Abstract
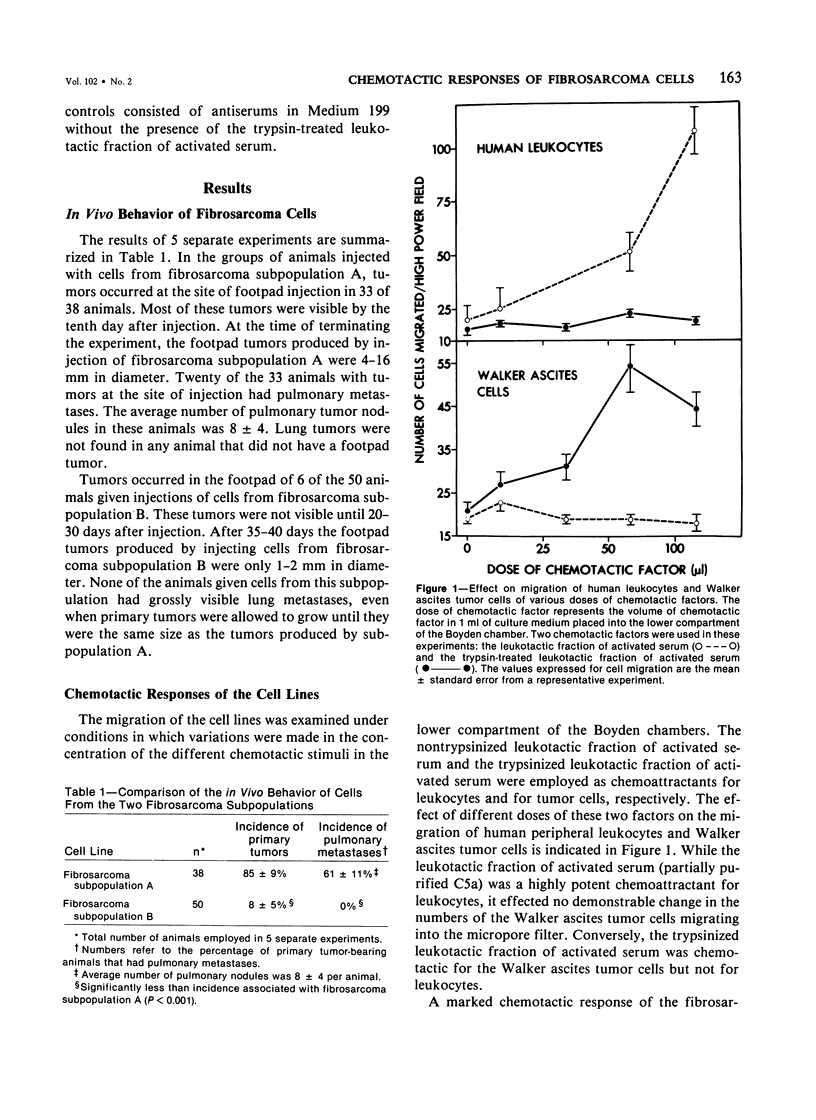
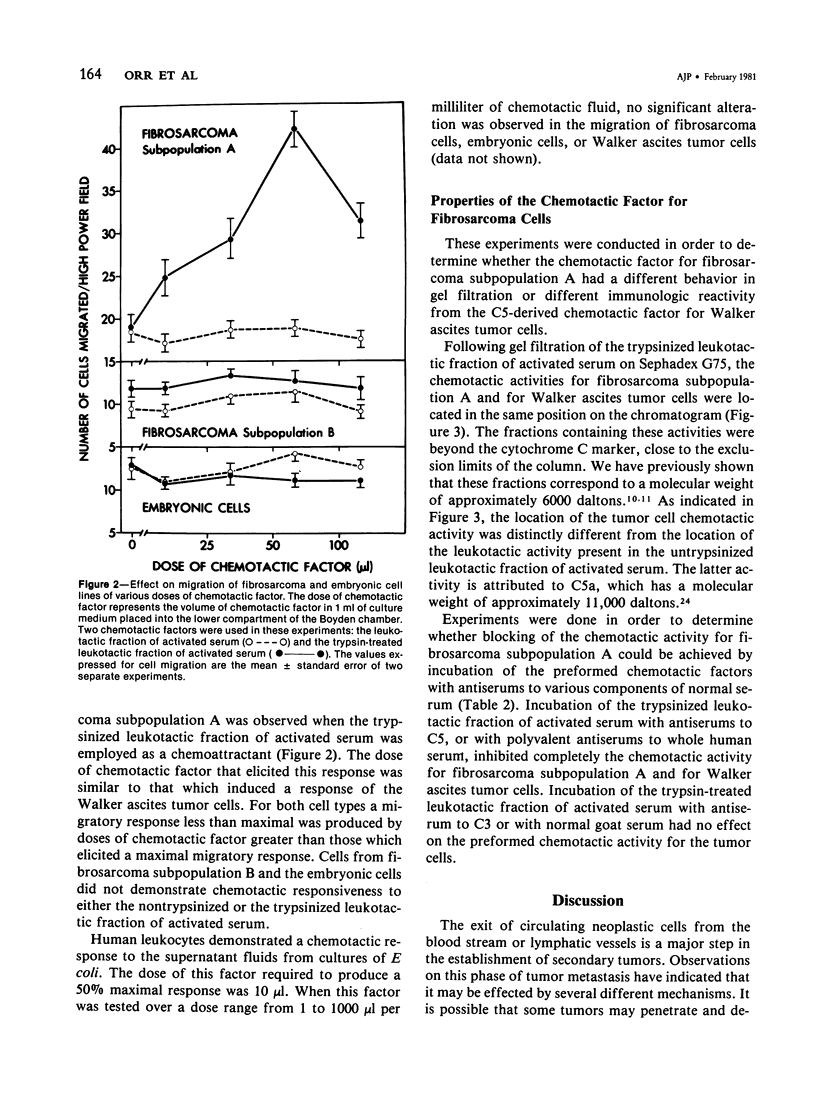
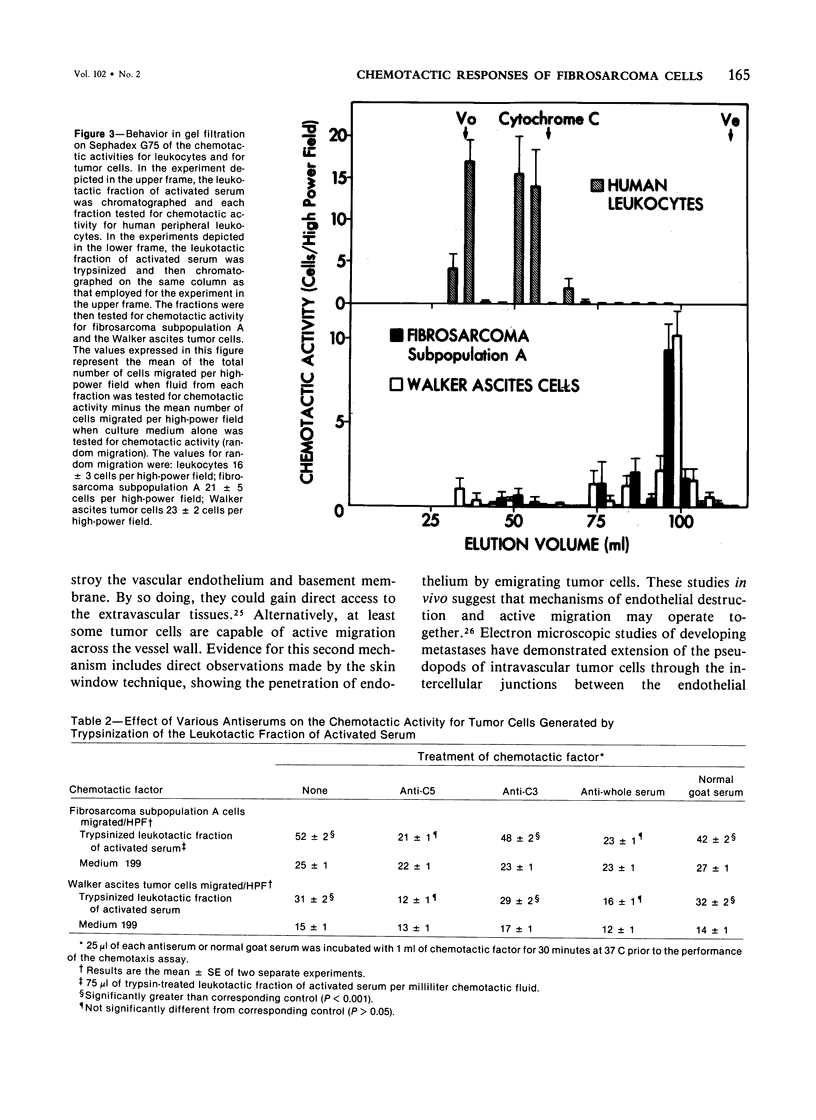
There are several points of similarity between the processes of cancer metastasis and inflammation. In both, cells circulate in the vasculature, arrest, and cross vessel walls, thereby entering the extravascular tissues. In vitro, leukocytes and some, but not all, tumor cells exhibit chemotaxis. Since the chemotactic response of leukocytes effect their transvascular migration, we propose that chemotactic responsiveness contributes to the ability of circulating tumor cells to localize in extravascular tissues. This study was done to seek a relationship between chemotactic responsiveness of tumor cells and their behavior in vivo. Two subpopulations of cells were isolated from a methylcholanthrene-induced fibrosarcoma. The two cell lines were compared with regard to their biologic behavior in vivo and their chemotactic responsiveness in vitro. In vivo one subpopulation was highly malignant. An injection of 2.0 x 10(5) cells into the footpad of syngeneic mice led to the development of primary tumors in 87% of the animals and lung metastases in 61% of the animals with primary tumors. This line demonstrated chemotaxis to a factor that behaved similarly in gel filtration and showed immunologic reactivity similar to that of a previously described tumor cell chemotactic factor derived from the fifth component of complement. In contrast, an injection of the same number of cells from the second subpopulation of fibrosarcoma cells led to the development of primary tumors in only 12% of syngeneic mice, and lung metastases did not occur. Neither this subpopulation nor normal embryonic fibroblasts demonstrated chemotactic responsiveness. We postulate that the ability of tumor cells to respond to specific chemotactic stimuli may be one of the many unique properties which distinguish malignant from benign tumor cells. This is the first report documenting the chemotactic responsiveness of non-ascites tumors and fibrosarcomas.