Abstract
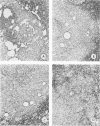
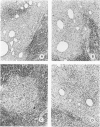
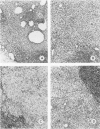
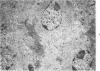
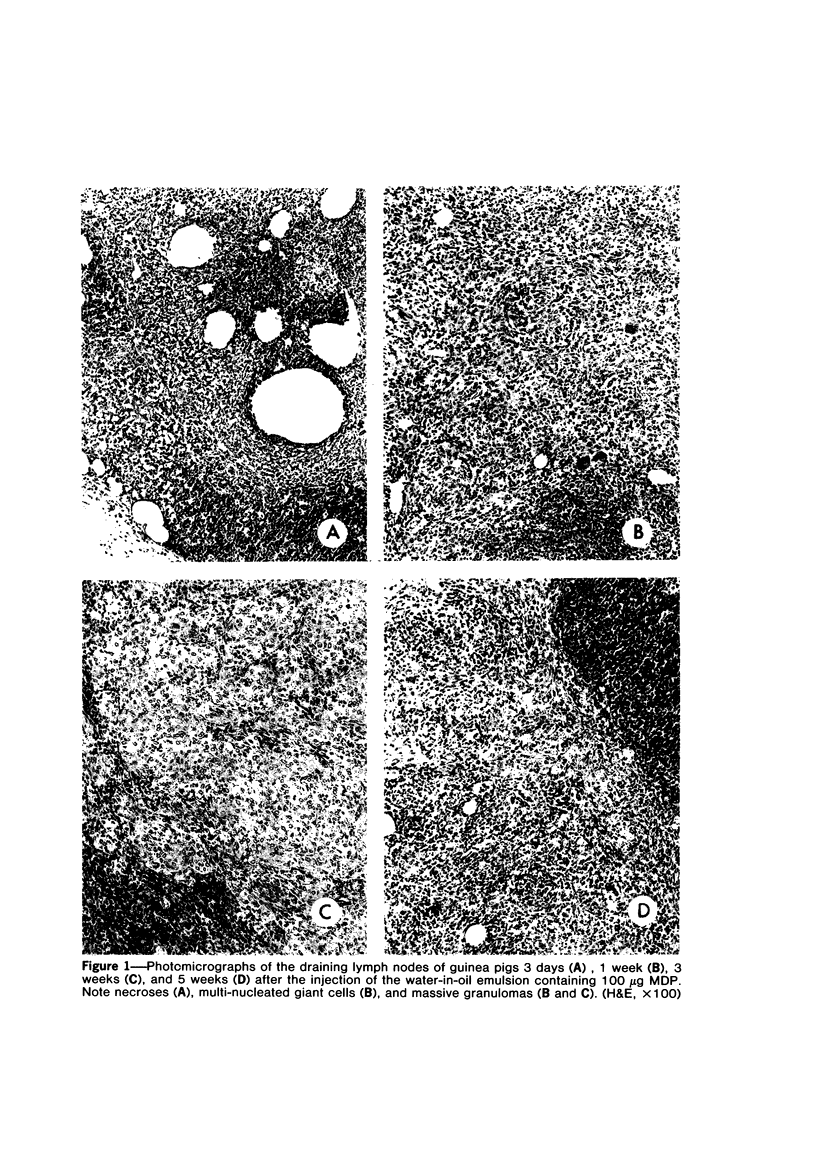
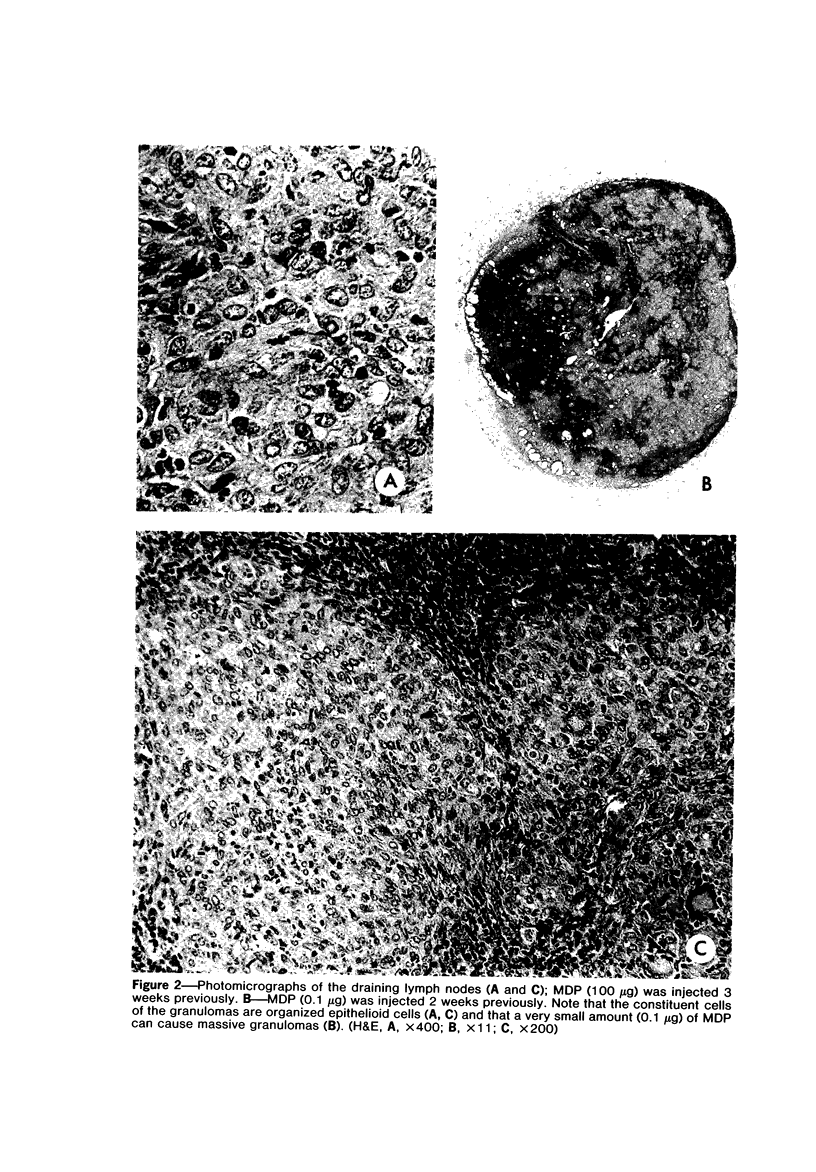
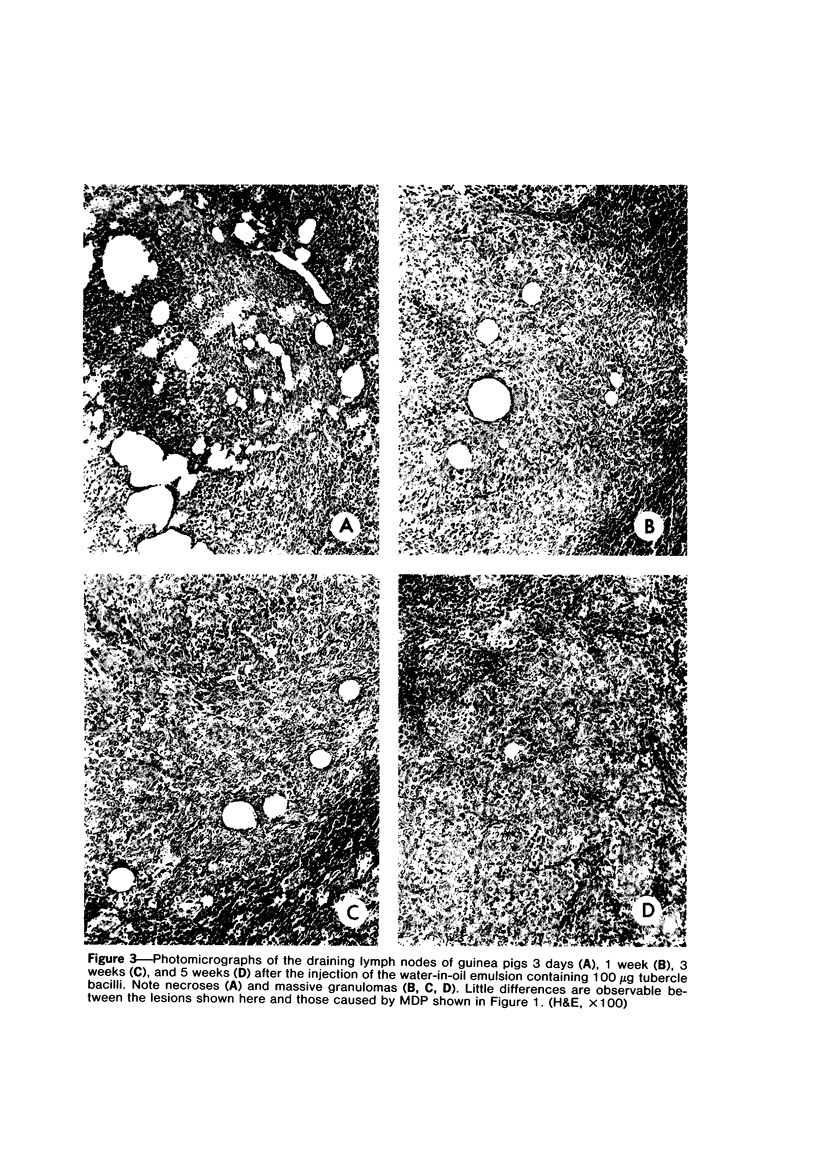
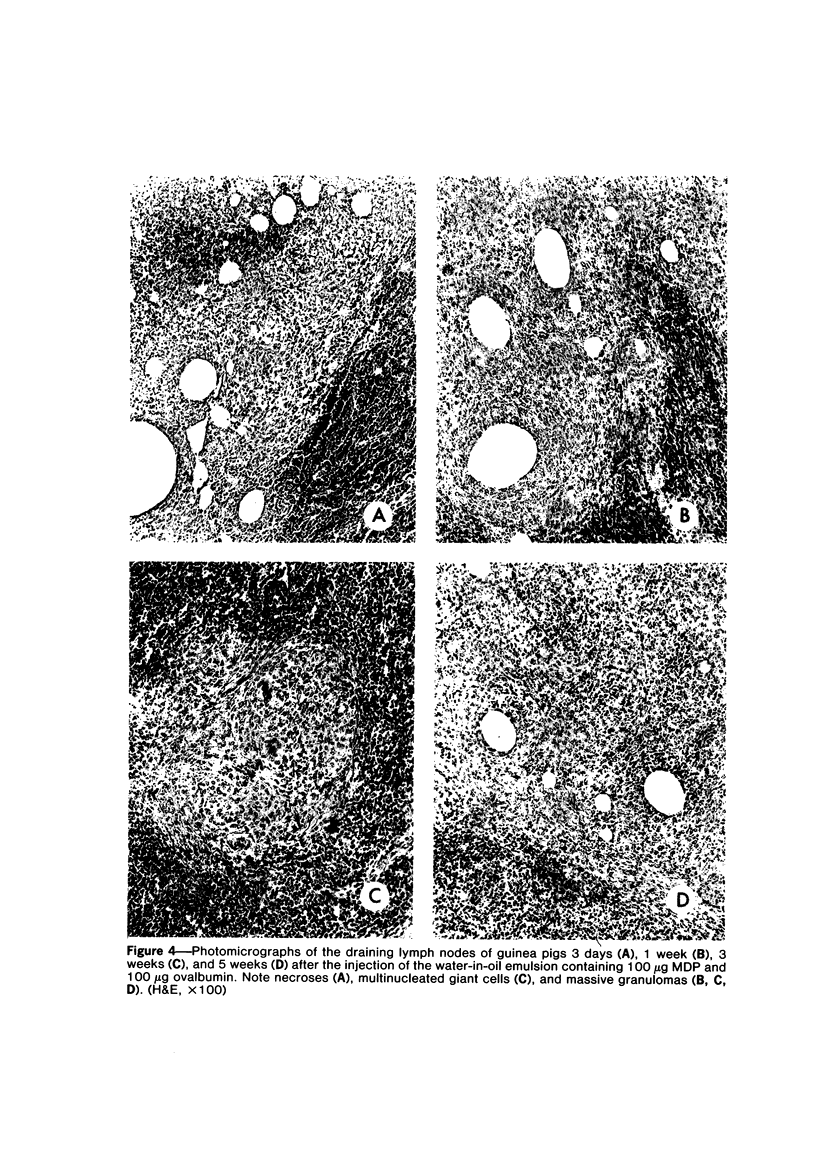
A synthetic muramyl dipeptide (MDP, N-acetylmuramyl-L-alanyl-D-isoglutamine) is a minimal essential structure that is contained generally in bacterial cell walls and is responsible for their many biologic activities such as adjuvant activity, pyrogenicity, and a capacity to confer resistance against bacterial and viral infections. We found that this MDP evoked dose-dependently massive organized epithelioid granulomas in guinea pigs, when injected in the form of Freund-type water-in-oil emulsion. Granuloma formation reached