Abstract
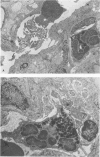
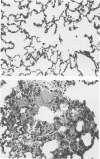
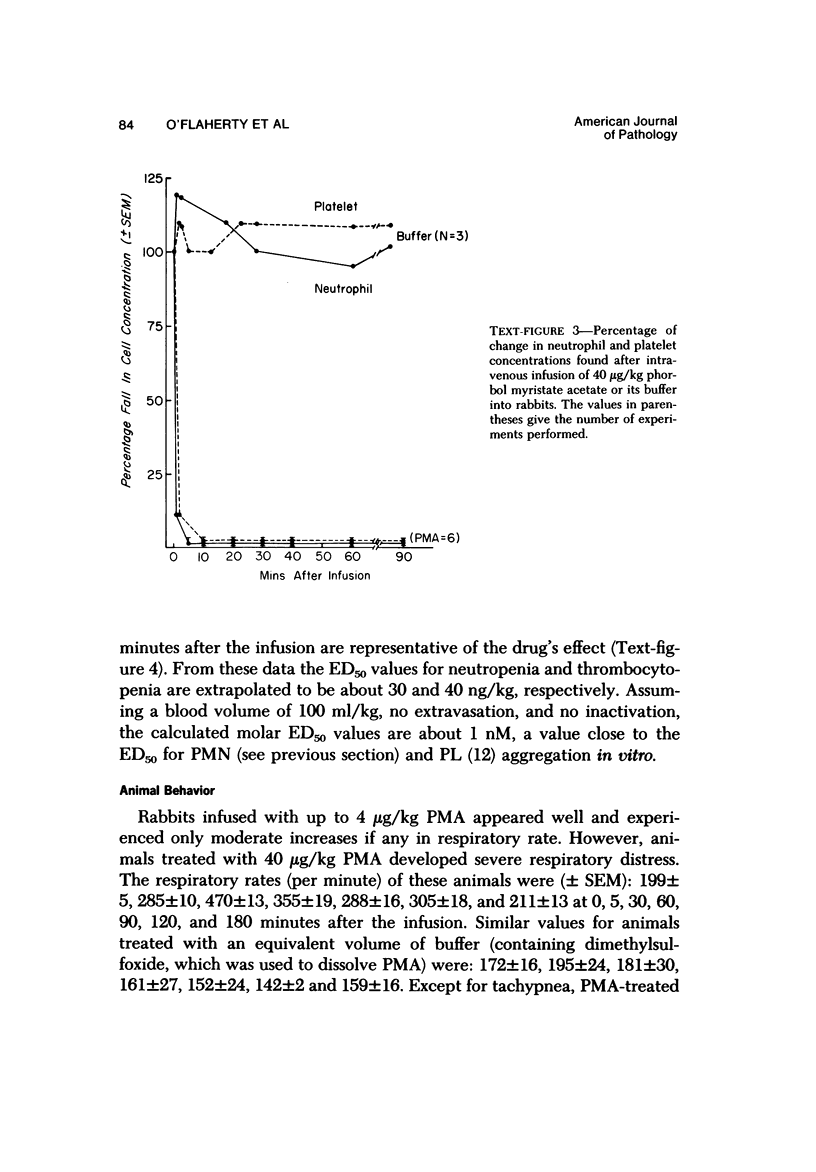
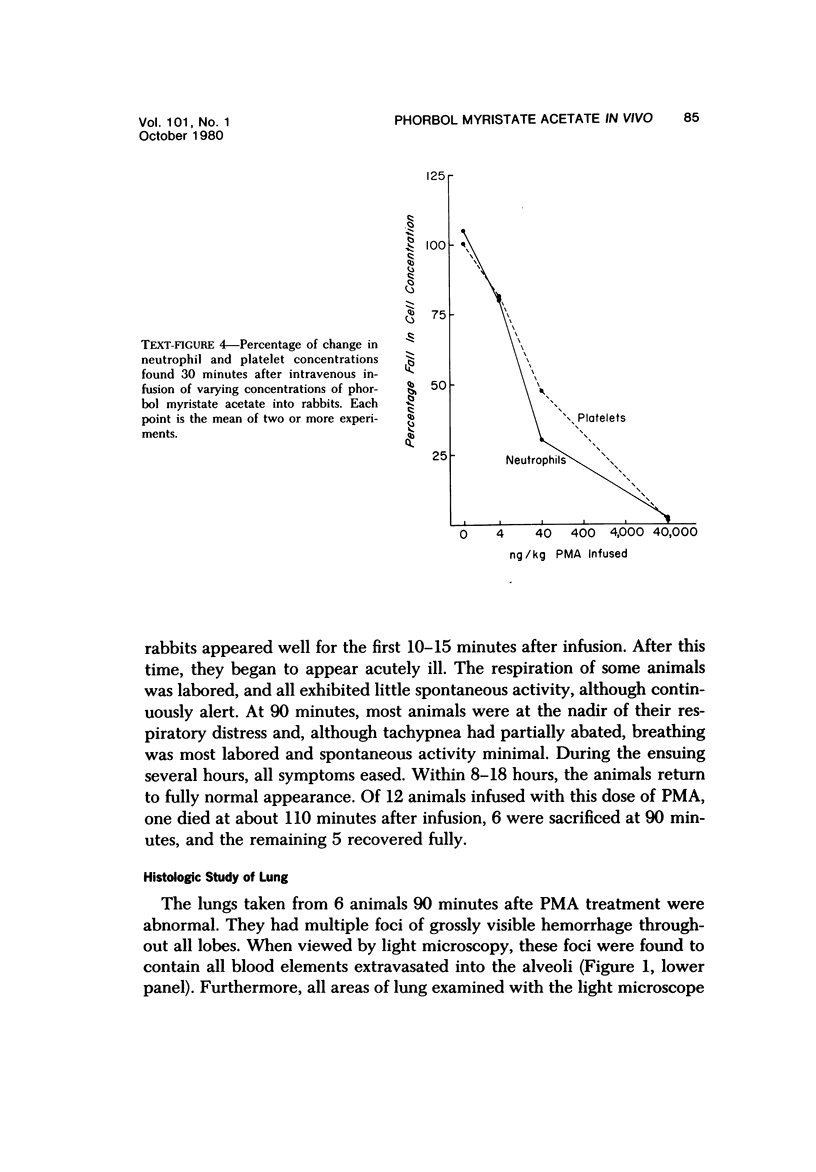
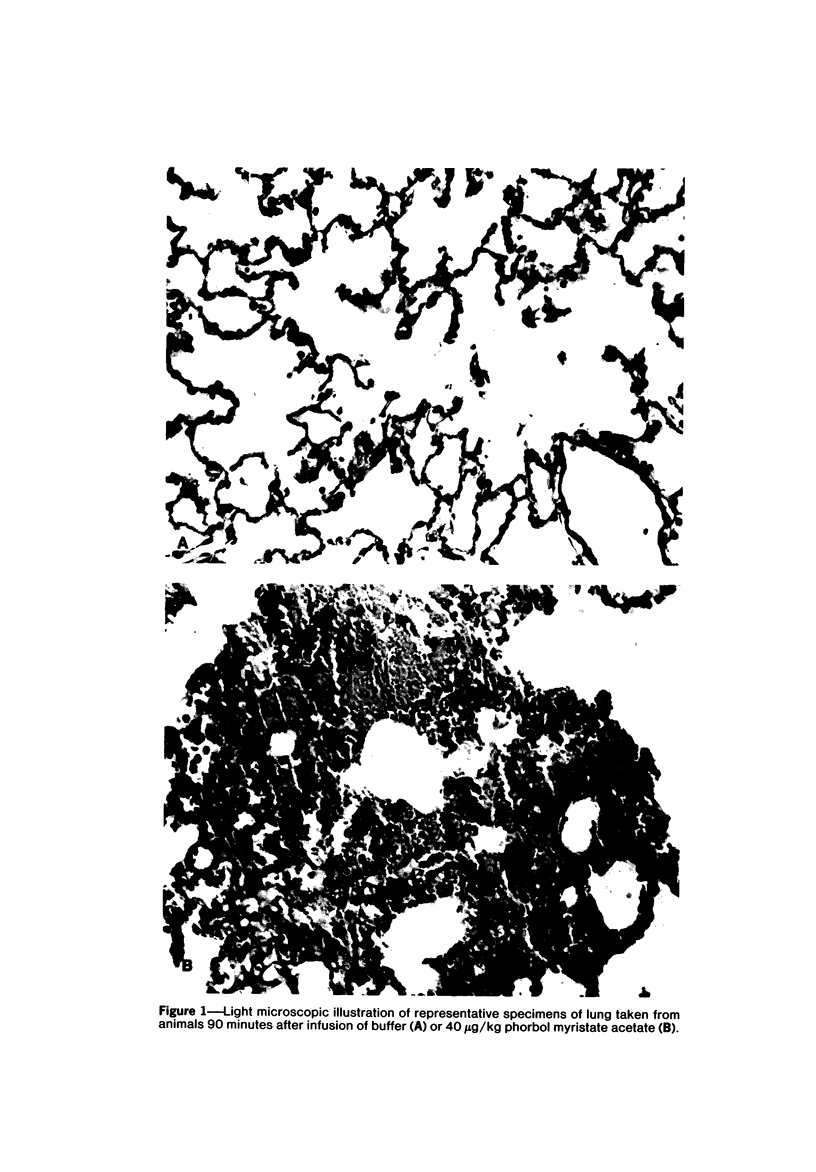
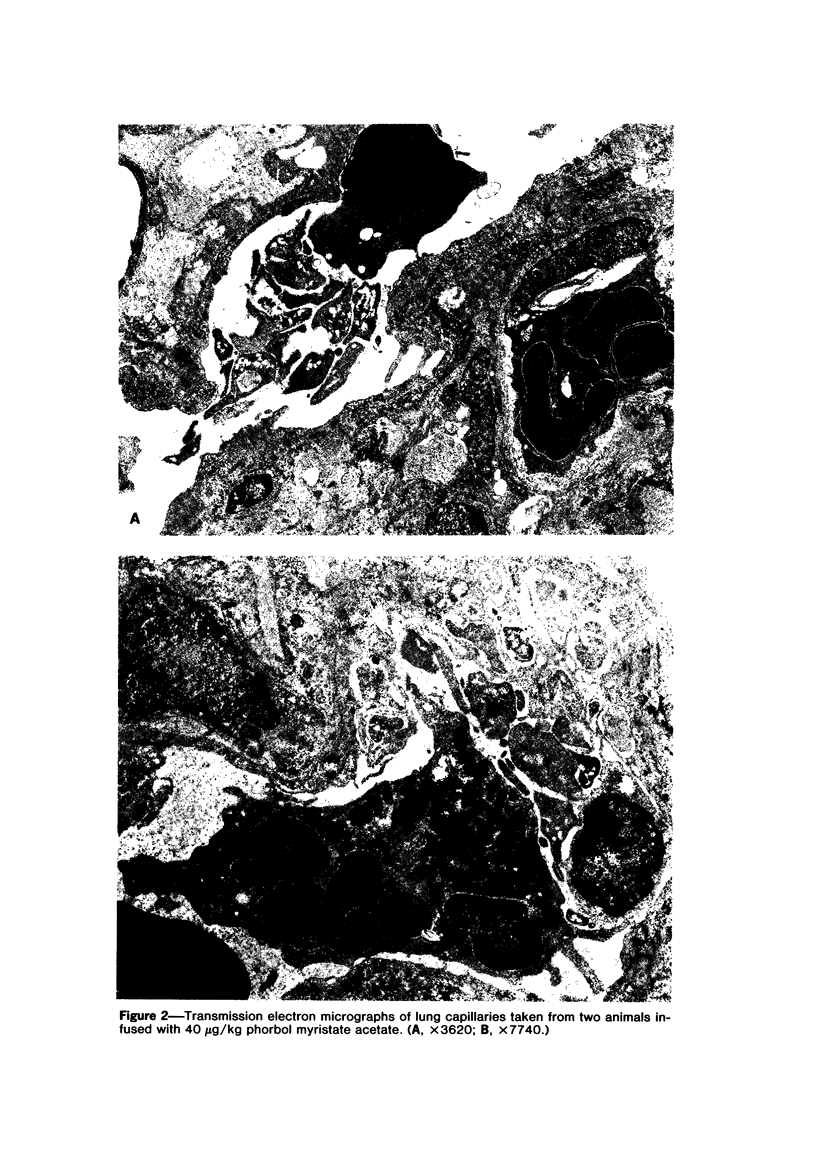
Phorbol myristate acetate is a potent aggregator of platelets. It was found that it was similarly potent in aggregating neutrophils and in producing striking thrombocytopenia and neutropenia when infused intravenously into rabbits. Aggregation and cytopenia were further correlated in that both types of responses developed abruptly and persisted for more than 90 minutes. Animals infused with 40 microgram/kg of the phorbol ester exhibited moderately severe respiratory distress. Their respiratory rate doubled shortly after the infusion, and this tachypnea persisted for more than 2 hours. At necroscopic examination, the lungs of these rabbits contained two outstanding abnormalities: numerous foci of alveolar hemorrhage and extensive intravascular accumulations of platelets and neutrophils. Thus, these animals had evidence of increased permeability and potential occlusion of the pulmonary microvasculature. Increased permeability, occlusion of lung blood vessels, or the occurrence of both processes was further indicated in studies on animals pre-infused with the plasma protein marker 125I-albumin: animals infused with the phorbol ester had a significantly increased amount of this label in their lungs in spite of thorough postmortem perfusion of their pulmonary vasculature with saline and fixative. We conclude that phorbol myristate acetate has actions in vivo that resemble those of a variety of other platelet (eg, arachidonic acid) and neutrophil (eg, chemotactic factors) aggregating agents that cause cytopenia and lung dysfunction. However, compared with these other agents, the phorbol ester produces respiratory distress of intermediate severity and greater duration. The drug, therefore, induces a syndrome that more closely resembles that seen in a variety of clinical and experimental conditions that associate shocklike states with cytopenia and lung dysfunction. It may serve as a useful tool in the study of the pathophysiology of these states as well as in those produced by other aggregating agents.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bass D. A., Dechatelet L. R., McCall C. E. Independent stimulation of motility and the oxidative metabolic burst of human polymorphonuclear leukocytes. J Immunol. 1978 Jul;121(1):172–178. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Clowes G. H., Jr, Zuschneid W., Dragacevic S., Turner M. The nonspecific pulmonary inflammatory reactions leading to respiratory failure after shock, gangrene, and sepsis. J Trauma. 1968 Sep;8(5):899–914. doi: 10.1097/00005373-196809000-00040. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., White J. G. Ultrastructural features on the platelet response to phorbol myristate acetate. Am J Pathol. 1974 Mar;74(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Influence of divalent cations upon complement-mediated enzyme release from human polymorphonuclear leukocytes. J Immunol. 1975 Sep;115(3):665–670. [PubMed] [Google Scholar]
- Goldstein I. M., Hoffstein S. T., Weissmann G. Mechanisms of lysosomal enzyme release from human polymorphonuclear leukocytes. Effects of phorbol myristate acetate. J Cell Biol. 1975 Sep;66(3):647–652. doi: 10.1083/jcb.66.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Arachidonic acid induced degranulation of rabbit peritoneal neutrophils. Biochem Biophys Res Commun. 1979 Mar 15;87(1):292–299. doi: 10.1016/0006-291x(79)91678-4. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Craddock P. R., Jacob H. S. Effect of intravascular complement activation on granulocyte adhesiveness and distribution. Blood. 1978 Apr;51(4):731–739. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Neutrophil aggregation and swelling induced by chemotactic agents. J Immunol. 1977 Jul;119(1):232–239. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. The influence of chemotactic factors on neutrophil adhesiveness. Inflammation. 1978 Mar;3(1):37–48. doi: 10.1007/BF00917320. [DOI] [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. J., Becker E. L., Ward P. A. Neutrophil aggregation and degranulation. Effect of arachidonic acid. Am J Pathol. 1979 May;95(2):433–444. [PMC free article] [PubMed] [Google Scholar]
- Pinckard R. N., Farr R. S., Hanahan D. J. Physicochemical and functional identity of rabbit platelet-activating factor (PAF) released in vivo during IgE anaphylaxis with PAF released in vitro from IgE sensitized basophils. J Immunol. 1979 Oct;123(4):1847–1857. [PubMed] [Google Scholar]
- Pinckard R. N., Halonen M., Palmer J. D., Butler C., Shaw J. O., Henson P. M. Intravascular aggregation and pulmonary sequestration of platelets during IgE-induced systemic anaphylaxis in the rabbit: abrogation of lethal anaphylactic shock by platelet depletion. J Immunol. 1977 Dec;119(6):2185–2193. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M. J., Hoch W., Kocsis J. J., Ingerman C. M., Smith J. B. Arachidonic acid causes sudden death in rabbits. Science. 