Abstract
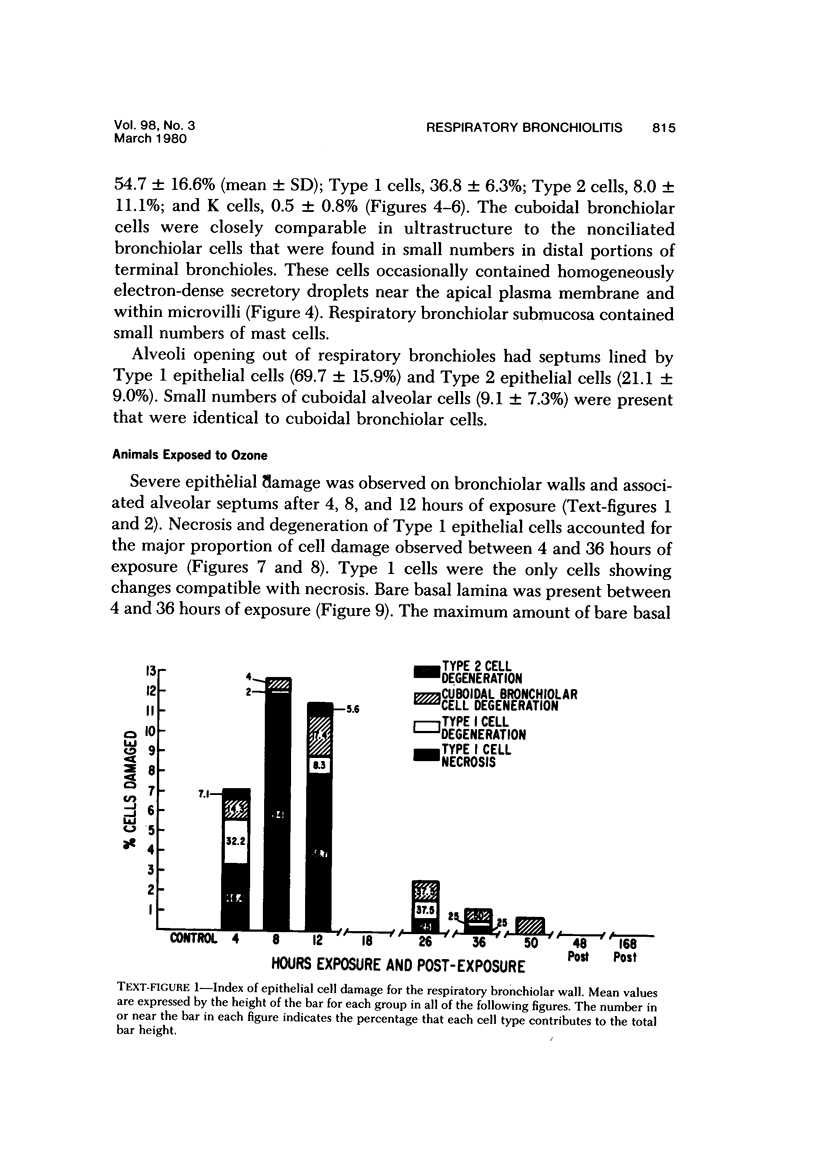
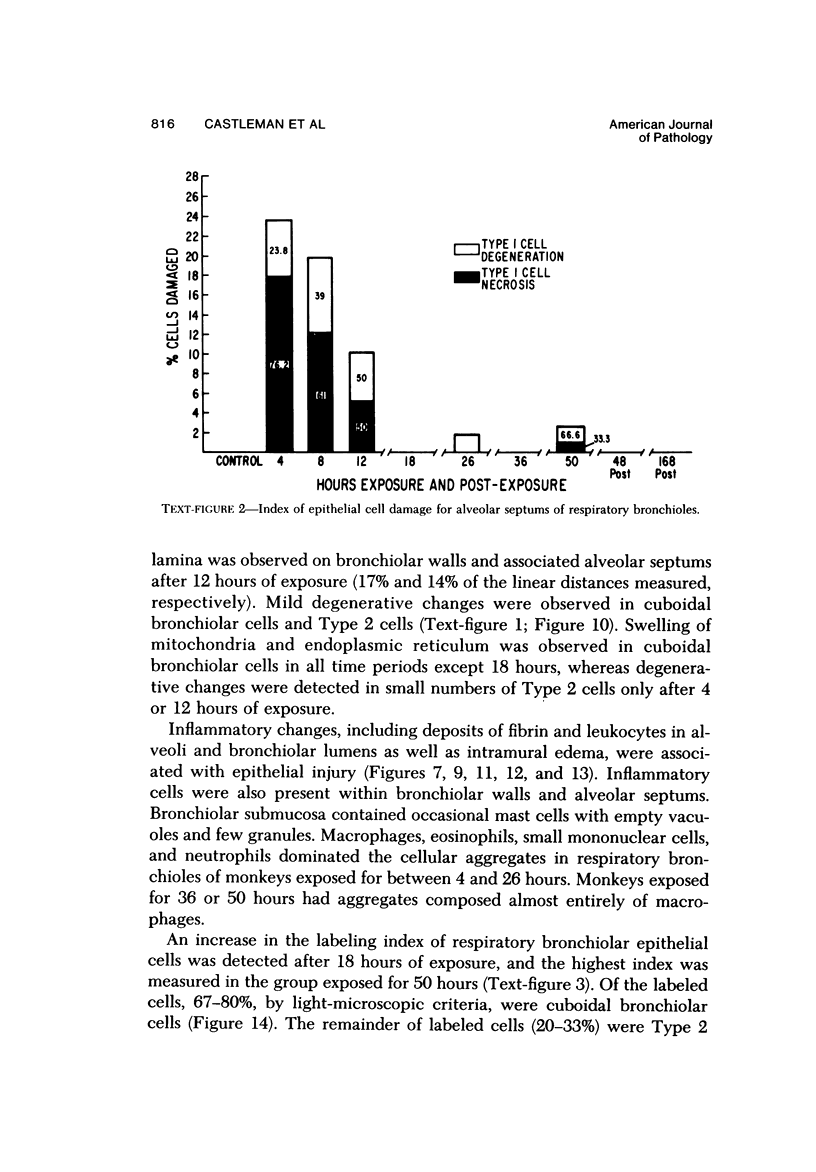
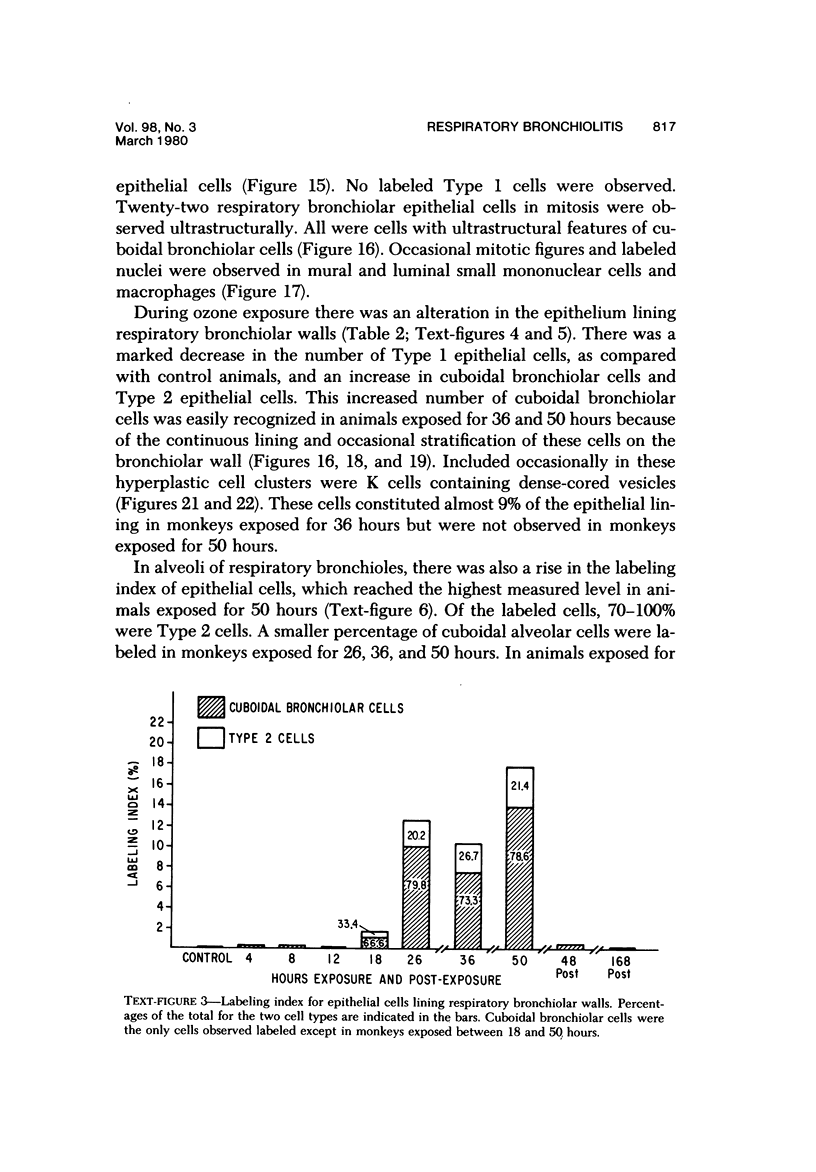
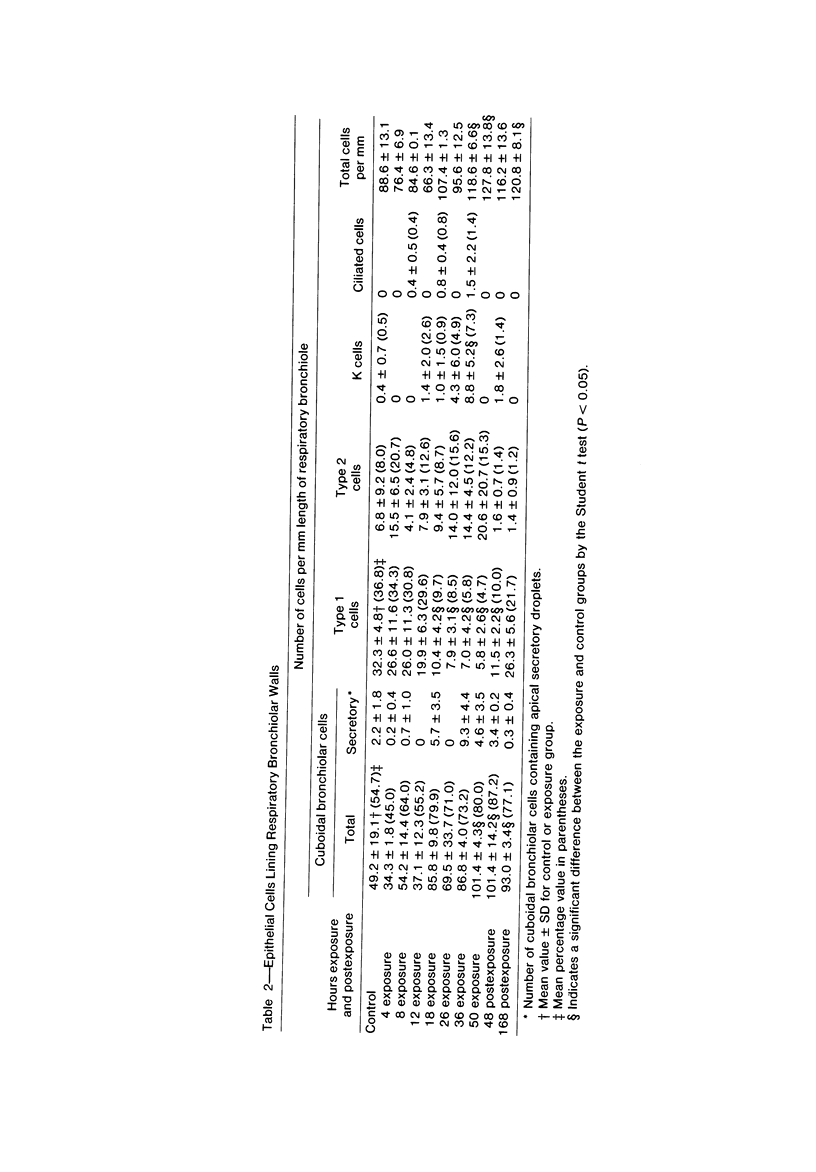
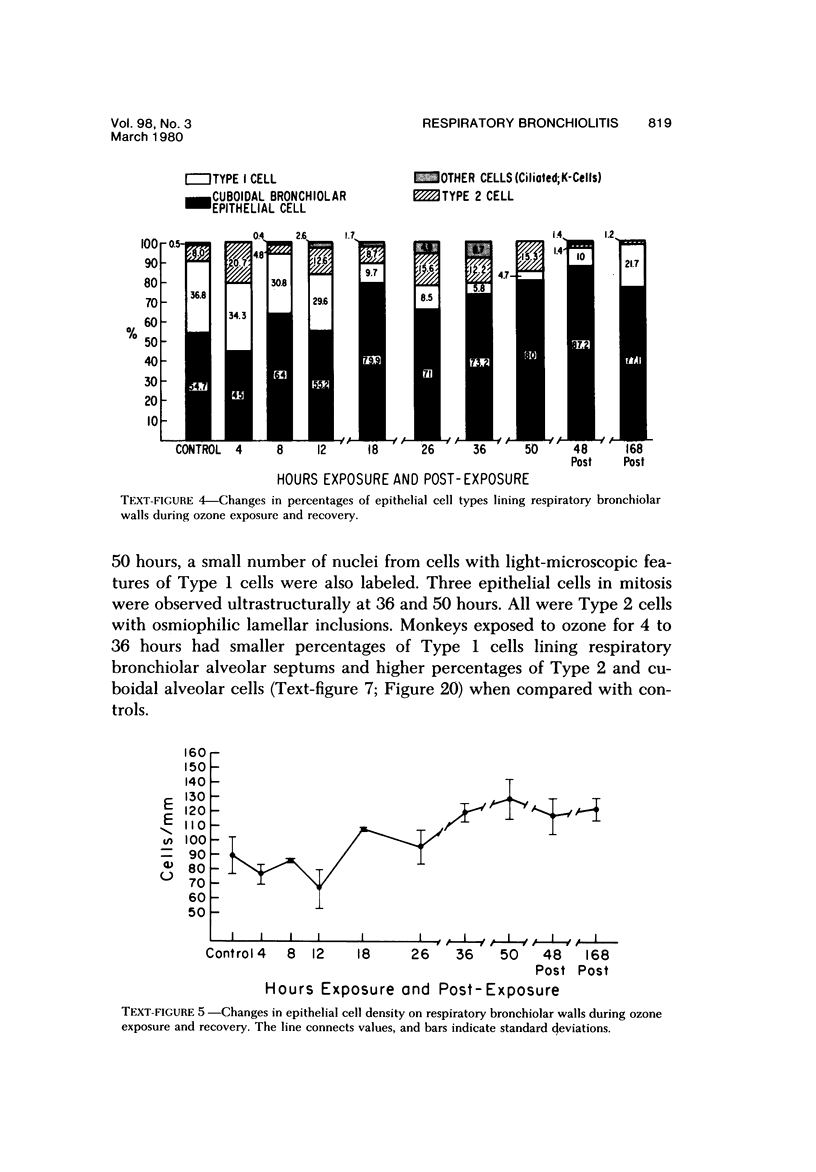
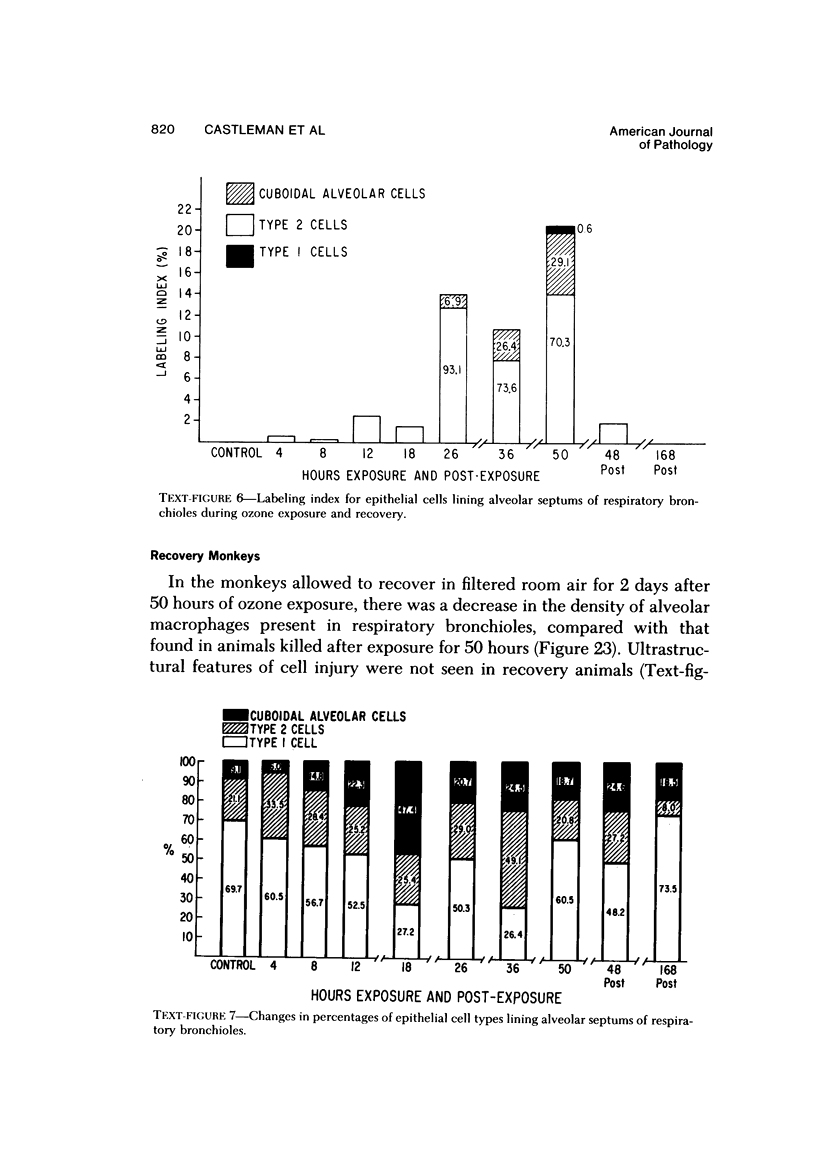
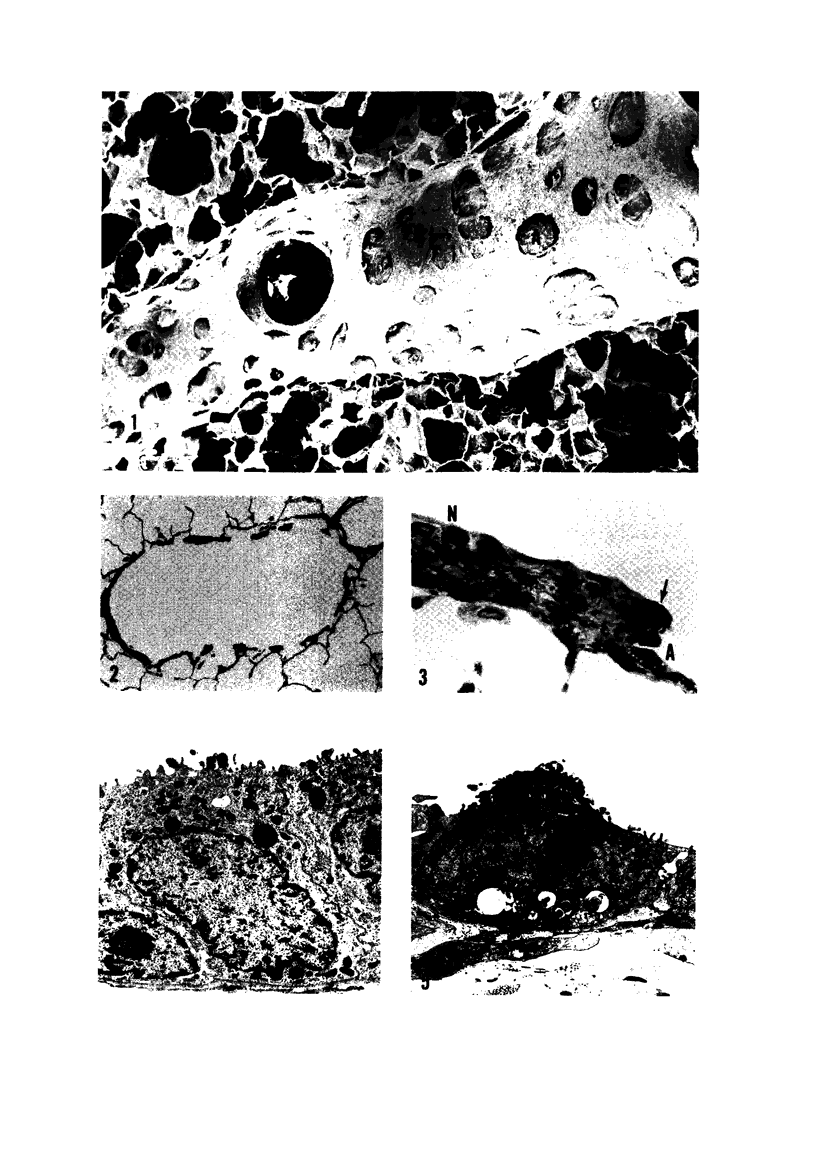
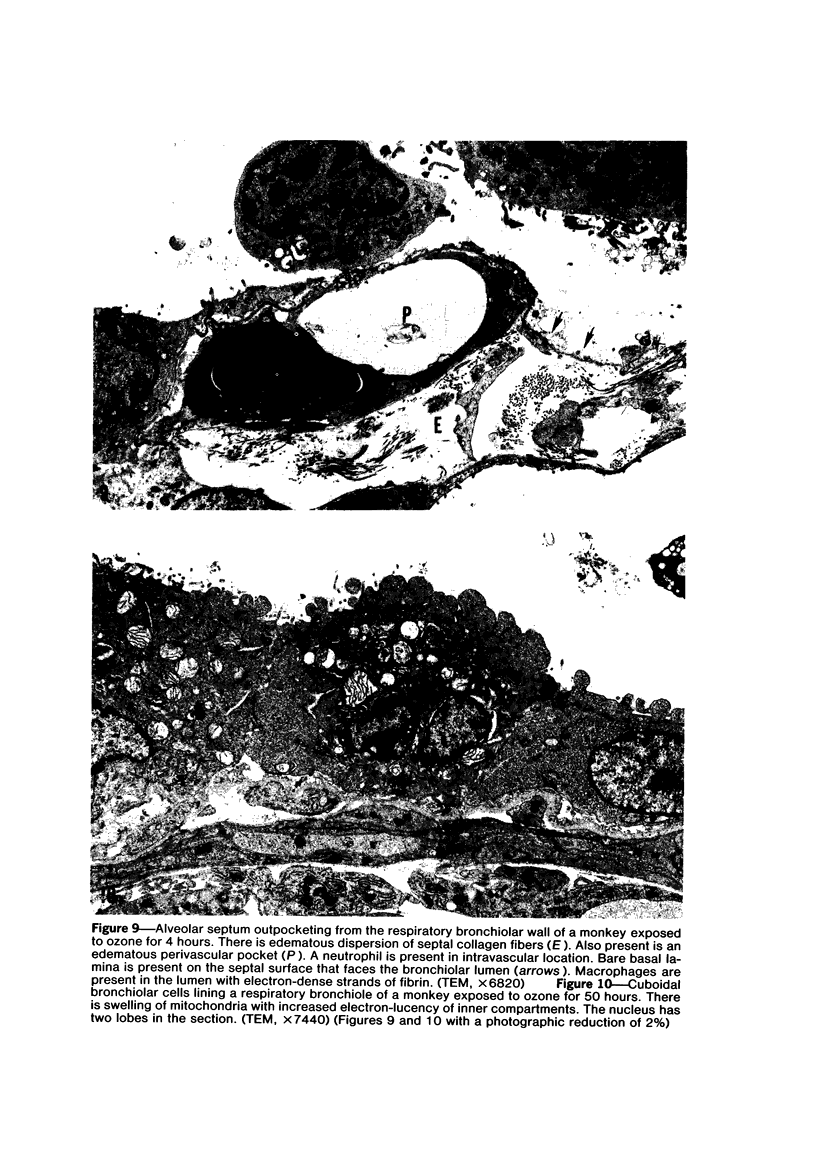
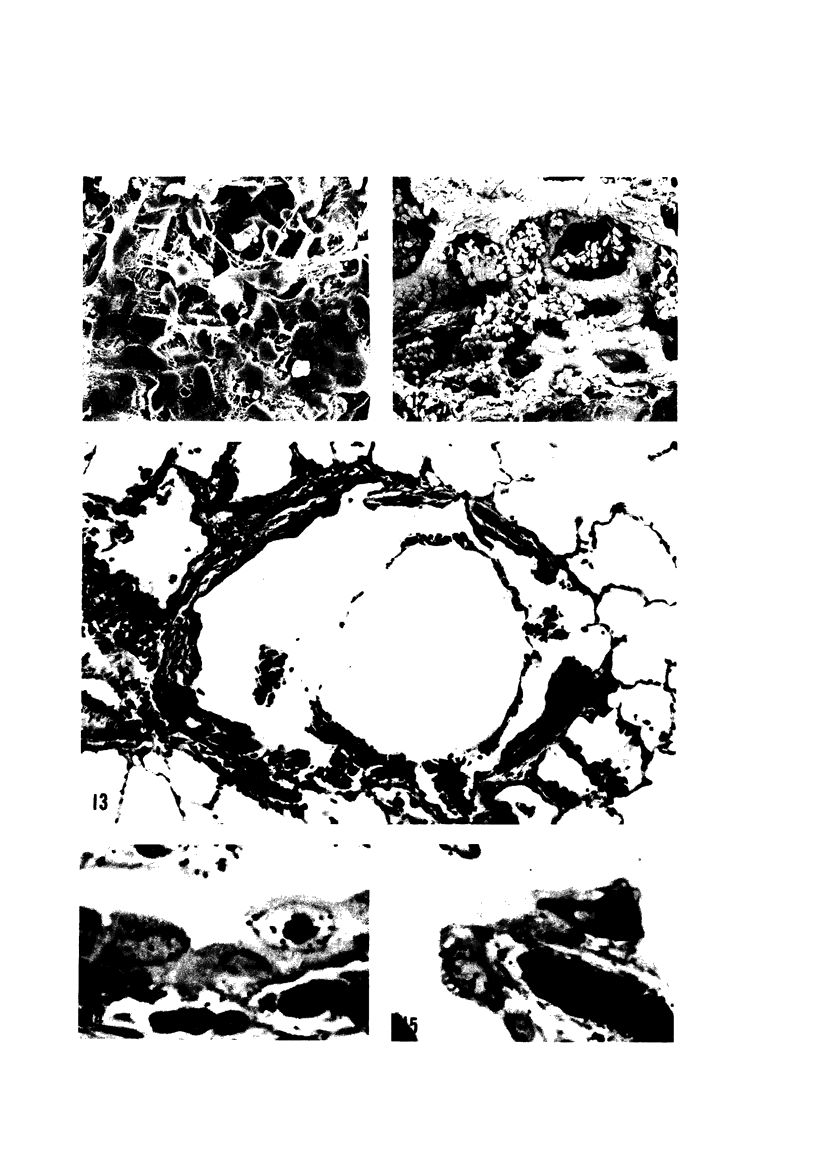
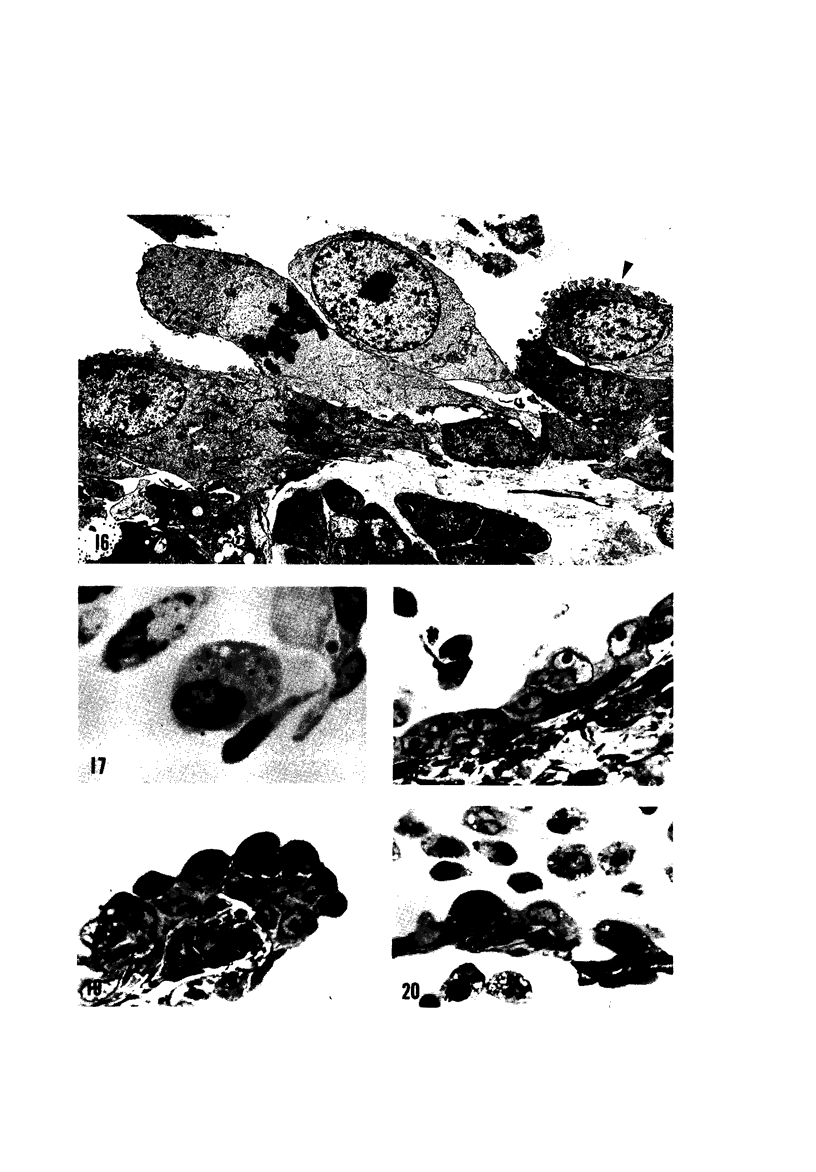
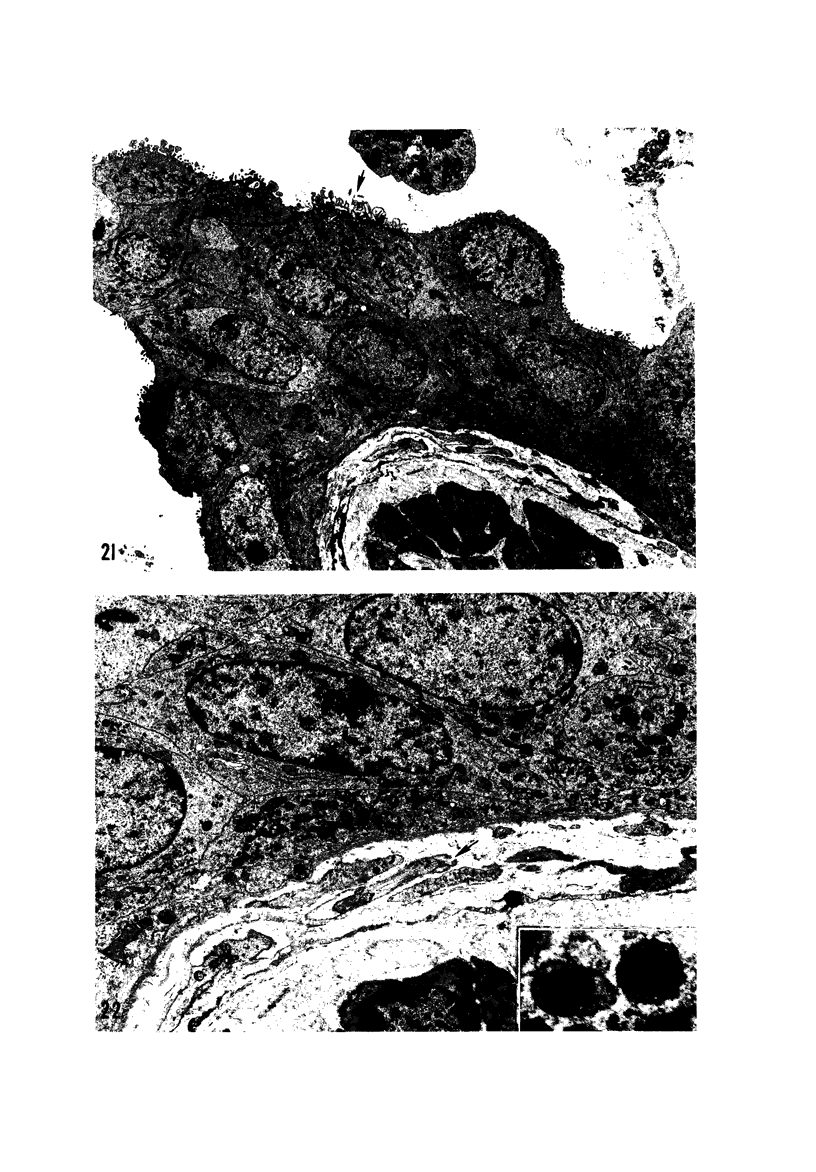
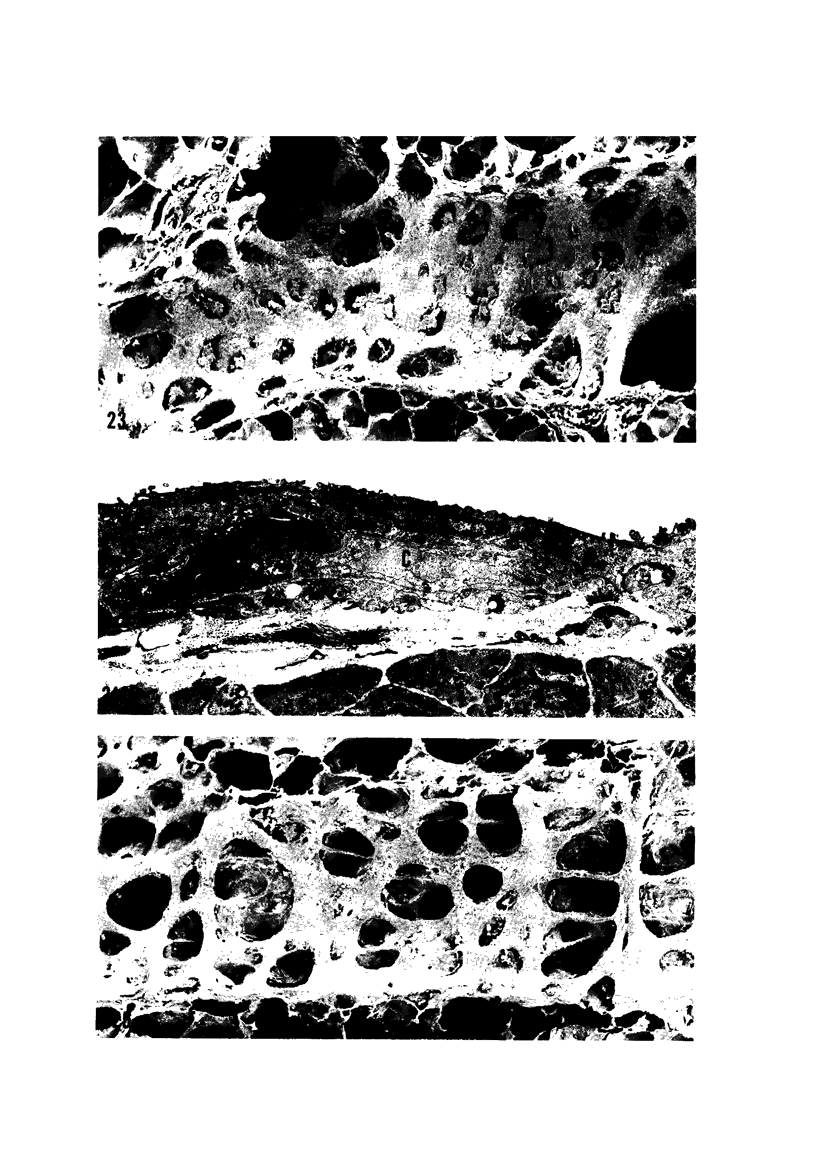
The pathogenesis of acute respiratory bronchiolitis was examined in rhesus monkeys exposed to 0.8 ppm ozone fpr 4--50 hours. Epithelial injury and renewal was qualitatively and quantitatively characterized by correlated techniques of scanning and transmission electron microscopy as well as by light-microscopic autoradiography following labeling with tritiated thymidine. Extensive degeneration and necrosis of Type 1 epithelial cells occurred on the respiratory bronchiolar wall during the initial 4--12 hours of exposure. Increased numbers of labeled epithelial cells were present in this region after 18 hours of exposure, and the highest labeling index (18% was measured after 50 hours of exposure. Most (67--80%) of the labeled cells and all the mitotic epithelial cells (22) observed ultrastructurally were