Abstract
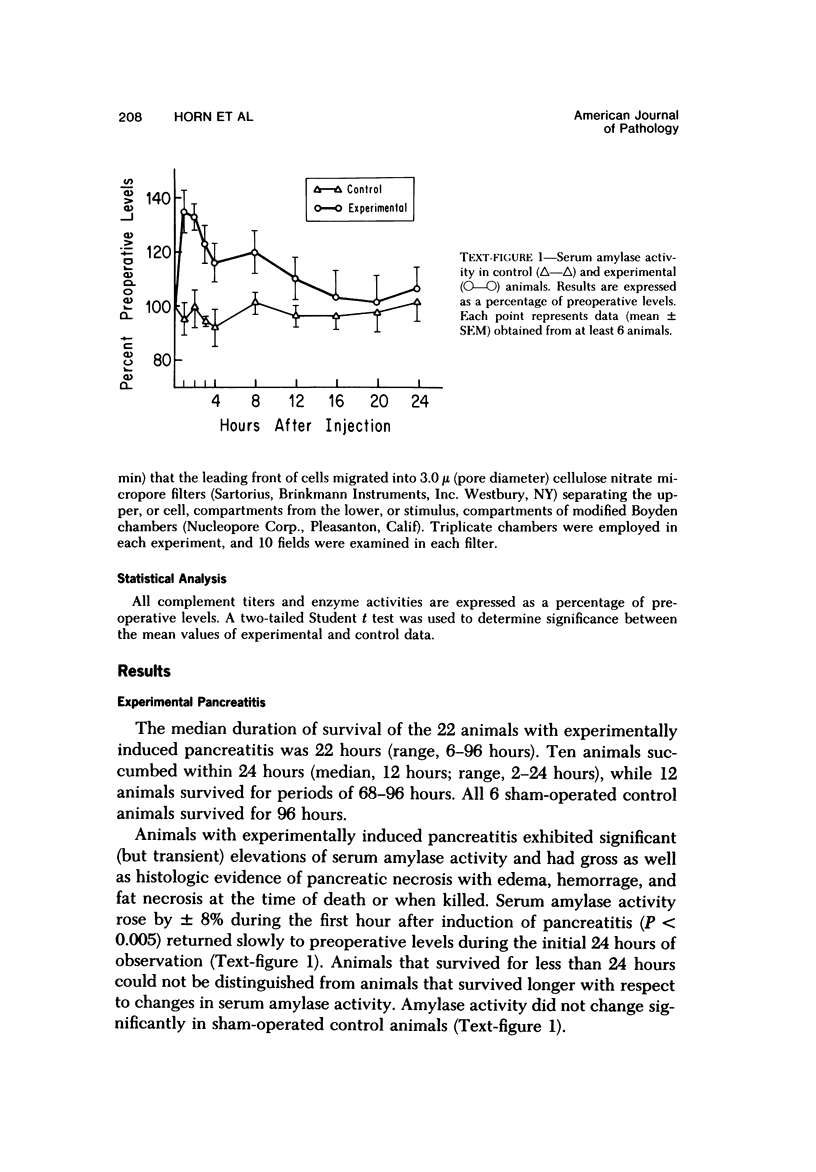
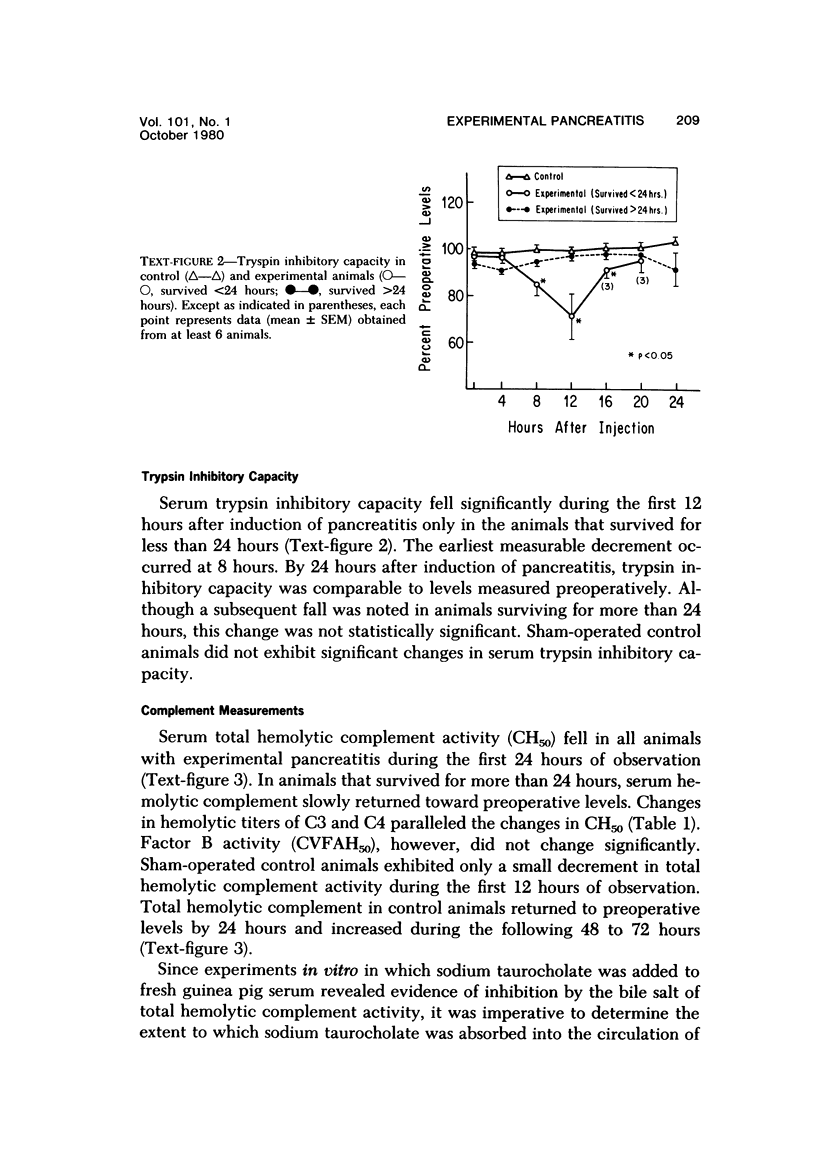
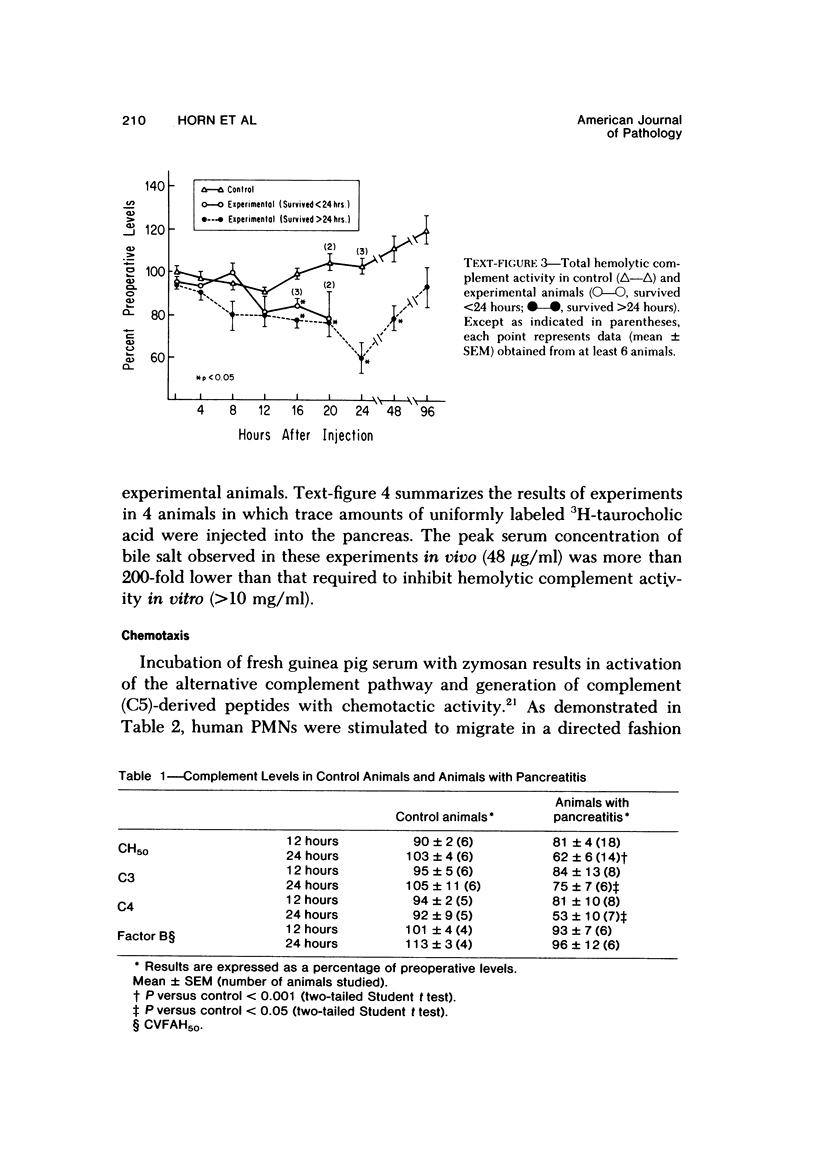
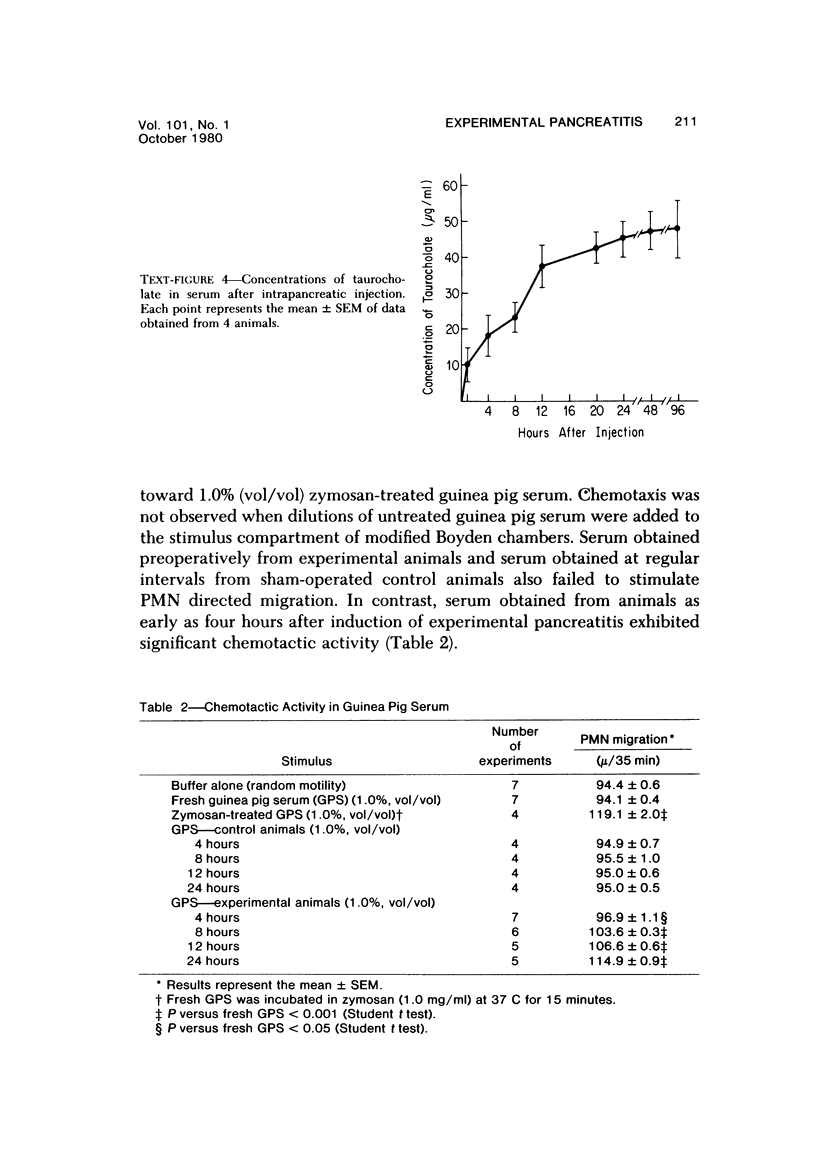
Serum specimens from guinea pigs with experimentally induced acute pancreatitis were examined for evidence of protease-antiprotease imbalance and complement catabolism. Pancreatitis was induced in 22 male Hartley guinea pigs by the injection of sodium taurocholate into the pancreatic parenchyma. Only a laparotomy was performed in 6 control animals. In 10 experimental animals that survived for less than 24 hours, there was a significant, early reduction of serum trypsin inhibitory capacity (a measure of antiprotease activity). Levels of total hemolytic complement as well as titers of hemolytic C3 and C4 fell significantly in all experimental animals during the first 24 hours. Factor B activity, however, did not change. Only serum from experimental animals contained chemotactic activity for human polymorphonuclear leukocytes. These findngs indicate that circulating complement components are cleaved during the course of experimental acute pancreatitis. As a consequence, complement-derived peptides are generated that may mediate local and systemic tissue injury.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNFELD P. Enzymes of starch degradation and synthesis. Adv Enzymol Relat Subj Biochem. 1951;12:379–428. doi: 10.1002/9780470122570.ch7. [DOI] [PubMed] [Google Scholar]