a peak at 3 weeks. A minimal effective dose of MDP was 0.1 microgram. Essentially, no difference was observed qualitatively among granulomas evoked by MDP, MDP plus antigen, and killed tubercle bacilli incorporated in the emulsion. Quantitatively, however, MDP was stronger in its granulomagenic capacity than tubercle bacilli. Antigenicity of MDP was not detectable. These findings support our proposal that MDP may be a chemical structure in tubercle bacilli essential for epithelioid granuloma formation and that the MDP-induced epithelioid granuloma may be of a nonallergic nature.
Full text
PDF






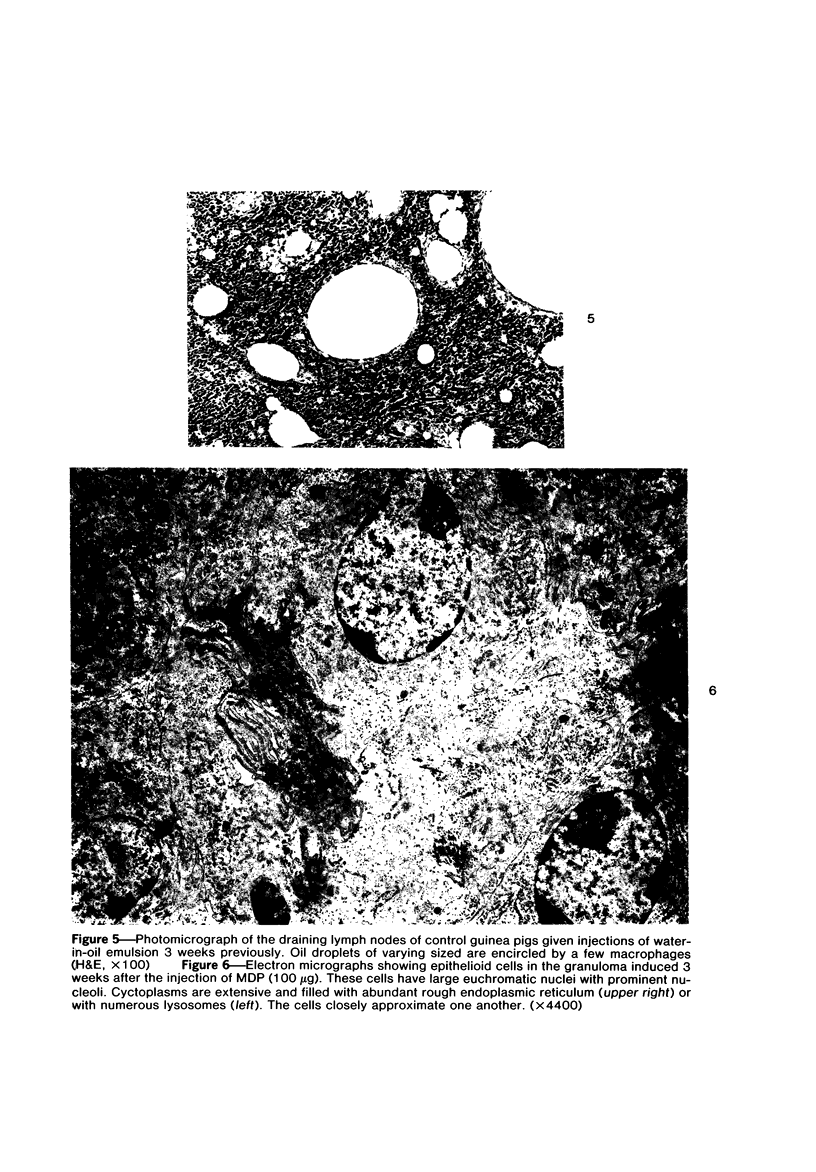
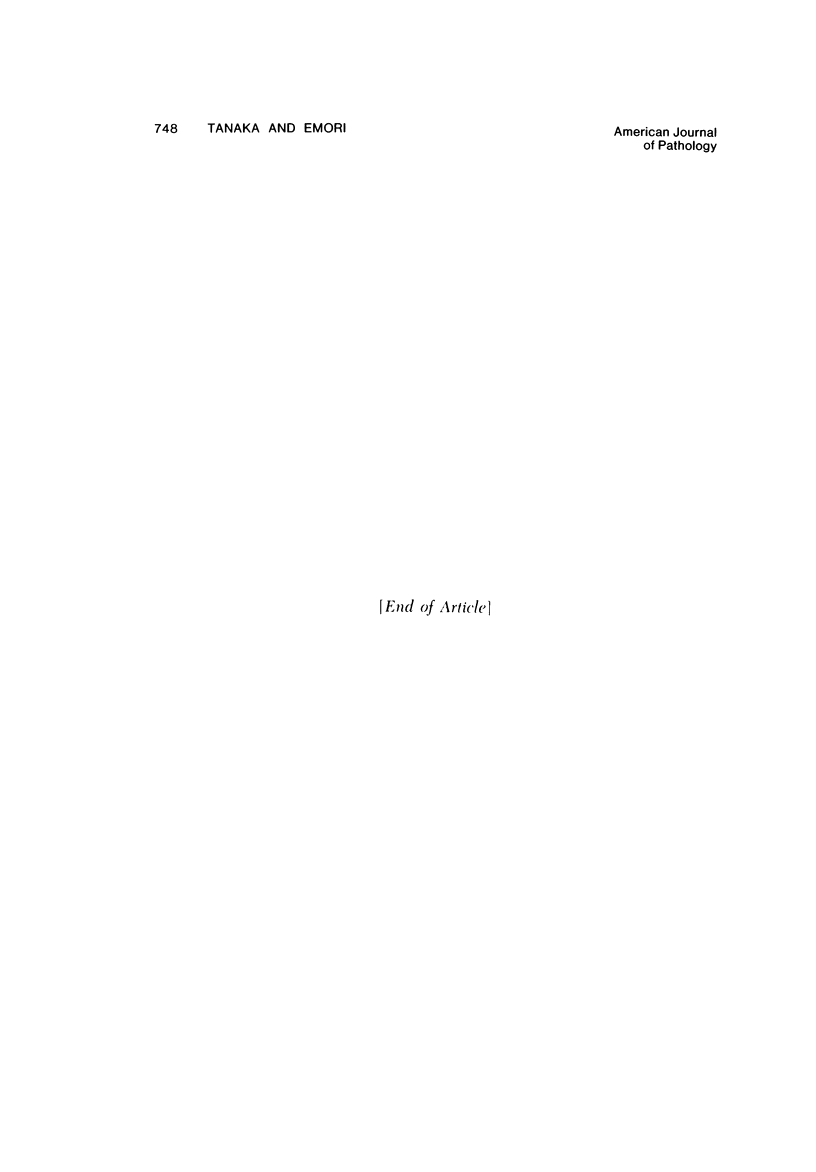
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. I. Sequential fine and histologic studies of the effect of Bacillus Calmette-Guerin (BCG). Am J Pathol. 1974 Jul;76(1):17–48. [PMC free article] [PubMed] [Google Scholar]