cuboidal bronchiolar epithelial cells. Of the labeled epithelial cells, 20--33% were Type 2 epithelial cells. After 50 hours of exposure the respiratory bronchiolar epithelium was hyperplastic. The predominant inflammatory cell in respiratory bronchiolar exudate was the alveolar macrophage. Monkeys that were exposed for 50 hours and allowed to recover in unozonized air for 7 days had incomplete resolution of respiratory bronchiolar epithelial hyperplasia. The results indicate that Type 1 epithelial cells lining respiratory bronchioles are the cell type most sensitive to injury and that both cuboidal bronchiolar epithelial cells and Type 2 epithelial cells function as stem cells in epithelial renewal.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Origin of ciliated alveolar epithelial cells in bleomycin-induced lung injury. Am J Pathol. 1977 Jun;87(3):569–580. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The pathogenesis of bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 1974 Nov;77(2):185–197. [PMC free article] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- André-Bougaran J., Pariente R., Legrand M., Cayrol E. Ultrastructure normale des petites bronches et des bronchioles chez l'homme. Pathol Biol (Paris) 1975 Oct;23(8):629–638. [PubMed] [Google Scholar]
- André-Bougaran J., Pariente R., Legrand M., Cayrol E. Ultrastructure normale des petites bronches et des bronchioles chez l'homme. Bronches. 1974 Jan-Feb;24(1):1–14. [PubMed] [Google Scholar]
- Bensch K. G., Corrin B., Pariente R., Spencer H. Oat-cell carcinoma of the lung. Its origin and relationship to bronchial carcinoid. Cancer. 1968 Dec;22(6):1163–1172. doi: 10.1002/1097-0142(196811)22:6<1163::aid-cncr2820220612>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bensch K. G., Gordon G. B., Miller L. R. Studies on the bronchial counterpart of the Kultschitzky (argentaffin) cell and innervation of bronchial glands. J Ultrastruct Res. 1965 Jun;12(5):668–686. doi: 10.1016/s0022-5320(65)80055-7. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Tyler W. S. Histochemically detected enzymatic alterations in rat lung exposed to ozone. Exp Mol Pathol. 1973 Dec;19(3):402–421. doi: 10.1016/0014-4800(73)90070-1. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Dungworth D. L., Tyler W. S. Intrapulmonary airway morphology in three species of monkeys: a correlated scanning and transmission electron microscopic study. Am J Anat. 1975 Jan;142(1):107–121. doi: 10.1002/aja.1001420108. [DOI] [PubMed] [Google Scholar]