- Bawnik J. B., Orda R., Wiznitzer T. Acute necrotizing pancreatitis. An experimental model. Am J Dig Dis. 1974 Dec;19(12):1143–1147. doi: 10.1007/BF01076150. [DOI] [PubMed] [Google Scholar]
- Brai M., Osler A. G. Studies of the C3 shunt activation in cobra venom induced lysis of unsensitized erythrocytes. Proc Soc Exp Biol Med. 1972 Jul;140(3):1116–1121. doi: 10.3181/00379727-140-36623. [DOI] [PubMed] [Google Scholar]
- Budzko D. B., Müller-Eberhard H. J. Cleavage of the fourth component of human complement (C4) by C1 esterase: isolation and characteristics of the low molecular weight product. Immunochemistry. 1970 Feb;7(2):227–234. doi: 10.1016/0019-2791(70)90158-8. [DOI] [PubMed] [Google Scholar]
- Carey L. C. Extra-abdominal manifestations of acute pancreatitis. Surgery. 1979 Aug;86(2):337–342. [PubMed] [Google Scholar]
- Clark R. A., Frank M. M., Kimball H. R. Generation of chemotactic factors in guinea pig serum via activation of the classical and alternate complement pathways. Clin Immunol Immunopathol. 1973 Apr;1(3):414–426. doi: 10.1016/0090-1229(73)90058-5. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968 Feb 1;127(2):371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Dalmasso A. P., Brighan K. L., Jacob H. S. Hemodialysis leukopenia. Pulmonary vascular leukostasis resulting from complement activation by dialyzer cellophane membranes. J Clin Invest. 1977 May;59(5):879–888. doi: 10.1172/JCI108710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock P. R., Hammerschmidt D., White J. G., Dalmosso A. P., Jacob H. S. Complement (C5-a)-induced granulocyte aggregation in vitro. A possible mechanism of complement-mediated leukostasis and leukopenia. J Clin Invest. 1977 Jul;60(1):260–264. doi: 10.1172/JCI108763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLANGER B. F., KOKOWSKY N., COHEN W. The preparation and properties of two new chromogenic substrates of trypsin. Arch Biochem Biophys. 1961 Nov;95:271–278. doi: 10.1016/0003-9861(61)90145-x. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Ruddy S., Schur P. H., McCabe W. R. Activation of the properdin pathway of complement in patients with gram-negative of bacteremia. N Engl J Med. 1975 May 1;292(18):937–940. doi: 10.1056/NEJM197505012921802. [DOI] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gaither T. A., Alling D. W., Frank M. M. A new one-step method for the functional assay of the fourth component (C4) of human and guinea pig complement. J Immunol. 1974 Aug;113(2):574–583. [PubMed] [Google Scholar]
