Abstract
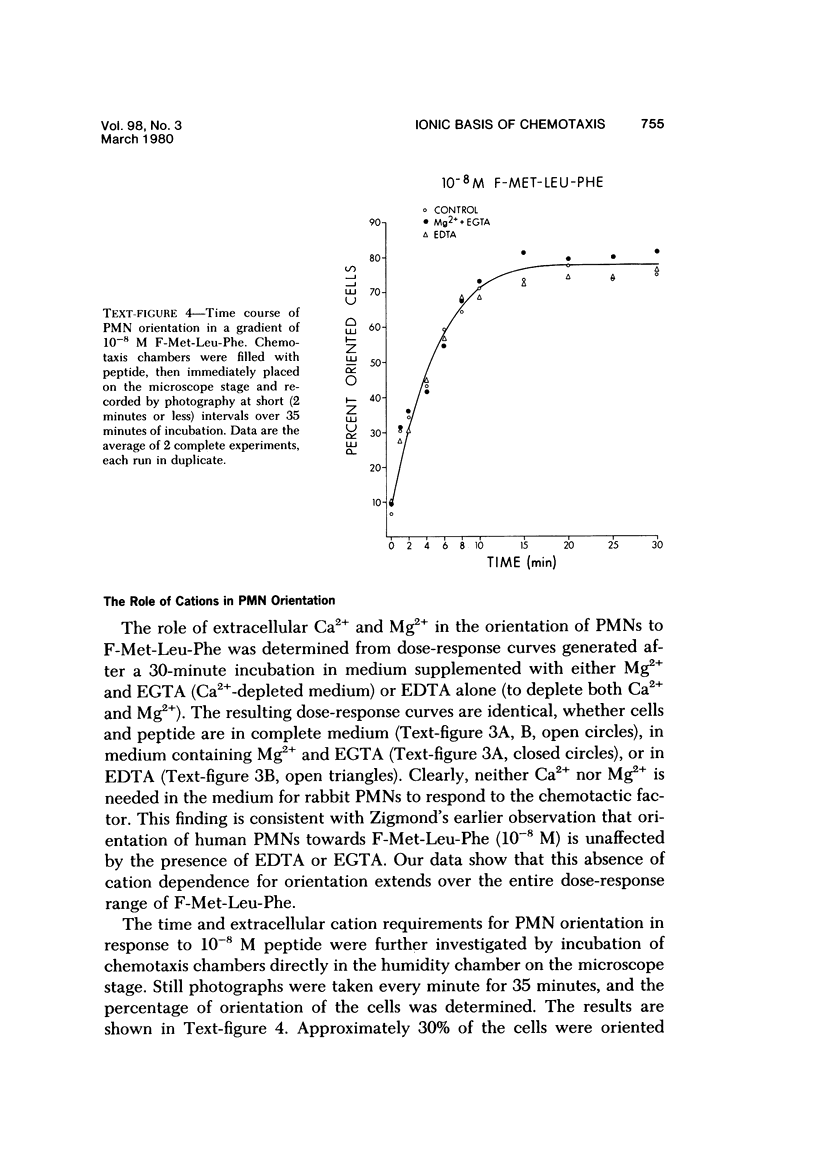
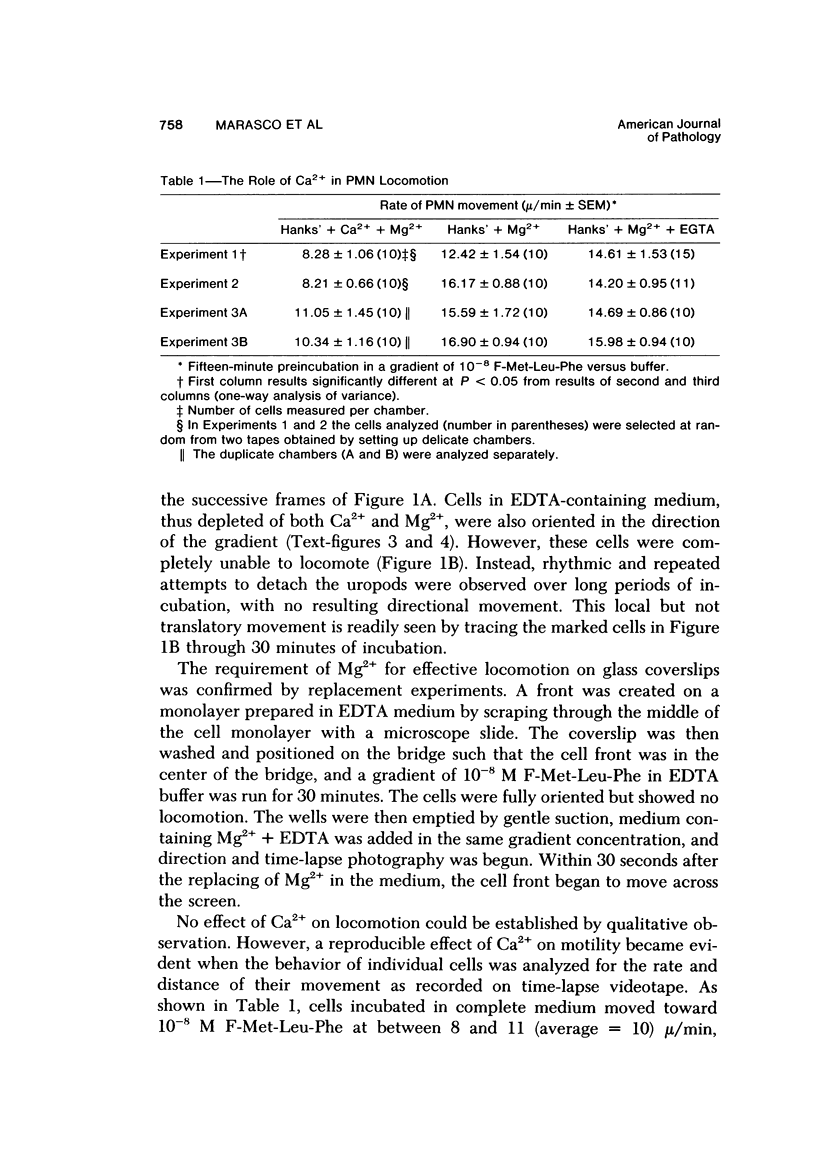
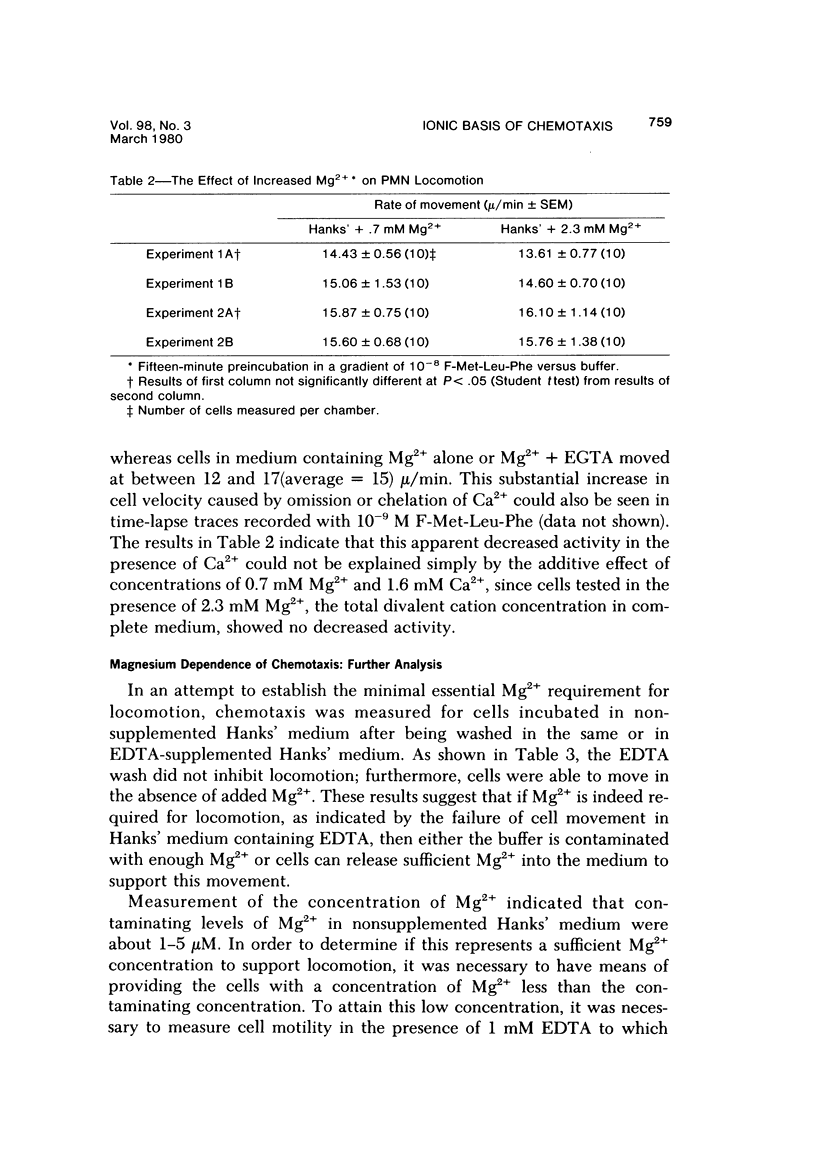
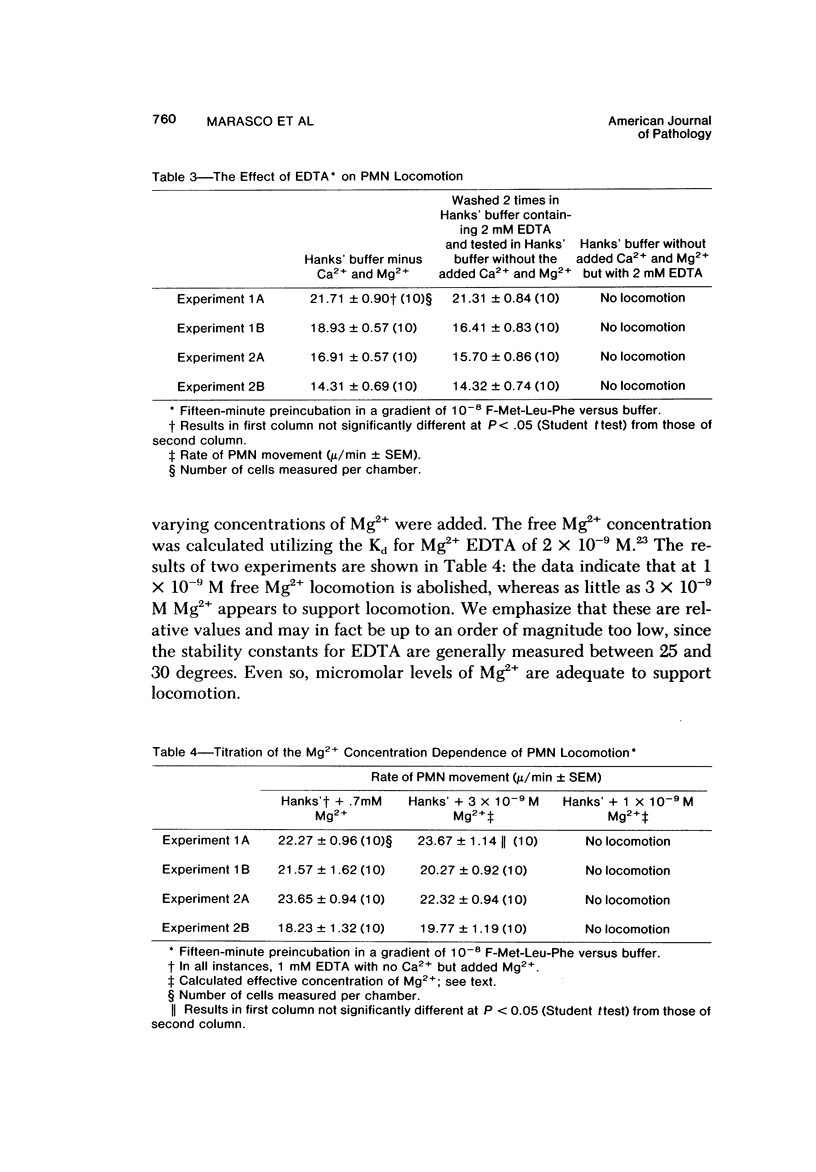
The behavior of cells undergoing chemotaxis may be analyzed in terms of their orientation, a static characteristic, and of their locomotion. We have examined the extracellular divalent cation requirements for orientation and locomotion of rabbit polymorphonuclear leukocytes (neutrophils) in a gradient of the chemotactic peptide N-formyl-methionyl-leu-cyl-phenylalanine (F-Met-Leu-Phe) using the chemotaxis chamber recently developed by Zigmond. This chamber allows direct observation of cells attached to glass coverslips as they move up a gradient of chemotactic agent established across a 1-mm bridge. The orientation of neutrophils in the direction of the gradient was equally efficient whether cells and F-Met-Leu-Phe were suspended in merium supplemented with both Ca2+ and Mg2+ (complete medium), with Mg2+ but not Ca2+ (by simple omission of Ca2+ or by addition of EGTA), or with nonsupplemented medium (by omission of Ca2+ and Mg2+ or by addition of EDTA). These data confirm and extend Zigmond's earlier observation that exogenous divalent cations are not required for polymorphonuclear leukocyte orientation toward the chemotactic peptide. In contrast, cell