- Castleman W. L., Tyler W. S., Dungworth D. L. Lesions in respiratory bronchioles and conducting airways of monkeys exposed to ambient levels of ozone. Exp Mol Pathol. 1977 Jun;26(3):384–400. doi: 10.1016/0014-4800(77)90041-7. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. Activities of pentose shunt and glycolytic enzymes in lungs of ozone-exposed rats. Arch Environ Health. 1973 Apr;26(4):205–208. doi: 10.1080/00039896.1973.10666257. [DOI] [PubMed] [Google Scholar]
- Chow C. K., Tappel A. L. An enzymatic protective mechanism against lipid peroxidation damage to lungs of ozone-exposed rats. Lipids. 1972 Aug;7(8):518–524. doi: 10.1007/BF02533017. [DOI] [PubMed] [Google Scholar]
- Cutz E., Chan W., Wong V., Conen P. E. Endocrine cells in rat fetal lungs. Ultrastructural and histochemical study. Lab Invest. 1974 Apr;30(4):458–464. [PubMed] [Google Scholar]
- Dungworth D. L., Castleman W. L., Chow C. K., Mellick P. W., Mustafa M. G., Tarkington B., Tyler W. S. Effect of ambient levels of ozone on monkeys. Fed Proc. 1975 Jul;34(8):1670–1674. [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Johnson L. V., Stephens R. J., Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest. 1976 Sep;35(3):246–257. [PubMed] [Google Scholar]
- Gmelich J. T., Bensch K. G., Liebow A. A. Cells of Kultschitzky type in bronchioles and their relation to the origin of peripheral carcinoid tumor. Lab Invest. 1967 Jul;17(1):88–98. [PubMed] [Google Scholar]
