Abstract
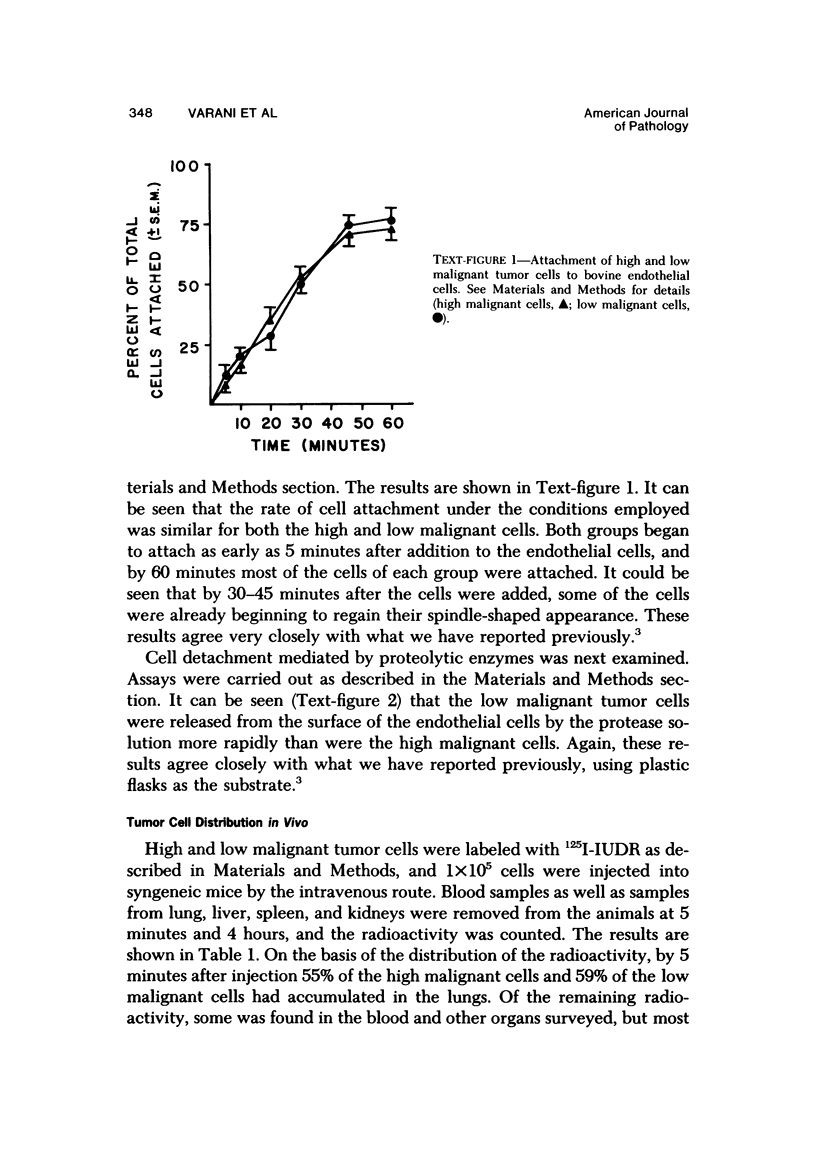
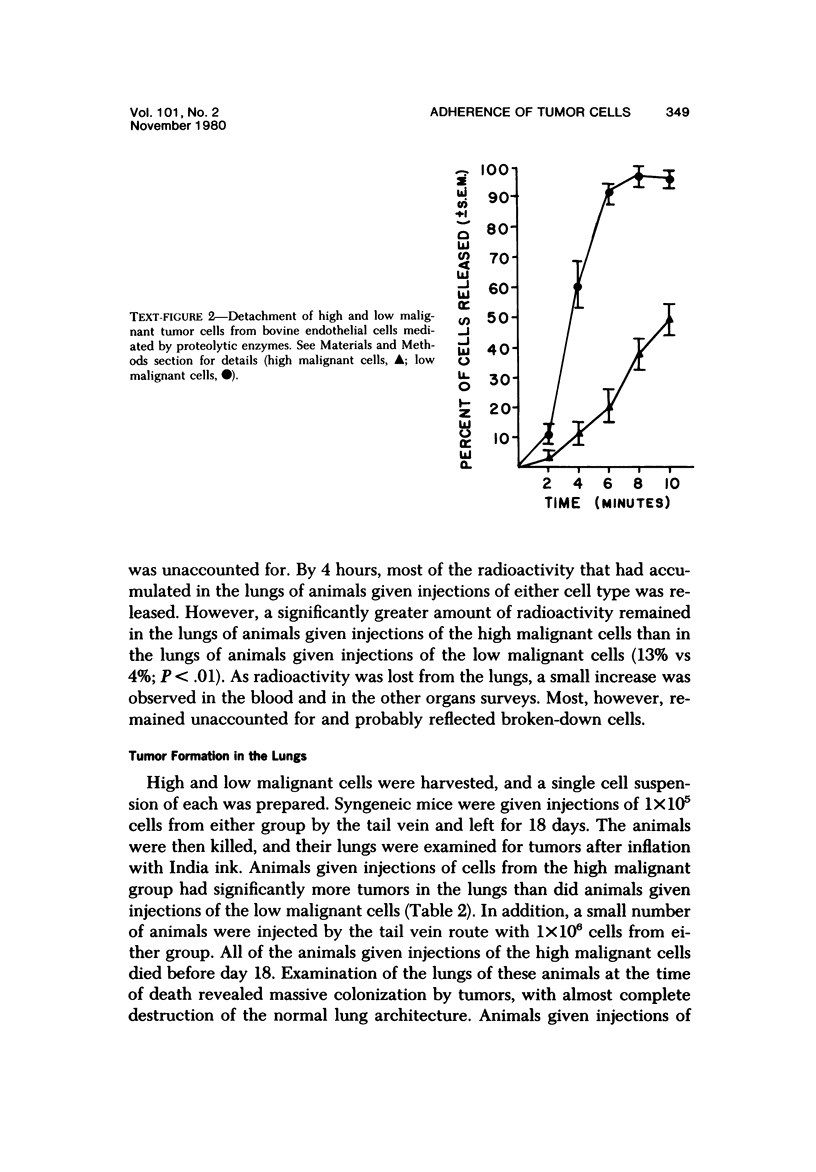
Murine fibrosarcoma variants that differed greatly in tumorgenicity, in vivo growth rate, and rate of spontaneous metastasis formation were compared for their ability to induce tumors in the lungs of syngeneic mice after intravenous injection of a suspension of single cells. Significantly more tumors were observed in the lungs of mice that received 1 x 10(5) of the cells of high malignant potential than in the lungs of animals that received 1 x 10(5) cells of lower malignant potential (54 tumors per animal vs 3 tumors per animal). When the tumor cells were prelabeled in culture for 24 hours with 125I-IUDR prior to intravenous injection, it was found that both the "high" and "low malignant" cells rapidly accumulated in the lungs (55-59% of the radioactivity was found in the lung tissue by 5 minutes after injection). However, by 4 hours only 4% of the low malignant cells (as indicated by the amount of radioactivity present) were still in the lungs, while a significantly higher percentage (13%) of the high malignant cells were still present in the lungs. The difference between the high and low malignant cells with regard to ability to remain sequestered in the lungs of syngeneic mice and to subsequently form tumors in the lungs of these animals correlated with the ability of the cells to form stable attachments to monolayers of endothelial cells in culture. While both the high and low malignant cells attached at the same rate to monolayers of bovine endothelial cells, once the cells were attached, the low malignant cells were released by trypsin treatment more easily than the high malignant cells. These observations suggest that the difference in malignant potential between the variants may be due, at least in part, to differences in ability to form stable attachments.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker B. E., Sanford K. K. Cytologic manifestations of neoplastic transformation in vitro. J Natl Cancer Inst. 1970 Jan;44(1):39–63. [PubMed] [Google Scholar]
- Briles E. B., Kornfeld S. Isolation and metastatic properties of detachment variants of B16 melanoma cells. J Natl Cancer Inst. 1978 Jun;60(6):1217–1222. doi: 10.1093/jnci/60.6.1217. [DOI] [PubMed] [Google Scholar]
