer and nonproducer cells. Cancer Res. 1976 Jan;36(1):200–208. [PubMed] [Google Scholar]
- Kono Y., Kobayashi K., Fukunaga Y. Antigenic drift of equine infectious anemia virus in chronically infected horses. Arch Gesamte Virusforsch. 1973;41(1):1–10. doi: 10.1007/BF01249923. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levy J. A. Autoimmunity and neoplasia. The possible role of C-type viruses. Am J Clin Pathol. 1974 Aug;62(2):258–280. doi: 10.1093/ajcp/62.2.258. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Chase J. Antigenic shift of visna virus in persistently infected sheep. Science. 1977 Jul 22;197(4301):376–378. doi: 10.1126/science.195339. [DOI] [PubMed] [Google Scholar]
- Narayan O., Griffin D. E., Silverstein A. M. Slow virus infection: replication and mechanisms of persistence of visna virus in sheep. J Infect Dis. 1977 May;135(5):800–806. doi: 10.1093/infdis/135.5.800. [DOI] [PubMed] [Google Scholar]
- Pétursson G., Nathanson N., Georgsson G., Panitch H., Pálsson P. A. Pathogenesis of visna. I. Sequential virologic, serologic, and pathologic studies. Lab Invest. 1976 Oct;35(4):402–412. [PubMed] [Google Scholar]
- Schumacher H. R., Jr Synovial membrane and fluid morphologic alterations in early rheumatoid arthritis: microvascular injury and virus-like particles. Ann N Y Acad Sci. 1975 Jun 13;256:39–64. doi: 10.1111/j.1749-6632.1975.tb36034.x. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren S. L., Marmor L., Liebes D. M., Hollins R. An active agent from human rheumatoid arthritis which is transmissible in mice. Arch Intern Med. 1969 Nov;124(5):629–634. [PubMed] [Google Scholar]
- Wilkes R. M., Simsarian J. P., Hopps H. E., Roth H., Decker J. L., Aptekar R. G., Meyer H. M., Jr Virologic studies on rheumatoid arthritis. Arthritis Rheum. 1973 Jul-Aug;16(4):446–454. doi: 10.1002/art.1780160403. [DOI] [PubMed] [Google Scholar]