- HEPPLESTON A. G. The pathological anatomy of simple pneumokoniosis in coal workers. J Pathol Bacteriol. 1953 Jul;66(1):235–246. doi: 10.1002/path.1700660127. [DOI] [PubMed] [Google Scholar]
- Hage E. Histochemistry and fine structure of endocrine cells in foetal lungs of the rabbit, mouse and guinea-pig. Cell Tissue Res. 1974 Jun 24;149(4):513–524. doi: 10.1007/BF00223029. [DOI] [PubMed] [Google Scholar]
- Hyde D., Orthoefer J., Dungworth D., Tyler W., Carter R., Lum H. Morphometric and morphologic evaluation of pulmonary lesions in beagle dogs chronically exposed to high ambient levels of air pollutants. Lab Invest. 1978 Apr;38(4):455–469. [PubMed] [Google Scholar]
- Kapanci Y., Weibel E. R., Kaplan H. P., Robinson F. R. Pathogenesis and reversibility of the pulmonary lesions of oxygen toxicity in monkeys. II. Ultrastructural and morphometric studies. Lab Invest. 1969 Jan;20(1):101–118. [PubMed] [Google Scholar]
- Kennedy A. R., Desrosiers A., Terzaghi M., Little J. B. Morphometric and histological analysis of the lungs of Syrian golden hamsters. J Anat. 1978 Mar;125(Pt 3):527–553. [PMC free article] [PubMed] [Google Scholar]
