Abstract
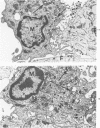
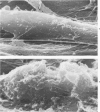
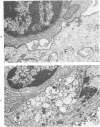
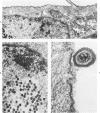
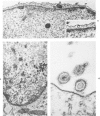
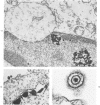
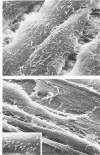
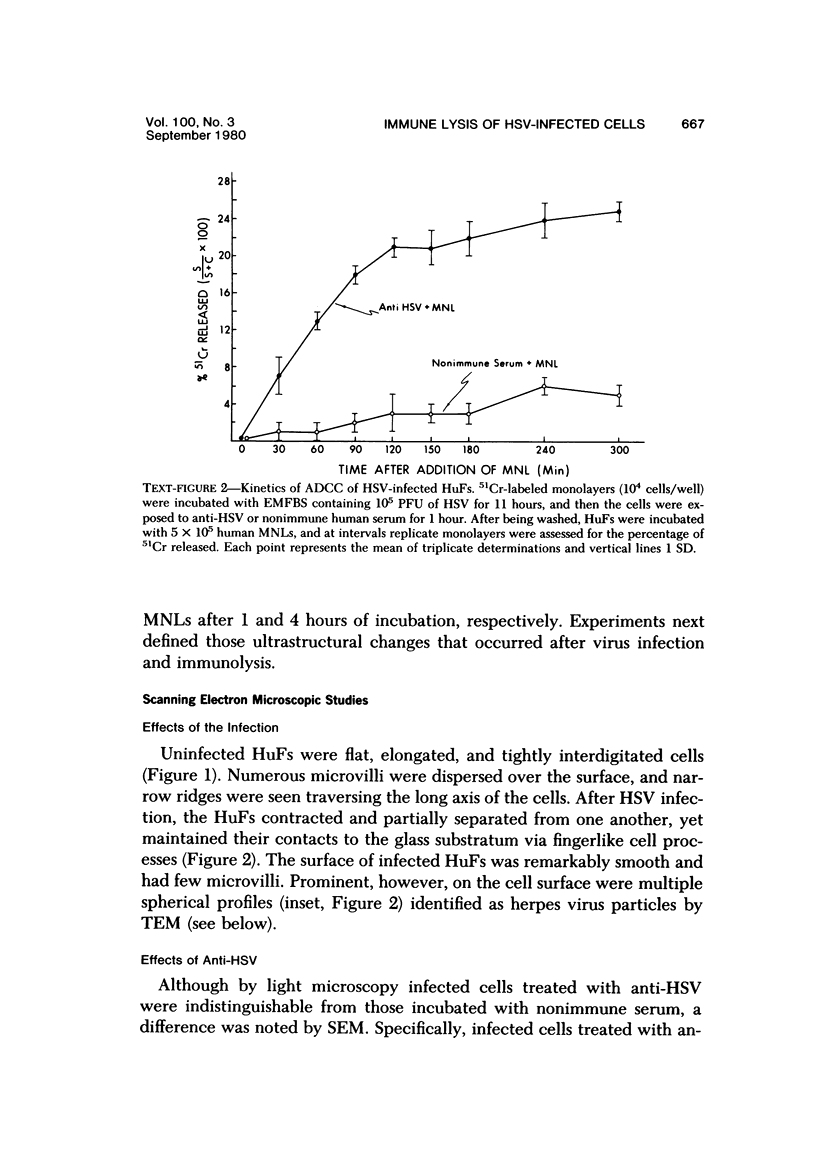
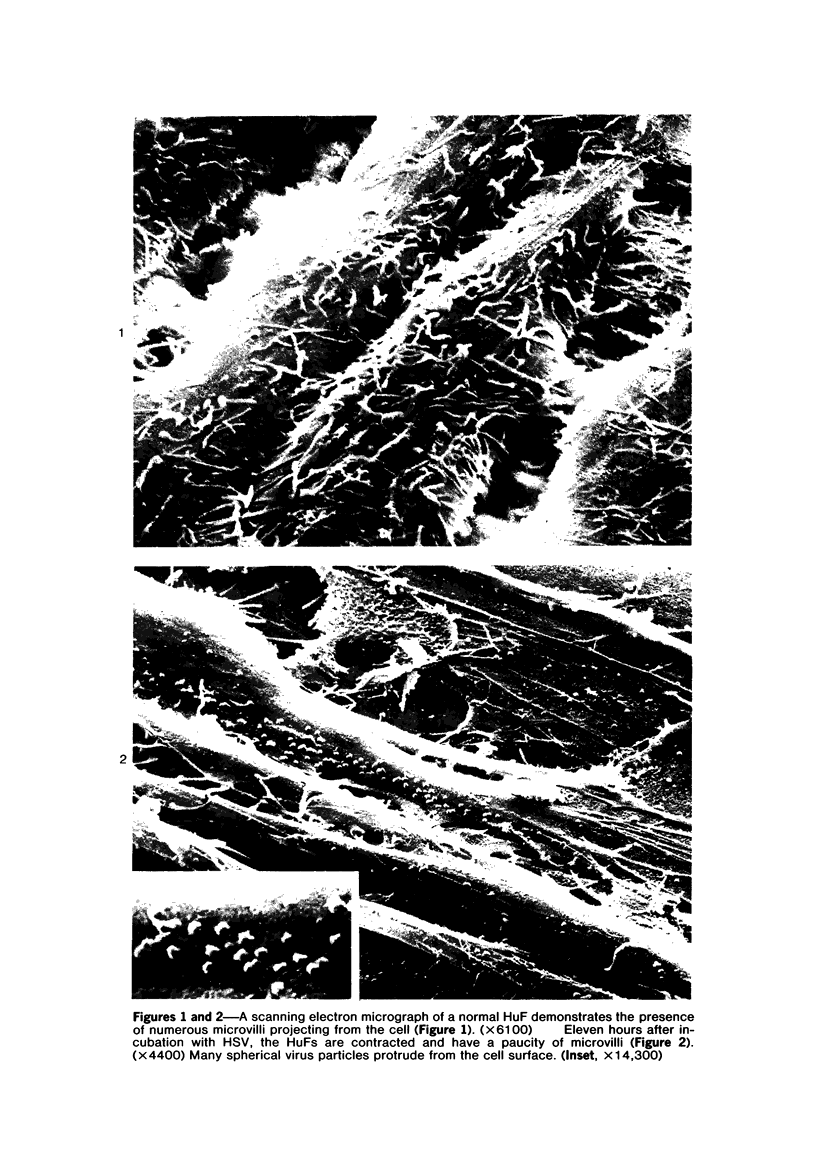
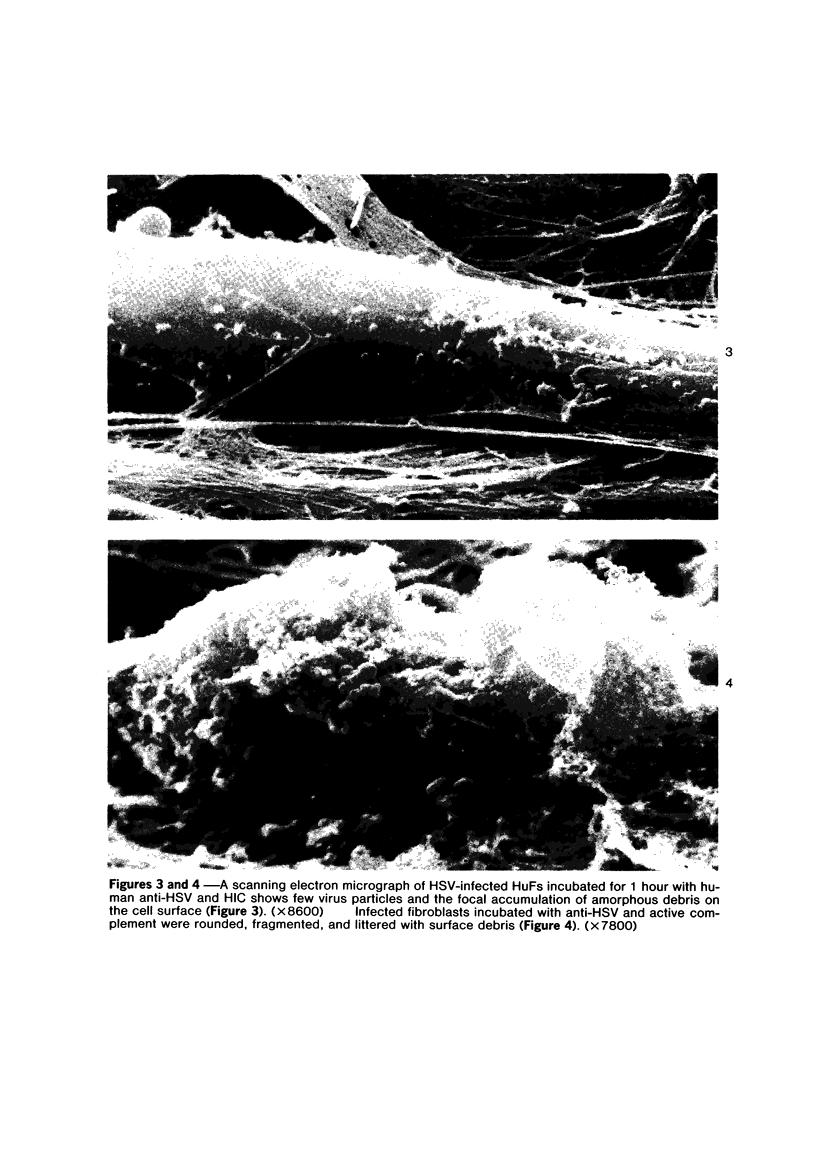
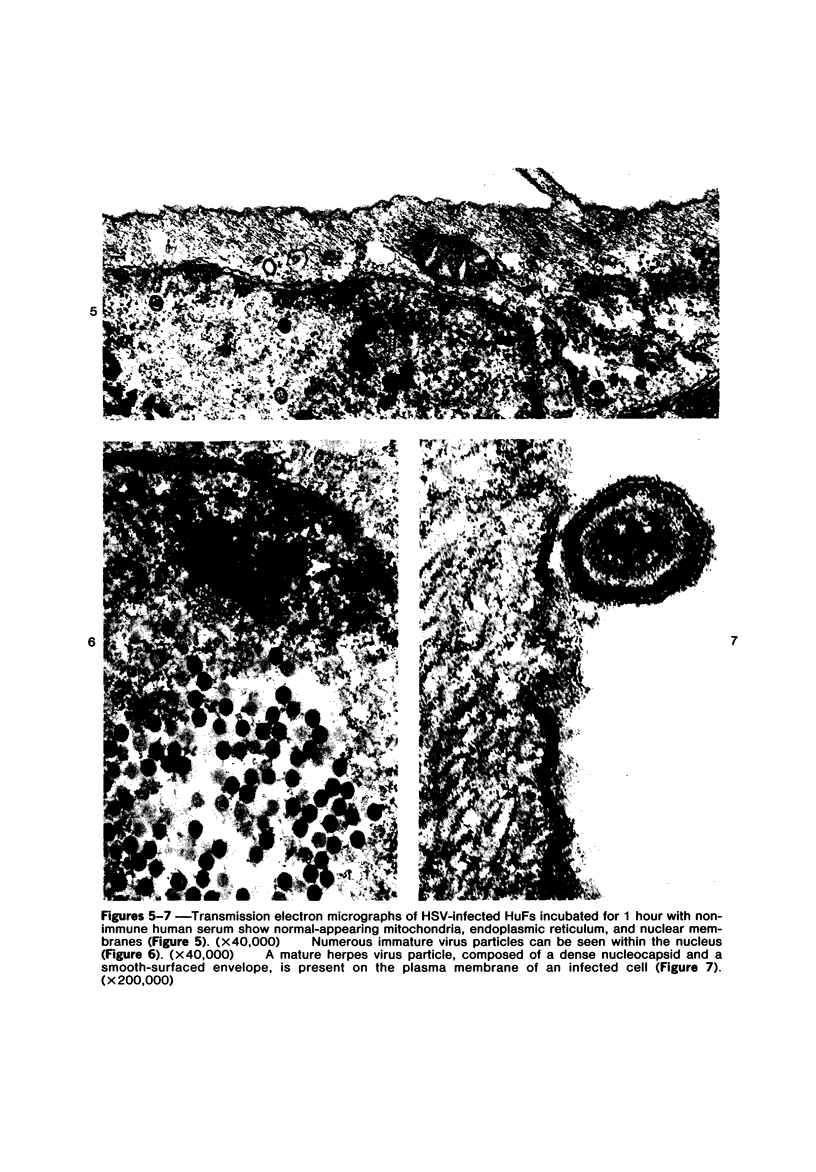
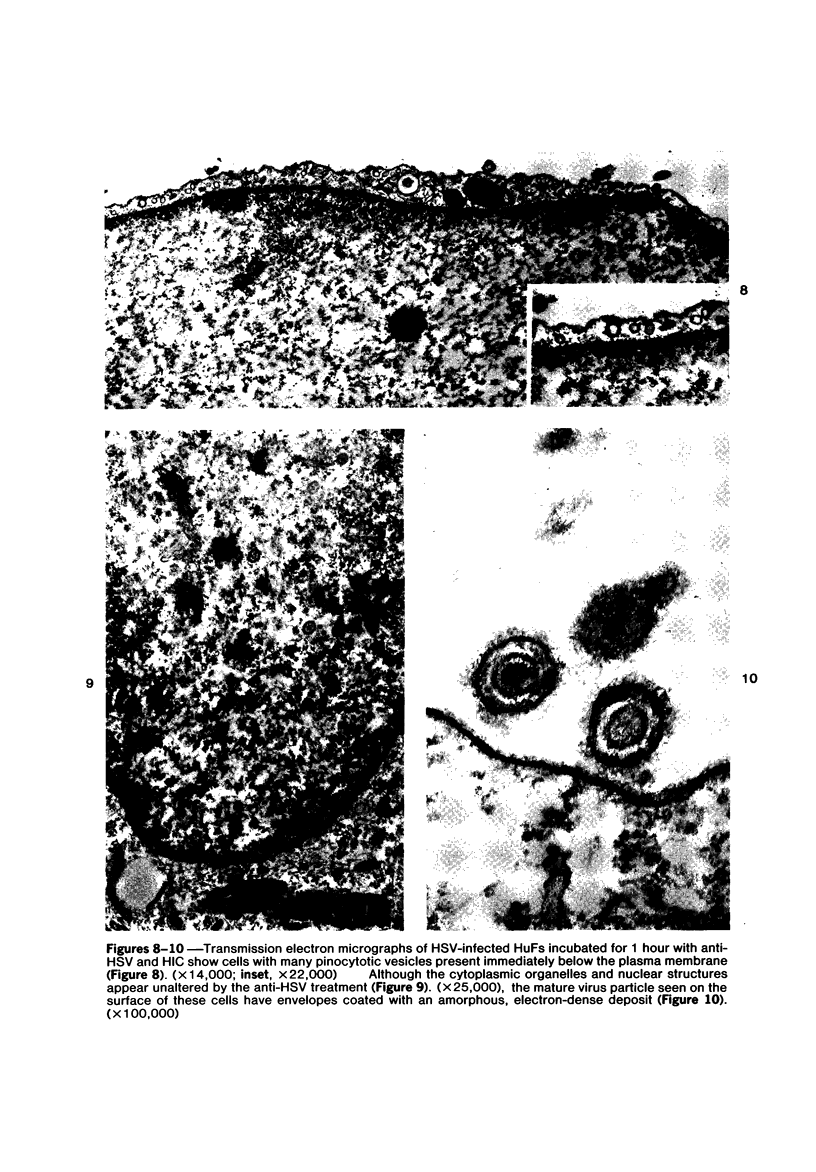
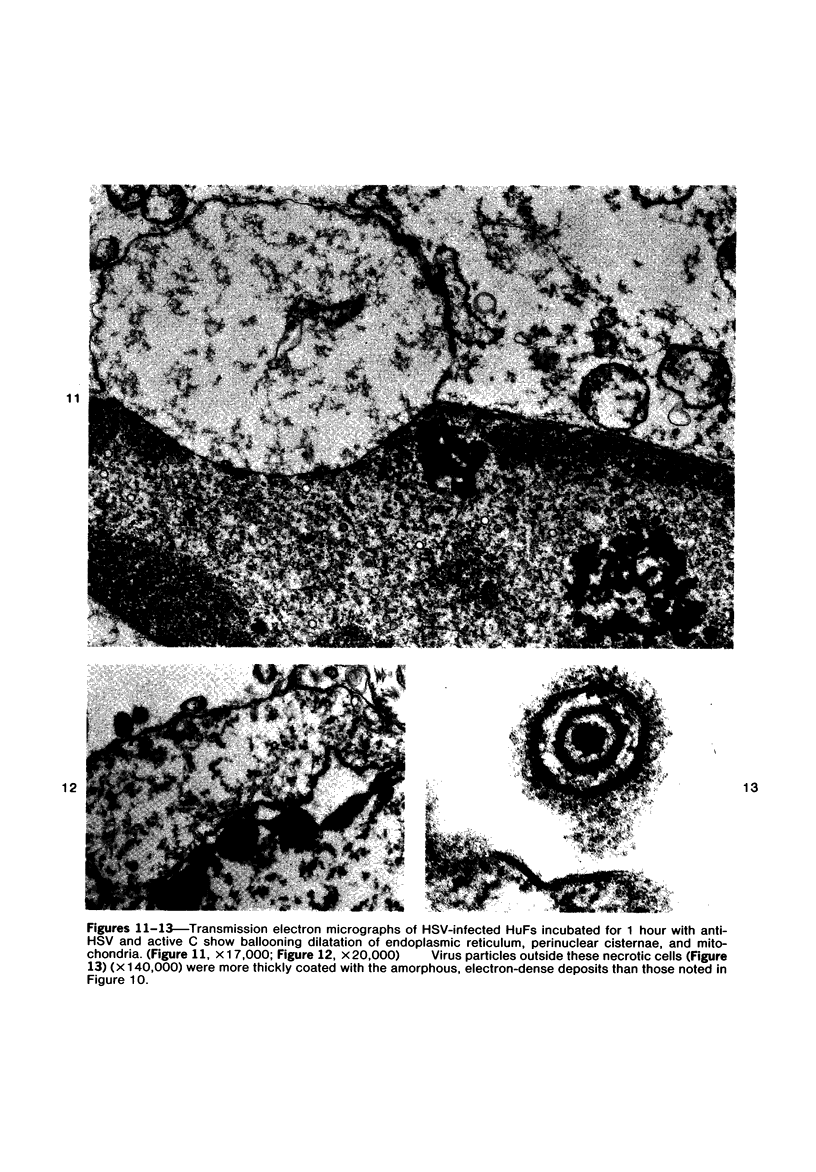
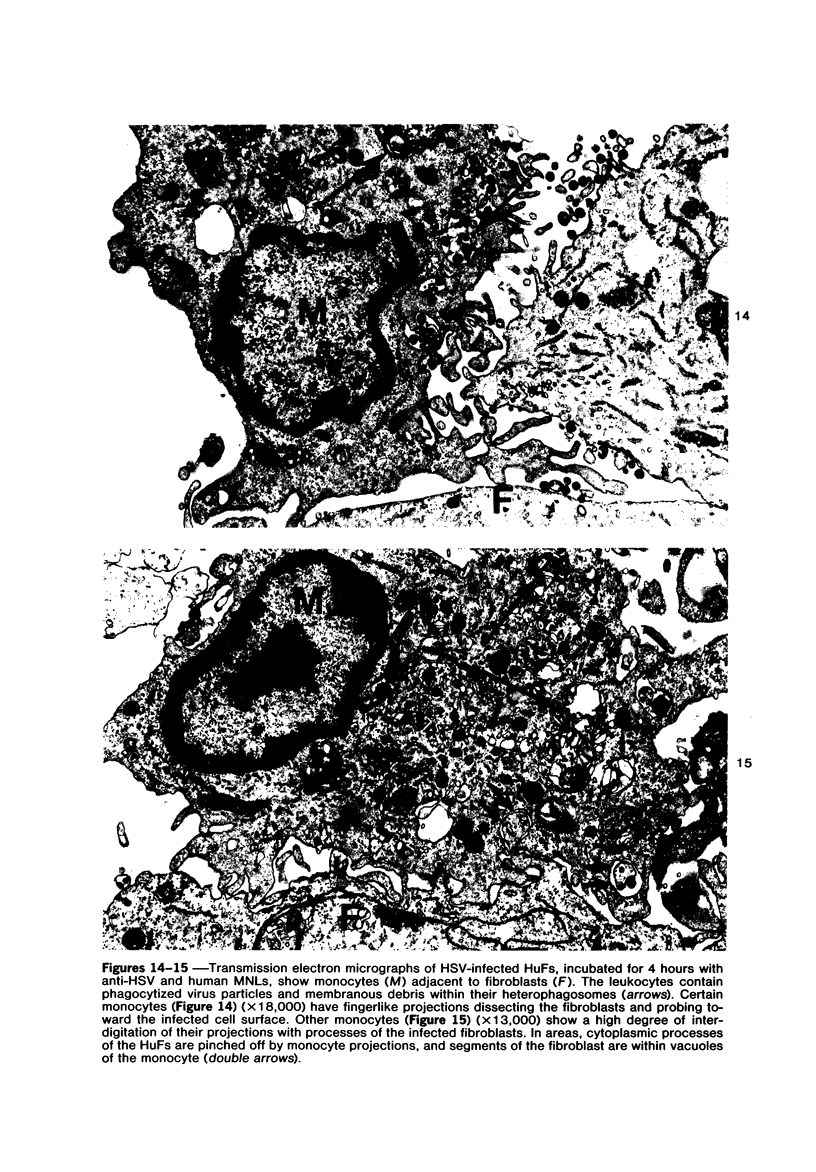
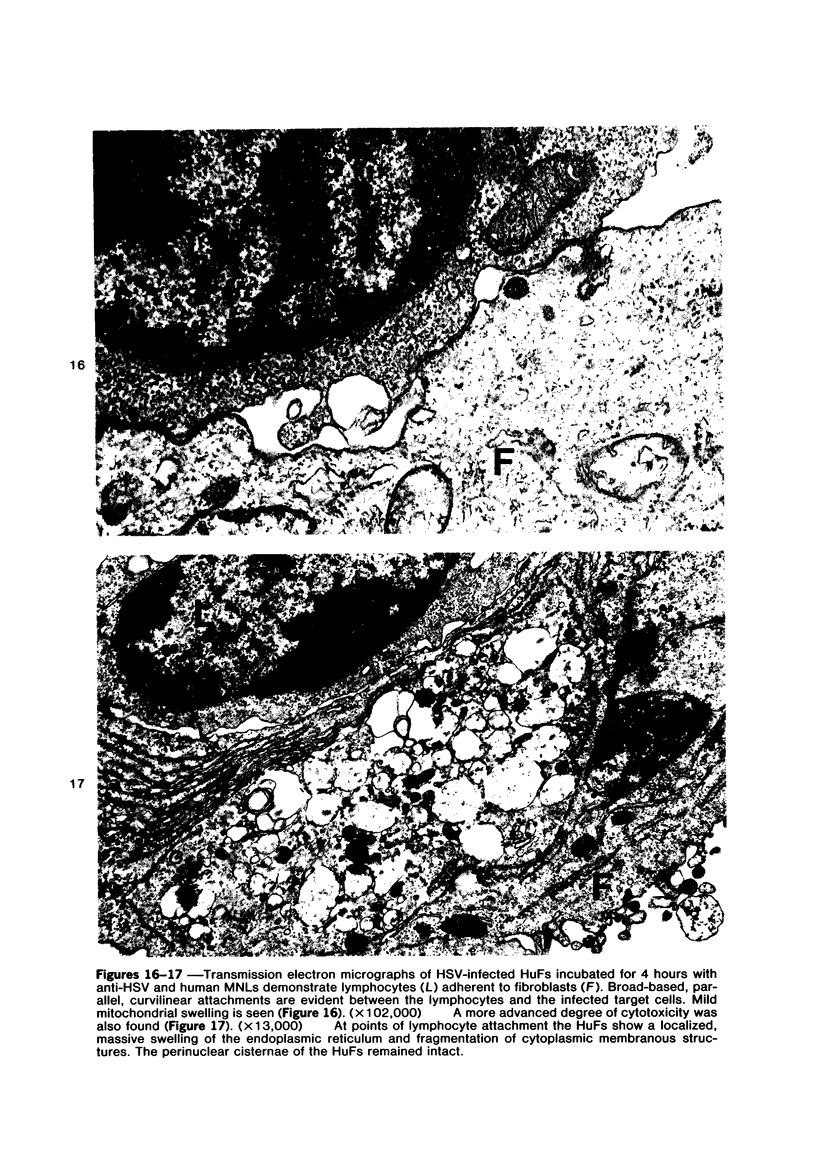
The morphologic aspects of complement-mediated and antibody-dependent cell-mediated cytolysis (ADCC) of human fibroblasts (HuFs) infected by herpes simplex virus (HSV) is described. Human antiviral antibody (antiHSV) was shown by transmission and scanning electron microscopy (TEM and SEM) to cause the deposition of an amorphous material over the surface of infected cells and virus particles. Associated with antiHSV treatment, the HuFs underwent endocytosis, with the appearance of pinocytotic vesicles immediately beneath the plasma membrane. The addition of complement resulted in lysis of the