locomotion, determined by linking the chemotaxis chamber to a time-lapse videocassette recorder and TV monitor, is markedly affected by the medium's content of divalent cations. Cells suspended in medium supplemented with Mg2+ but not calcium (by omission or chelation) or in nonsupplemented medium moved on the average 25% more rapidly than cells in complete Ca2+ and Mg2+ medium. Although the simple omission of Mg2+ does not prevent chemotaxis, chelation of Mg2+ in the medium completely abolishes leukocyte locomotion. Addition of varying concentrations of Mg2+ to the buffer in the presence of EDTA established that cell movement is fully restored by Mg2+ concentrations in the range of 3 X 10(-9) M, concentrations easily attained in the absence of added Mg2+. It was concluded that neither Ca2+ nor Mg2+ is needed for orientation in response to F-Met-Leu-Phe. However, low levels of exogenous Mg2+ but not Ca2+ are required for effective locomotion of neutrophils in the Zigmond changer. This result contrasts with data obtained in the Boyden chamber, where exogenous Ca2+ is considered essential for maximum chemotactic response.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Showell H. J. The effect of Ca2+ and Mg2+ on the chemotactic responsiveness and spontaneous motility of rabbit polymorphonuclear leukocytes. Z Immunitatsforsch Exp Klin Immunol. 1972 Jun;143(5):466–476. [PubMed] [Google Scholar]
