Abstract
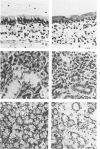
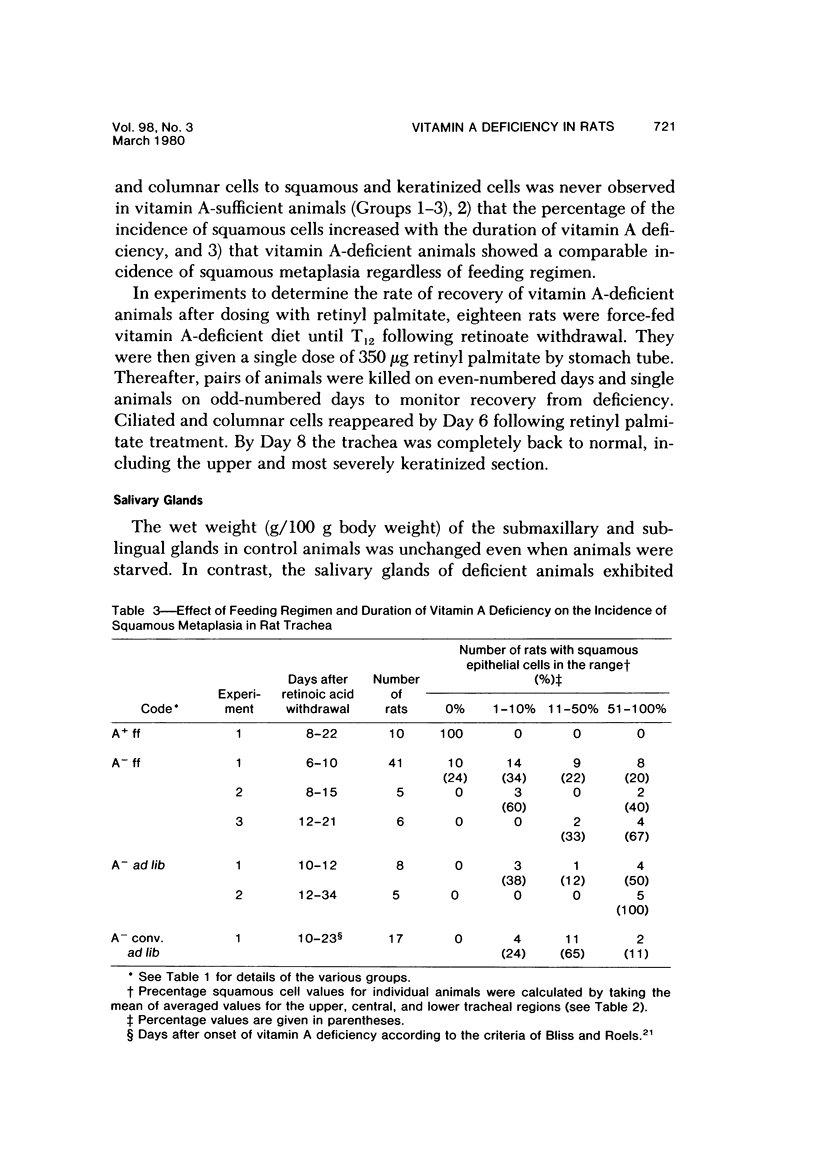
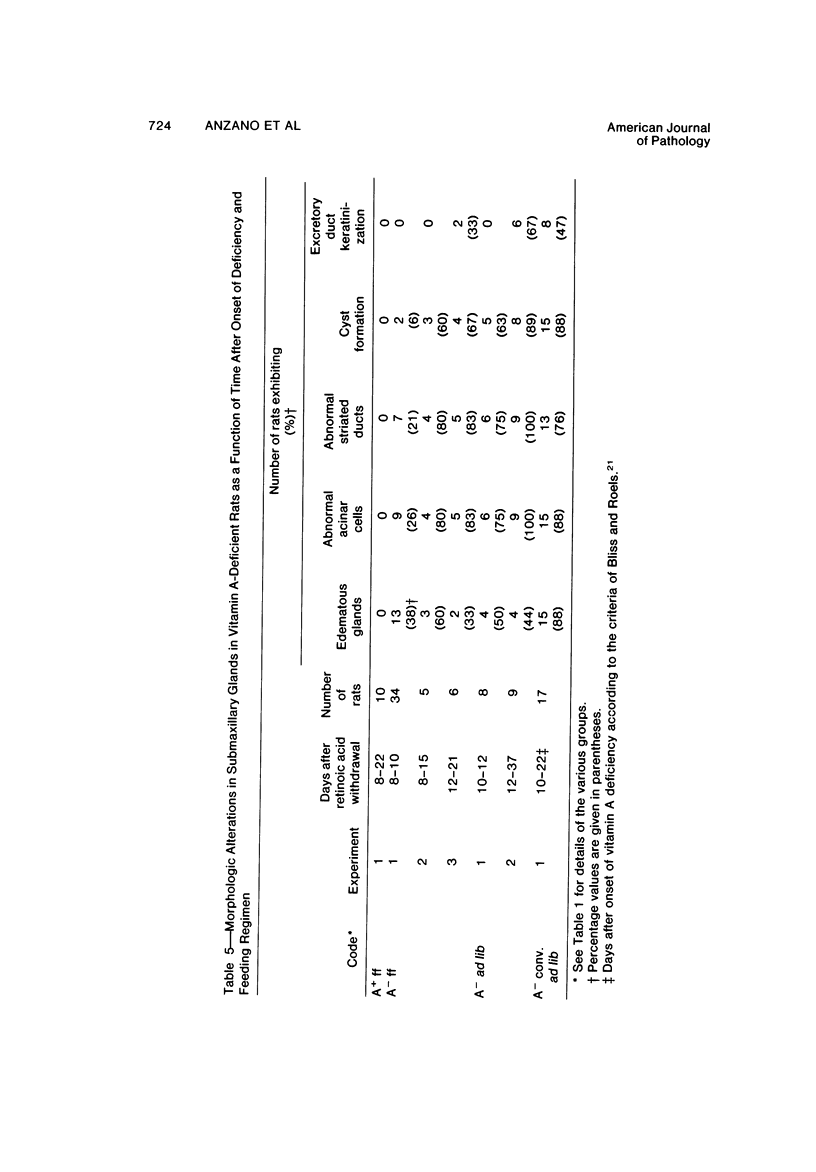
The use of the synchronous induction method enables both assessment of the sequence and reliability of the appearance of morphologic signs of vitamin A deficiency, and their accurate correlation with biochemical and physiologic abnormalities. In the trachea, hyperplasia of basal epithelial cells was observed by Day 4 (T4) following the withdrawal of retinoic acid from retinoate-cycled, stringently deficient rats. Keratinization was observed by Day 6, the upper part of the trachea showing the highest incidence of keratinization. All such metaplastic changes originated in the narrow strip of tissue directly cojoining the esophagus. In the submaxillary glands, atrophy of the acini, an increase in interlobular spaces, and fibrosis and dilatation of the ducts was observed by Day 10. In more advanced stages of deficiency (T14-T18), cyst formation associated with suppuration and extensive cell atrophy was observed. Morphologic changes were less marked in the sublingual glands, although mucin levels were noticeably depressed by Day 12 of deficiency. Following the oral dosing of deficient animals (T12) with 350 micrograms retinyl palmitate, all such changes were reversed within 6 days in the trachea and within 10 days in the submaxillary and sublingual glands. Similar patterns were observed whether animals were force-fed or were fed ad libitum. Apart, therefore, from cause-effect considerations per se, morphologic changes are also potentially valuable reference indicators of deficiency, particularly in time course studies, or where force-feeding attenuates other signs of deficiency.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemiya T. Vitamin A and the retina. Eye Ear Nose Throat Mon. 1971 Sep;50(9):341–346. [PubMed] [Google Scholar]