- LEOPOLD J. G., GOUGH J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957 Sep;12(3):219–235. doi: 10.1136/thx.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langloss J. M., Hoover E. A., Kahn D. E. Ultrastructural morphogenesis of acute viral pneumonia produced by feline calicivirus. Am J Vet Res. 1978 Oct;39(10):1577–1583. [PubMed] [Google Scholar]
- Lauweryns J. M., Cokelaere M., Theunynck P. Neuro-epithelial bodies in the respiratory mucosa of various mammals. A light optical, histochemical and ultrastructural investigation. Z Zellforsch Mikrosk Anat. 1972;135(4):569–592. doi: 10.1007/BF00583438. [DOI] [PubMed] [Google Scholar]
- Lauweryns J. M., Goddeeris P. Neuroepithelial bodies in the human child and adult lung. Am Rev Respir Dis. 1975 Apr;111(4):469–476. doi: 10.1164/arrd.1975.111.4.469. [DOI] [PubMed] [Google Scholar]
- Lum H., Schwartz L. W., Dungworth D. L., Tyler W. S. A comparative study of cell renewal after exposure to ozone or oxygen. Response of terminal bronchiolar epithelium in the rat. Am Rev Respir Dis. 1978 Aug;118(2):335–345. doi: 10.1164/arrd.1978.118.2.335. [DOI] [PubMed] [Google Scholar]
- McDowell E. M., Barrett L. A., Trump B. F. Observations on small granule cells in adult human bronchial epithelium and in carcinoid and oat cell tumors. Lab Invest. 1976 Feb;34(2):202–206. [PubMed] [Google Scholar]