- Becker E. L. Some interrelations of neutrophil chemotaxis, lysosomal enzyme secretion, and phagocytosis as revealed by synthetic peptides. Am J Pathol. 1976 Nov;85(2):385–394. [PMC free article] [PubMed] [Google Scholar]
- Becker E. L. Stimulated neutrophil locomotion: chemokinesis and chemotaxis. Arch Pathol Lab Med. 1977 Oct;101(10):509–513. [PubMed] [Google Scholar]
- Becker E. L. The relationship of the chemotactic behavior of the complement-derived factors, C3a, C5a, and C567, and a bacterial chemotactic factor to their ability to activate the proesterase 1 of rabbit polymorphonuclear leukocytes. J Exp Med. 1972 Feb 1;135(2):376–387. doi: 10.1084/jem.135.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucek M. M., Snyderman R. Calcium influx requirement for human neutrophil chemotaxis: inhibition by lanthanum chloride. Science. 1976 Sep 3;193(4256):905–907. doi: 10.1126/science.948752. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., DesPrez R. M., VanWay M. H., Rogers D. E. Studies on human leukocyte motility. I. Effects of alterations in pH, electrolyte concentration, and phagocytosis on leukocyte migration, adhesiveness, and aggregation. J Exp Med. 1966 Sep 1;124(3):483–499. doi: 10.1084/jem.124.