- Culp L. A., Black P. H. Release of macromolecules from BALB-c mouse cell lines treated with chelating agents. Biochemistry. 1972 May 23;11(11):2161–2172. doi: 10.1021/bi00761a024. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Biological behavior of malignant melanoma cells correlated to their survival in vivo. Cancer Res. 1975 Jan;35(1):218–224. [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J., Nicolson G. L. Tumor cell and host properties affecting the implantation and survival of blood-borne metastatic variants of B16 melanoma. Isr J Med Sci. 1978 Jan;14(1):38–50. [PubMed] [Google Scholar]
- Nicolson G. L., Winkelhake J. L. Organ specificity of blood-borne tumour metastasis determined by cell adhesion? Nature. 1975 May 15;255(5505):230–232. doi: 10.1038/255230a0. [DOI] [PubMed] [Google Scholar]
- Shields R., Pollock K. The adhesion of BHK and PyBHK cells to the substratum. Cell. 1974 Sep;3(1):31–38. doi: 10.1016/0092-8674(74)90034-8. [DOI] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Adhesive characteristics of tumor cell variants of high and low tumorigenic potential. J Natl Cancer Inst. 1980 May;64(5):1173–1178. [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Comparison of subpopulations of tumor cells with altered migratory activity, attachment characteristics, enzyme levels and in vivo behavior. Eur J Cancer. 1979 Apr;15(4):585–592. doi: 10.1016/0014-2964(79)90096-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Hydrolytic enzyme activities, migratory activity, and in vivo growth and metastatic potential of recent tumor isolates. Cancer Res. 1979 Jul;39(7 Pt 1):2376–2380. [PubMed] [Google Scholar]
- Wexler H. Accurate identification of experimental pulmonary metastases. J Natl Cancer Inst. 1966 Apr;36(4):641–645. doi: 10.1093/jnci/36.4.641. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Determination of adhesive properties of variant metastatic melanoma cells to BALB/3T3 cells and their virus-transformed derivatives by a monolayer attachment assay. J Natl Cancer Inst. 1976 Feb;56(2):285–291. doi: 10.1093/jnci/56.2.285. [DOI] [PubMed] [Google Scholar]