- Geokas M. C., Brodrick J. W., Johnson J. H., Largman C. Pancreatic elastase in human serum. Determination by radioimmunoassay. J Biol Chem. 1977 Jan 10;252(1):61–67. [PubMed] [Google Scholar]
- Geokas M. C., Wollesen F., Rinderknecht H. Radioimmunoassay for pancreatic carboxypeptidase B in human serum. J Lab Clin Med. 1974 Oct;84(4):574–583. [PubMed] [Google Scholar]
- Goldstein I. M., Cala D., Radin A., Kaplan H. B., Horn J., Ranson J. Evidence of complement catabolism in acute pancreatitis. Am J Med Sci. 1978 May-Jun;275(3):257–264. doi: 10.1097/00000441-197805000-00003. [DOI] [PubMed] [Google Scholar]
- Hahn-Pedersen J., Sorensen H., Kehlet H. Complement activation during surgical procedures. Surg Gynecol Obstet. 1978 Jan;146(1):66–68. [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Ohlsson K. Experimental pancreatitis in the dog. Appearance of complexes between proteases and trypsin inhibitors in ascitic fluid, lymph, and plasma. Scand J Gastroenterol. 1971;6(7):645–652. doi: 10.3109/00365527109181147. [DOI] [PubMed] [Google Scholar]
- Perez H. D., Lipton M., Goldstein I. M. A specific inhibitor of complement (C5)-derived chemotactic activity in serum from patients with systemic lupus erythematosus. J Clin Invest. 