- Anzano M. A., Lamb A. J., Olson J. A. Growth, appetite, sequence of pathological signs and survival following the induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1979 Aug;109(8):1419–1431. doi: 10.1093/jn/109.8.1419. [DOI] [PubMed] [Google Scholar]
- BEAVER D. L. Vitamin A deficiency in the germ-free rat. Am J Pathol. 1961 Mar;38:335–357. [PMC free article] [PubMed] [Google Scholar]
- Berkman M. D., Kronman J. H. A histochemical study of the effects of castration and testosterone administration on the major salivary glands of Swiss mice. Acta Anat (Basel) 1970;76(2):200–219. doi: 10.1159/000143492. [DOI] [PubMed] [Google Scholar]
- Bonanní F., Levinson S. S., Wolf G., De Luca L. Glycoproteins from the hamster respiratory tract and their response to vitamin A. Biochim Biophys Acta. 1973 Feb 28;297(2):441–451. doi: 10.1016/0304-4165(73)90091-3. [DOI] [PubMed] [Google Scholar]
- CAPURRO P., ANGRIST A., BLACK J., MOUMGIS B. Studies in squamous metaplasia in rat bladder. I. Effects of hypovitaminosis A, foreign bodies, and methylcholanthrene. Cancer Res. 1960 May;20:563–567. [PubMed] [Google Scholar]
- Crocker T. T., Sanders L. L. Influence of vitamin A and 3,7-dimethyl-2,6-octadienal (citral) on the effect of benzo(a)pyrene on hamster trachea in organ culture. Cancer Res. 1970 May;30(5):1312–1318. [PubMed] [Google Scholar]
- De Luca L., Little E. P., Wolf G. Vitamin A and protein synthesis by rat intestinal mucosa. J Biol Chem. 1969 Feb 25;244(4):701–708. [PubMed] [Google Scholar]
- De Luca L., Wolf G. Mechanism of action of vitamin A in differentiation of mucus-secreting epithelia. J Agric Food Chem. 1972 May-Jun;20(3):474–476. doi: 10.1021/jf60181a034. [DOI] [PubMed] [Google Scholar]