- Adams D. O. The structure of mononuclear phagocytes differentiating in vivo. II. The effect of Mycobacterium tuberculosis. Am J Pathol. 1975 Jul;80(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Audibert F., Chedid L., Hannoun C. Augmentation de la réponse immunitaire au vaccin grippal par un glycopeptide synthétique adjuvant (N-acétyl muramyl-L-alanyl-D-isoglutamine). C R Acad Sci Hebd Seances Acad Sci D. 1977 Sep 12;285(4):467–470. [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-L-alanyl-D-isoglutamine) and several analogs. Proc Natl Acad Sci U S A. 1977 May;74(5):2089–2093. doi: 10.1073/pnas.74.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAUNAY A., ASSELINEAU J., LEDERER E. Réactions histologiques provoquées chez le cobaye par l'injection de lipopolysaccharides extraits de bacilles de Koch. C R Seances Soc Biol Fil. 1951 May;145(9-10):650–652. [PubMed] [Google Scholar]
- Dinarello C. A., Elin R. J., Chedid L., Wolff S. M. The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. J Infect Dis. 1978 Dec;138(6):760–767. doi: 10.1093/infdis/138.6.760. [DOI] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Emori K., Tanaka A. Granuloma formation by synthetic bacterial cell wall fragment: muramyl dipeptide. Infect Immun. 1978 Feb;19(2):613–620. doi: 10.1128/iai.19.2.613-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W. L. Cutaneous granulomas. Int J Dermatol. 1977 Sep;16(7):574–579. doi: 10.1111/j.1365-4362.1977.tb00782.x. [DOI] [PubMed] [Google Scholar]
- Epstein W. L. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- Fevrier M., Birrien J. L., Leclerc C., Chedid L., Liacopoulos P. The macrophage, target cell of the synthetic adjuvant muramyl dipeptide. Eur J Immunol. 