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Reusch M. E., Epstein M. L., Hill H. R. Role of Ca2+ and Mg2+ in some human neutrophil functions as indicated by ionophore A23187. Infect Immun. 1976 Jan;13(1):146–151. doi: 10.1128/iai.13.1.146-151.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvin J. E. Effects of divalent cations on adhesiveness of rat polymorphonuclear neutrophils in vitro. J Cell Physiol. 1968 Dec;72(3):197–212. doi: 10.1002/jcp.1040720307. [DOI] [PubMed] [Google Scholar]
- Hawkins R. A., Berlin R. D. Purine transport in polymorphonuclear leukocytes. Biochim Biophys Acta. 1969 Mar 11;173(2):324–337. doi: 10.1016/0005-2736(69)90115-1. [DOI] [PubMed] [Google Scholar]
- Korchak H. M., Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Transport of sodium, potassium, and calcium across rabbit polymorphonuclear leukocyte membranes. Effect of chemotactic factor. J Cell Biol. 1977 May;73(2):428–444. doi: 10.1083/jcb.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Volpi M., Showell H. J., Becker E. L., Sha'afi R. I. Chemotactic factor-induced release of membrane calcium in rabbit neutrophils. Science. 1979 Feb 2;203(4379):461–463. doi: 10.1126/science.760200. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Krawiec J. A., Becker E. L. The distribution of actin during chemotaxis in rabbit neutrophils. J Reticuloendothel Soc. 1978 Dec;24(6):697–704. [PubMed] [Google Scholar]