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dingemans K. P. Invasion of liver tissue by blood-borne mammary carcinoma cells. J Natl Cancer Inst. 1974 Dec;53(6):1813–1824. doi: 10.1093/jnci/53.6.1813. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Quantitative characteristics of the transformation of hamster cells by PARA (defective simian virus 40)-adenovirus 7. J Virol. 1970 May;5(5):568–577. doi: 10.1128/jvi.5.5.568-577.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Fidler I. J., Cifone M. A. Properties of metastatic and nonmetastatic cloned subpopulations of an ultraviolet-light-induced murine fibrosarcoma of recent origin. Am J Pathol. 1979 Dec;97(3):633–648. [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Hart I. R. The selection and characterization of an invasive variant of the B16 melanoma. Am J Pathol. 1979 Dec;97(3):587–600. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCHESI V. T. The site of leucocyte emigration during inflammation. Q J Exp Physiol Cogn Med Sci. 1961 Apr;46:115–118. doi: 10.1113/expphysiol.1961.sp001522. [DOI] [PubMed] [Google Scholar]
- Mundy G. R., Varani J., Orr W., Gondek M. D., Ward P. A. Resorbing bone is chemotactic for monocytes. Nature. 1978 Sep 14;275(5676):132–135. doi: 10.1038/275132a0. [DOI] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Gondek M. D., Ward P. A., Mundy G. R. Partial characterization of a bone-derived chemotactic factor for tumor cells. Am J Pathol. 1980 Apr;99(1):43–52. [PMC free article] [PubMed] [Google Scholar]
- Orr F. W., Varani J., Kreutzer D. L., Senior R. M., Ward P. A. Digestion of the fifth component of complement by leukocyte enzymes. Sequential generation of chemotactic activities for leukocytes and for tumor cells. Am J Pathol. 1979 Jan;94(1):75–83. [PMC free article] [PubMed] [Google Scholar]