- FELL H. B., MELLANBY E. Metaplasia produced in cultures of chick ectoderm by high vitamin A. J Physiol. 1953 Mar;119(4):470–488. doi: 10.1113/jphysiol.1953.sp004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grangaud R., Nicol M., Desplanques D. Effect of vitamin A on enzymic conversion of the delta-5-3-beta-hydroxy- into delta-4-3-oxosteroids by adrenals of the rat. Am J Clin Nutr. 1969 Aug;22(8):991–1002. doi: 10.1093/ajcn/22.8.991. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Sporn M. B., Kaufman D. G., Smith J. M., Jackson F. E., Saffiotti U. Histogenesis of squamous metaplasia in the hamster tracheal epithelium caused by vitamin A deficiency or benzo[a]pyrene-Ferric oxide. J Natl Cancer Inst. 1972 Mar;48(3):743–761. [PubMed] [Google Scholar]
- Hayes K. C. Vitamin A, differentiation and reproduction. Comments. Am J Clin Nutr. 1969 Aug;22(8):1081–1084. doi: 10.1093/ajcn/22.8.1081. [DOI] [PubMed] [Google Scholar]
- JACOBY F., LEESON C. R. The postnatal development of the rat submaxillary gland. J Anat. 1959 Apr;93(2):201–216. [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE D. J., BERN H. A. Mucous metaplasia and mucous gland formation in keratinized adult epithelium in situ treated with vitamin A. Exp Cell Res. 1960 Nov;21:443–446. doi: 10.1016/0014-4827(60)90277-9. [DOI] [PubMed] [Google Scholar]
- Lamb A. J., Apiwatanaporn P., Olson J. A. Induction of rapid, synchronous vitamin A deficiency in the rat. J Nutr. 1974 Sep;104(9):1140–1148. doi: 10.1093/jn/104.9.1140. [DOI] [PubMed] [Google Scholar]
- Olson J. A. The biological role of vitamin A in maintaining epithelial tissues. Isr J Med Sci. 1972 Aug-Sep;8(8):1170–1178. [PubMed] [Google Scholar]
- Oomen H. A. Clinical epidemiology of xerophthalamia in man. Am J Clin Nutr. 1969 Aug;22(8):1098–1105. doi: 10.1093/ajcn/22.8.1098. [DOI] [PubMed] [Google Scholar]
- Orfanos C. E., Pullmann H., Runne U., Kurka M., Strunk V., Künzig M., Dierlich E. Behandlung der Psoriasis mit Vitamin A, Vitamin-A-Säure und oralen Retinoiden. Hautarzt. 1979 Mar;30(3):124–133. [PubMed] [Google Scholar]
- QUINTARELLI G., CHAUNCEY H. H. Metachromatic reactivity of mammalian submaxillary glands. Arch Oral Biol. 1960 Jul;2:162–166. doi: 10.1016/0003-9969(60)90065-0. [DOI] [PubMed] [Google Scholar]