- McLaughlin R. F., Jr, Tyler W. S., Canada R. O. Subgross pulmonary anatomy of the rabbit, rat, and guinea pig, with additional notes on the human lung. Am Rev Respir Dis. 1966 Sep;94(3):380–387. doi: 10.1164/arrd.1966.94.3.380. [DOI] [PubMed] [Google Scholar]
- Mellick P. W., Dungworth D. L., Schwartz L. W., Tyler W. S. Short term morphologic effects of high ambient levels of ozone on lungs of rhesus monkeys. Lab Invest. 1977 Jan;36(1):82–90. [PubMed] [Google Scholar]
- Niewoehner D. E., Kleinerman J., Rice D. B. Pathologic changes in the peripheral airways of young cigarette smokers. N Engl J Med. 1974 Oct 10;291(15):755–758. doi: 10.1056/NEJM197410102911503. [DOI] [PubMed] [Google Scholar]
- Nowell J. A., Tyler W. S. Scanning electron microscopy of the surface morphology of mammalian lungs. Am Rev Respir Dis. 1971 Mar;103(3):313–328. doi: 10.1164/arrd.1971.103.3.313. [DOI] [PubMed] [Google Scholar]
- Penha P. D., Werthamer S. Pulmonary lesions induced by long-term exposure to ozone. II. Ultrastructure observations of proliferative and regressive lesions. Arch Environ Health. 1974 Nov;29(5):282–289. doi: 10.1080/00039896.1974.10666588. [DOI] [PubMed] [Google Scholar]
- Plopper C. G., Dungworth D. L., Tyler W. S. Pulmonary lesions in rats exposed to ozone. A correlated light and electron microscopic study. Am J Pathol. 