- Orr W., Phan S. H., Varani J., Ward P. A., Kreutzer D. L., Webster R. O., Henson P. M. Chemotactic factor for tumor cells derived from the C5a fragment of complement component C5. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1986–1989. doi: 10.1073/pnas.76.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr W., Varani J., Ward P. A. Characteristics of the chemotactic response of neoplastic cells to a factor derived from the fifth component of complement. Am J Pathol. 1978 Nov;93(2):405–422. [PMC free article] [PubMed] [Google Scholar]
- Ozaki T., Yoshida K., Ushijima K., Hayashi H. Studies on the mechanisms of invasion in cancer. II. In vivo effects of a factor chemotactic for cancer cells. Int J Cancer. 1971 Jan 15;7(1):93–100. doi: 10.1002/ijc.2910070111. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Seyer J. M., Kang A. H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc Natl Acad Sci U S A. 1978 Feb;75(2):871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. Generation of a fibroblast chemotactic factor in serum by activation of complement. J Clin Invest. 1979 Nov;64(5):1379–1385. doi: 10.1172/JCI109595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Snyderman R., Kang A. H. The chemotactic attraction of human fibroblasts to a lymphocyte-derived factor. J Exp Med. 1976 Nov 2;144(5):1188–1203. doi: 10.1084/jem.144.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romualdez A. G., Jr, Ward P. A. A unique complement derived chemotactic factor for tumor cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4128–4132. doi: 10.1073/pnas.72.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romualdez A. G., Ward P. A. Further studies on the C5-derived chemotactic factor for tumor cells. Prog Clin Biol Res. 1976;9:65–71. [PubMed] [Google Scholar]
- Sindelar W. F., Tralka T. S., Ketcham A. S. Electron microscopic observations on formation of pulmonary metastases. J Surg Res. 1975 Feb;18(2):137–161. doi: 10.1016/0022-4804(75)90010-4. [DOI] [PubMed] [Google Scholar]
- Sugarbaker E. V., Ketcham A. S. Mechanisms and prevention of cancer dissemination: an overview. Semin Oncol. 1977 Mar;4(1):19–32. [PubMed] [Google Scholar]