infected HuFs and massive dilatation of the perinuclear cisternae, but the virus particles associated with the cell surface did not appear lysed. Instead, an additional deposit was noted on the enveloped particles after the addition of complement (C). Human peripheral blood mononuclear leukocytes (MNLs) also lysed the antibody-coated, infected HuFs. Lymphocytes formed broad-based areas of attachment to the antiHSV-treated cells. Beneath these areas of contact occurred focal cytoplasmic changes that preceded cell lysis. Monocytes showed multiple points of binding and sent cytoplasmic projections over the surface of the infected HuFs. Virus particles and segments of target cell cytoplasm were gathered into vacuoles of the monocyte. In accord with the above morphologic findings, the relative roles that antibody, C, and leukocytes may play in human viral diseases is discussed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Able M. E., Lee J. C., Rosenau W. Lymphocyte--target cell interaction in vitro. Ultrastructural and cinematographic studies. Am J Pathol. 1970 Sep;60(3):421–434. [PMC free article] [PubMed] [Google Scholar]
- Almeida J. D., Waterson A. P. The morphology of virus-antibody interaction. Adv Virus Res. 1969;15:307–338. doi: 10.1016/S0065-3527(08)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn W. R. Pathobiology of nucleocytoplasmic exchange. Pathobiol Annu. 1971;1:1–31. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chambers V. C., Weiser R. S. The ultrastructure of target L-cells and immune macrophages during their interaction in vivo. Cancer Res. 1971 Dec;31(12):2059–2066. [PubMed] [Google Scholar]
- Chambers V. C., Weiser R. S. The ultrastructure of target cells and immune macrophages during their interaction in vitro. Cancer Res. 1969 Feb;29(2):301–317. [PubMed] [Google Scholar]
- Cooper J. A., Jr, Daniels C. A., Trofatter E. F., Jr The effect of prednisolone on antibody-dependent cell-mediated cytotoxicity and the growth of type I herpes simplex virus in human cells. Invest Ophthalmol Vis Sci. 1978 Apr;17(4):381–385. [PubMed] [Google Scholar]
- Daniels C. A., Borsos T., Rapp H. J., Snyderman R., Notkins A. L. Neutralization of sensitized virus by purified components of complement. Proc Natl Acad Sci U S A. 1970 Mar;65(3):528–535. doi: 10.1073/pnas.65.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., Borsos T., Rapp H. J., Snyderman R., Notkins A. L. Neutralization of sensitized virus by the fourth component of complement. Science. 1969 Aug 1;165(3892):508–509. doi: 10.1126/science.165.3892.508. [DOI] [PubMed] [Google Scholar]