1978 Aug;8(8):558–562. doi: 10.1002/eji.1830080804. [DOI] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Harada K., Shiba T. Correlation between the immunoadjuvant activities and pyrogenicities of synthetic N-acetylmuramyl-peptides or -amino acids. Biken J. 1976 Mar;19(1):9–13. [PubMed] [Google Scholar]
- Nagao S., Tanaka A., Yamamoto Y., Koga T., Onoue K., Shiba T., Kusumoto K., Kotani S. Inhibition of macrophage migration by muramyl peptides. Infect Immun. 1979 May;24(2):308–312. doi: 10.1128/iai.24.2.308-312.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Nagao R., Kotani S., Shiba T., Kusumoto S. Stimulation of the reticuloendothelial system of mice by muramyl dipeptide. Infect Immun. 1979 May;24(2):302–307. doi: 10.1128/iai.24.2.302-307.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Nagao S., Saito R., Kotani S., Kusumoto S., Shiba T. Correlation of stereochemically specific structure in muramyl dipeptide between macrophage activation and adjuvant activity. Biochem Biophys Res Commun. 1977 Jul 25;77(2):621–627. doi: 10.1016/s0006-291x(77)80024-7. [DOI] [PubMed] [Google Scholar]
- WHITE R. G., COONS A. H., CONNOLLY J. M. Studies on antibody production. IV. The role of a wax fraction of Mycobacterium tuberculosis in adjuvant emulsions on the production of antibody to egg albumin. J Exp Med. 1955 Jul 1;102(1):83–104. doi: 10.1084/jem.102.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Yamamoto Y., Nagao S., Tanaka A., Koga T., Onoue K. Inhibition of macrophage migration by synthetic muramyl dipeptide. Biochem Biophys Res Commun. 1978 Feb 28;80(4):923–928. doi: 10.1016/0006-291x(78)91333-5. [DOI] [PubMed] [Google Scholar]