- Vallota E. H., Müller-Eberhard H. J. Formation of C3a and C5a anaphylatoxins in whole human serum after inhibition of the anaphylatoxin inactivator. J Exp Med. 1973 May 1;137(5):1109–1123. doi: 10.1084/jem.137.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. A comparison of the migration patterns of normal and malignant cells in two assay systems. Am J Pathol. 1978 Jan;90(1):159–172. [PMC free article] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Comparison of cell attachment and caseinolytic activities of five tumour cell types. J Cell Sci. 1978 Dec;34:133–144. doi: 10.1242/jcs.34.1.133. [DOI] [PubMed] [Google Scholar]
- Varani J., Orr W., Ward P. A. Comparison of subpopulations of tumor cells with altered migratory activity, attachment characteristics, enzyme levels and in vivo behavior. Eur J Cancer. 1979 Apr;15(4):585–592. doi: 10.1016/0014-2964(79)90096-3. [DOI] [PubMed] [Google Scholar]
- WOOD S., Jr Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch Pathol. 1958 Oct;66(4):550–568. [PubMed] [Google Scholar]
- Ward P. A., Lepow I. H., Newman L. J. Bacterial factors chemotactic for polymorphonuclear leukocytes. Am J Pathol. 1968 Apr;52(4):725–736. [PMC free article] [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Complement-derived leukotactic factors in inflammatory synovial fluids of humans. J Clin Invest. 1971 Mar;50(3):606–616. doi: 10.1172/JCI106531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. A pathobiologic overview of metastasis. Semin Oncol. 1977 Mar;4(1):5–17. [PubMed] [Google Scholar]
- Wiener S., Lendvai S., Rogers B., Urivetzky M., Meilman E. Nonimmune chemotaxis in vivo: inhibition by complement depletion with cobra factor. Am J Pathol. 1973 Dec;73(3):807–816. [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., Ozaki T., Ushijima K., Hayashi H. Studies on the mechanisms of invasion in cancer. I. Isolation and purification of a factor chemotactic for cancer cells. Int J Cancer. 1970 Jul 15;6(1):123–132. doi: 10.1002/ijc.2910060116. [DOI] [PubMed] [Google Scholar]