- Daniels C. A., Kleinerman E. S., Snyderman R. Abortive and productive infections of human mononuclear phagocytes by type I herpes simplex virus. Am J Pathol. 1978 Apr;91(1):119–136. [PMC free article] [PubMed] [Google Scholar]
- Daniels C. A., LeGoff S. G. Shedding of infectious virus/antibody complexes from vesicular lesions of patients with recurrent herpes labialis. Lancet. 1975 Sep 20;2(7934):524–528. doi: 10.1016/s0140-6736(75)90896-x. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Ruth W. A., Wells M. A. Host defense mechanisms against infection with influenza virus. I. Effect of sensitized spleen cells on infection in vitro. J Infect Dis. 1974 Sep;130(3):248–256. doi: 10.1093/infdis/130.3.248. [DOI] [PubMed] [Google Scholar]
- GOLDBERG B., GREEN H. The cytotoxic action of immune gamma globulin and complement on Krebs ascites tumor cells. I. Ultrastructural studies. J Exp Med. 1959 May 1;109(5):505–510. doi: 10.1084/jem.109.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn F. L., Shelburne J. D., Trump B. F. Disorders of cell volume regulation. I. Effects of inhibition of plasma membrane adenosine triphosphatase with ouabain. Am J Pathol. 1968 Dec;53(6):1041–1071. [PMC free article] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Inglis J. R., Penhale W. J., Farmer A., Irvine W. J., Williams A. E. Observations on the antibody-dependent cytotoxic cell by scanning electron microscopy. Clin Exp Immunol. 1975 Aug;21(2):216–225. [PMC free article] [PubMed] [Google Scholar]
- Joseph B. S., Oldstone M. B. Immunologic injury in measles virus infection. II. Suppression of immune injury through antigenic modulation. J Exp Med. 1975 Oct 1;142(4):864–876. doi: 10.1084/jem.142.4.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman E. S., Snyderman R., Daniels C. A. Depression of human monocyte chemotaxis by herpes simplex and influenza viruses. J Immunol. 1974 Nov;113(5):1562–1567. [PubMed] [Google Scholar]
- Kohl S., Starr S. E., oleske J. M., Shore S. L., Ashman R. B., Nahmias A. J. Human monocyte-macrophage-mediated antibody-dependent cytotoxicity to herpes simplex virus-infected cells. J Immunol. 1977 Mar;118(3):729–735. [PubMed] [Google Scholar]