1974 Mar 15;183(4129):1085–1087. doi: 10.1126/science.183.4129.1085. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Stimler N. P., Hugli T. E., Bloor C. M. Pulmonary injury induced by C3a and C5a anaphylatoxins. Am J Pathol. 1980 Aug;100(2):327–348. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Estensen R. D. Cytochemical electron microscopic studies of the action of phorbol myristate acetate on platelets. Am J Pathol. 1974 Mar;74(3):453–466. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Estensen R. D. Selective labilization of specific granules in polymorphonuclear leukocytes by phorbol myristate acetate. Am J Pathol. 1974 Apr;75(1):45–60. [PMC free article] [PubMed] [Google Scholar]
- White J. G., Rao G. H., Estensen R. D. Investigation of the release reaction in platelets exposed to phorbol myristate acetate. Am J Pathol. 1974 May;75(2):301–314. [PMC free article] [PubMed] [Google Scholar]
- Willis A. L. Isolation of a chemical trigger for thrombosis. Prostaglandins. 1974 Jan 10;5(1):1–25. doi: 10.1016/s0090-6980(74)80126-7. [DOI] [PubMed] [Google Scholar]
- Willis A. L., Vane F. M., Kuhn D. C., Scott C. G., Petrin M. An endoperoxide aggregator (Lass), formed in platelets in response to thrombotic stimuli: purification, identification and unique biological significance. Prostaglandins. 1974 Dec 25;8(6):453–507. doi: 10.1016/0090-6980(74)90062-8. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Troll W., Belman S. The tumor-promoter phorbol ester (12-O-tetradecanoyl-phorbol-13-acetate), a potent aggregating agent for blood platelets. J Cell Biol. 1974 Feb;60(2):325–336. doi: 10.1083/jcb.60.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]