1978 Jul;62(1):29–38. doi: 10.1172/JCI109110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson J. H., Roses D. F., Fink S. D. Early respiratory insufficiency in acute pancreatitis. Ann Surg. 1973 Jul;178(1):75–79. doi: 10.1097/00000658-197307000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson J. H., Turner J. W., Roses D. F., Rifkind K. M., Spencer F. C. Respiratory complications in acute pancreatitis. Ann Surg. 1974 May;179(5):557–566. doi: 10.1097/00000658-197405000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig R., Lankisch P. G., Koop H., Winckler K., Kaboth U., Seelig H. P. Complement system in sodium taurocholate pancreatitis in the rat. Res Exp Med (Berl) 1978 Dec 27;174(1):57–65. doi: 10.1007/BF01851939. [DOI] [PubMed] [Google Scholar]
- Seelig R., Seelig H. P. Complement-mediated acinar cell necroses in pancreatitis induced by basement membrane antibodies. Virchows Arch A Pathol Anat Histol. 1976 Aug 19;371(1):69–77. doi: 10.1007/BF00433716. [DOI] [PubMed] [Google Scholar]
- Vallota E. H., Müller-Eberhard H. J. Formation of C3a and C5a anaphylatoxins in whole human serum after inhibition of the anaphylatoxin inactivator. J Exp Med. 1973 May 1;137(5):1109–1123. doi: 10.1084/jem.137.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R., Hourithane D. O. Systemic candidiasis at autopsy in Dublin. Ir J Med Sci. 1977 Jan;146(1):1–5. doi: 10.1007/BF03030918. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]