- Koren H. S., Ax W., Freund-Moelbert E. Morphological observations on the contact-induced lysis of target cells. Eur J Immunol. 1973 Jan;3(1):32–37. doi: 10.1002/eji.1830030108. [DOI] [PubMed] [Google Scholar]
- Linscott W. D., Levinson W. E. Complement components required for virus neutralization by early immunoglobulin antibody. Proc Natl Acad Sci U S A. 1969 Oct;64(2):520–527. doi: 10.1073/pnas.64.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K., Morgan C., Hsu K. C., Hampar B. Differentiation by immunoferritin of herpes simplex antigens with the use of rabbit 7S and 19S antibodies from early (7-day) and late (7-week) immune sera. J Natl Cancer Inst. 1971 Mar;46(3):629–646. [PubMed] [Google Scholar]
- Oldstone M. B., Cooper N. R., Larson D. L. Formation and biologic role of polyoma virus-antibody complexes. A critical role for complement. J Exp Med. 1974 Aug 1;140(2):549–565. doi: 10.1084/jem.140.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Virus neutralization and virus-induced immune complex disease. Virus-antibody union resulting in immunoprotection or immunologic injury--two sides of the same coin. Prog Med Virol. 1975;19:84–119. [PubMed] [Google Scholar]
- Oleske J. M., Ashman R. B., Kohl S., Shore S. L., Starr S. E., Wood P., Nahmias A. J. Human polymorphonuclear leucocytes as mediators of antibody-dependent cellular cytotoxicity to herpes simplex virus-infected cells. Clin Exp Immunol. 1977 Mar;27(3):446–453. [PMC free article] [PubMed] [Google Scholar]
- Oroszlan S., Gilden R. V. Immune virolysis: effect of antibody and complement on C-type RNA virus. Science. 1970 Jun 19;168(3938):1478–1480. doi: 10.1126/science.168.3938.1478. [DOI] [PubMed] [Google Scholar]
- Penfold P. L., Greenberg A. H., Roitt I. M. Characteristics of the effector cells mediating cytotoxicity against antibody-coated target cells. III. Ultrastructural studies. Clin Exp Immunol. 1976 Jan;23(1):91–97. [PMC free article] [PubMed] [Google Scholar]
- Radwan A. I., Crawford T. B. The mechanisms of neutralization of sensitized equine arteritis virus by complement components. J Gen Virol. 1974 Nov;25(2):229–237. doi: 10.1099/0022-1317-25-2-229. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Dubois-Dalcq M. Intramembrane changes occurring during maturation of herpes simplex virus type 1: freeze-fracture study. J Virol. 1978 May;26(2):435–447. doi: 10.1128/jvi.26.2.435-447.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Norrild B. Early damage of herpes-infected cells by antibody-dependent cellular cytotoxicity: relative roles of virus-specified cell-surface antigens and input virus. J Immunol. 1979 Nov;123(5):2239–2244. [PubMed] [Google Scholar]
- Shore S. L., Cromeans T. L., Romano T. J. Immune destruction of virus-infected cells early in the infectious cycle. Nature. 1976 Aug 19;262(5570):695–696. doi: 10.1038/262695a0. [DOI] [PubMed] [Google Scholar]
- Shore S. L., Melewicz F. M., Gordon D. S. The mononuclear cell in human blood which mediates antibody-dependent cellular cytotoxicity to virus-infected target cells. I. Identification of the population of effector cells. J Immunol. 1977 Feb;118(2):558–566. [PubMed] [Google Scholar]
- Snodgrass M. J., Hanna M. G., Jr Ultrastructural studies of histiocyte-tumor cell interactions during tumor regression after intralesional injection of Mycobacterium bovis. Cancer Res. 1973 Apr;33(4):701–716. [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Trofatter K. F., Jr, Daniels C. A. Interaction of human cells with prostaglandins and cyclic AMP modulators. I. Effects on complement-mediated lysis and antibody-dependent cell-mediated cytolysis of herpes simplex virus-infected human fibroblasts. J Immunol. 1979 Apr;122(4):1363–1370. [PubMed] [Google Scholar]