- Petroski R. J., Naccache P. H., Becker E. L., Sha'afi R. I. Effect of the chemotactic factor formyl methionyl- leucyl-phenylalanine and cytochalasin B on the cellular levels of calcium in rabbit neutrophils. FEBS Lett. 1979 Apr 1;100(1):161–165. doi: 10.1016/0014-5793(79)81155-2. [DOI] [PubMed] [Google Scholar]
- Ramsey W. S. Analysis of individual leucocyte behavior during chemotaxis.. Exp Cell Res. 1972 Jan;70(1):129–139. doi: 10.1016/0014-4827(72)90190-5. [DOI] [PubMed] [Google Scholar]
- Ramsey W. S. Locomotion of human polymorphonuclear leucocytes. Exp Cell Res. 1972 Jun;72(2):489–501. doi: 10.1016/0014-4827(72)90019-5. [DOI] [PubMed] [Google Scholar]
- Wilkinson P. C. Leucocyte locomotion and chemotaxis. The influence of divalent cations and cation ionophores. Exp Cell Res. 1975 Jul;93(2):420–426. doi: 10.1016/0014-4827(75)90468-1. [DOI] [PubMed] [Google Scholar]
- Wolf H. U. Studies on a Ca 2+ -dependent ATPase of human erythrocyte membranes. Effects of Ca 2+ and H + . Biochim Biophys Acta. 1972 May 9;266(2):361–375. doi: 10.1016/0005-2736(72)90094-6. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H. Ability of polymorphonuclear leukocytes to orient in gradients of chemotactic factors. J Cell Biol. 1977 Nov;75(2 Pt 1):606–616. doi: 10.1083/jcb.75.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]