1973 Jun;71(3):375–394. [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. W., Christman C. A. Alveolar macrophage migration. Influence of lung lining material and acute lung insult. Am Rev Respir Dis. 1979 Aug;120(2):429–439. doi: 10.1164/arrd.1979.120.2.429. [DOI] [PubMed] [Google Scholar]
- Schwartz L. W., Dungworth D. L., Mustafa M. G., Tarkington B. K., Tyler W. S. Pulmonary responses of rats to ambient levels of ozone: effects of 7-day intermittent or continuous exposure. Lab Invest. 1976 Jun;34(6):565–578. [PubMed] [Google Scholar]
- Stephens R. J., Freeman G., Evans M. J. Early response of lungs to low levels of nitrogen dioxide. Light and electron microscopy. Arch Environ Health. 1972 Mar;24(3):160–179. doi: 10.1080/00039896.1972.10666066. [DOI] [PubMed] [Google Scholar]
- Stephens R. J., Sloan M. F., Evans M. J., Freeman G. Early response of lung to low levels of ozone. Am J Pathol. 1974 Jan;74(1):31–58. [PMC free article] [PubMed] [Google Scholar]
- Strauss R. H., Palmer K. C., Hayes J. A. Acute lung injury induced by cadmium aerosol. I. Evolution of alveolar cell damage. Am J Pathol. 1976 Sep;84(3):561–578. [PMC free article] [PubMed] [Google Scholar]
- TYLER W. S., PEARSE A. G. OXIDATIVE ENZYMES OF THE INTERALVEOLAR SEPTUM OF THE RAT. Thorax. 1965 Mar;20:149–152. doi: 10.1136/thx.20.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]