- ROE D. A., WESTON M. O. POTENTIAL SIGNIFICANCE OF FREE TAURINE IN THE DIET. Nature. 1965 Jan 16;205:287–288. doi: 10.1038/205287a0. [DOI] [PubMed] [Google Scholar]
- Rogers W. E., Jr, Bieri J. G., McDaniel E. G. Vitamin A deficiency in the germfree state. Fed Proc. 1971 Nov-Dec;30(6):1773–1778. [PubMed] [Google Scholar]
- Rojanapo W., Lamb A. J., Olson J. A. The prevalence, metabolism and migration of goblet cells in rat intestine following the induction of rapid, synchronous vitamin A deficiency. J Nutr. 1980 Jan;110(1):178–188. doi: 10.1093/jn/110.1.178. [DOI] [PubMed] [Google Scholar]
- SALLEY J. J., BRYSON W. F. Vitamin A deficiency in the hamster. J Dent Res. 1957 Dec;36(6):935–944. doi: 10.1177/00220345570360062001. [DOI] [PubMed] [Google Scholar]
- SHERMAN B. S. The effect of vitamin A on epithelial mitosis in vitro and in vivo. J Invest Dermatol. 1961 Dec;37:469–480. doi: 10.1038/jid.1961.146. [DOI] [PubMed] [Google Scholar]
- SPICER S. S., DUVENCI J. HISTOCHEMICAL CHARACTERISTICS OF MUCOPOLYSACCHARIDES IN SALIVARY AND EXORBITAL LACRIMAL GLANDS. Anat Rec. 1964 Jul;149:333–357. doi: 10.1002/ar.1091490304. [DOI] [PubMed] [Google Scholar]
- Trowbridge H. O. Salivary gland changes in vitamin-A-deficient rats. Arch Oral Biol. 1969 Aug;14(8):891–900. doi: 10.1016/0003-9969(69)90267-2. [DOI] [PubMed] [Google Scholar]
- WILHELM D. L. Regeneration of the tracheal epithelium in the vitamin-A-deficient rat. J Pathol Bacteriol. 1954 Apr;67(2):361–365. doi: 10.1002/path.1700670209. [DOI] [PubMed] [Google Scholar]
- Wong Y. C., Buck R. C. An electron microscopic study of metaplasia of the rat tracheal epithelium in vitamin A deficiency. Lab Invest. 1971 Jan;24(1):55–66. [PubMed] [Google Scholar]
- Zile M., DeLuca H. F. Retinoic acid: some aspects of growth-promoting activity in the albino rat. J Nutr. 1968 Mar;94(3):302–308. doi: 10.1093/jn/94.3.302. [DOI] [PubMed] [Google Scholar]