Abstract
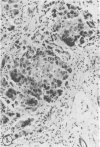
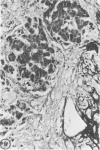
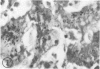
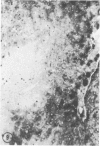
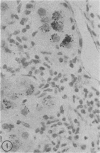
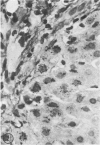
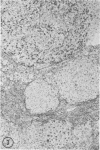
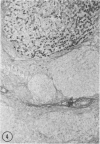
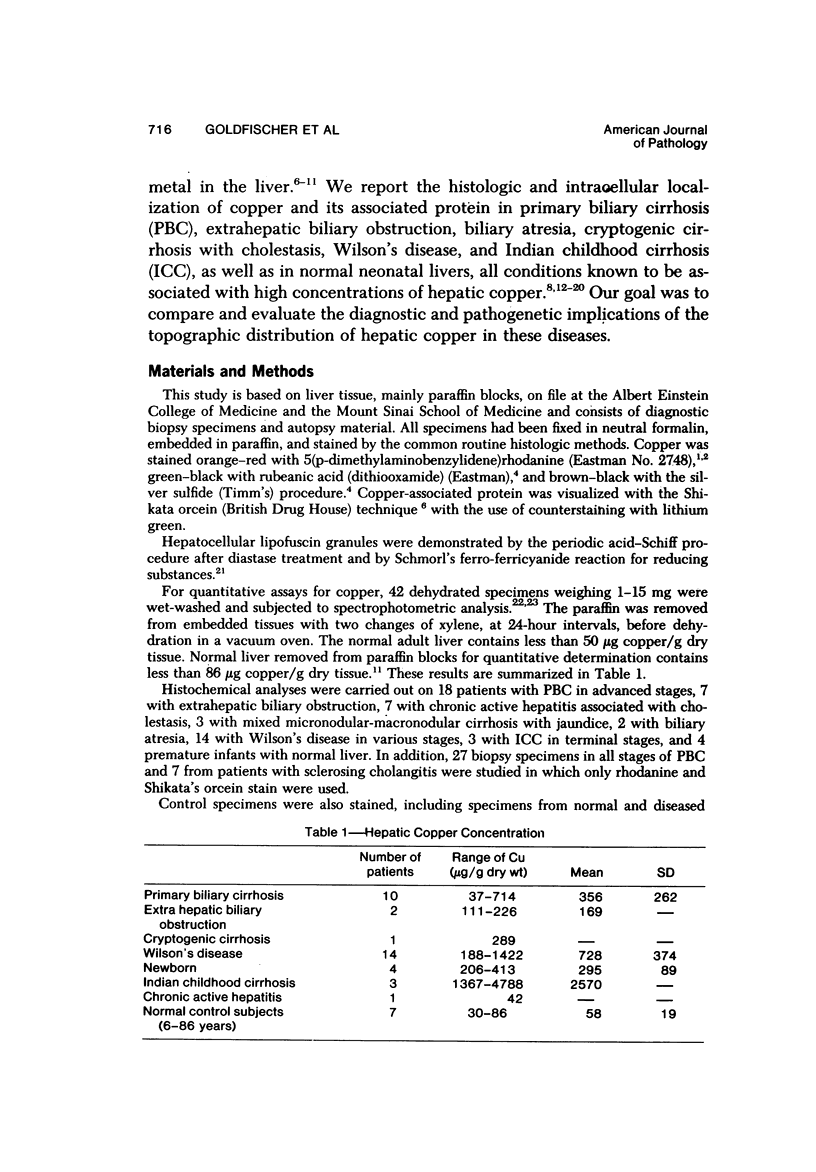
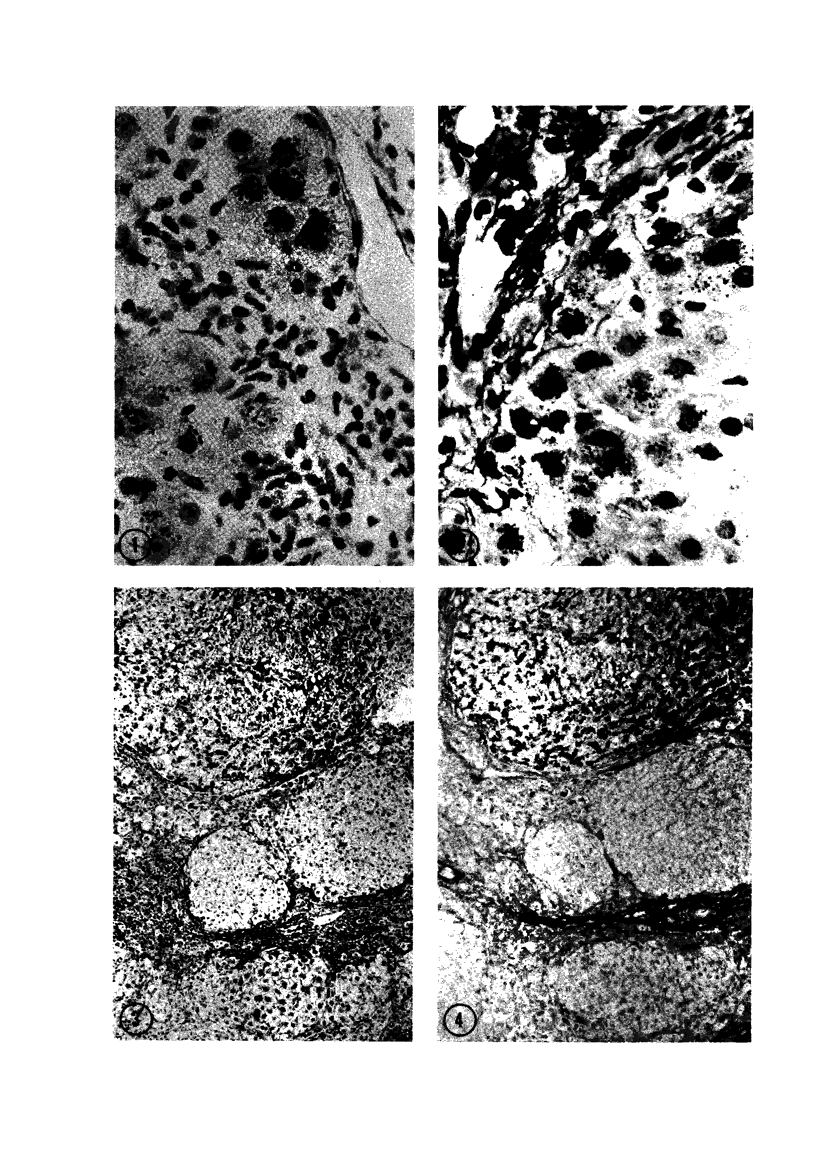
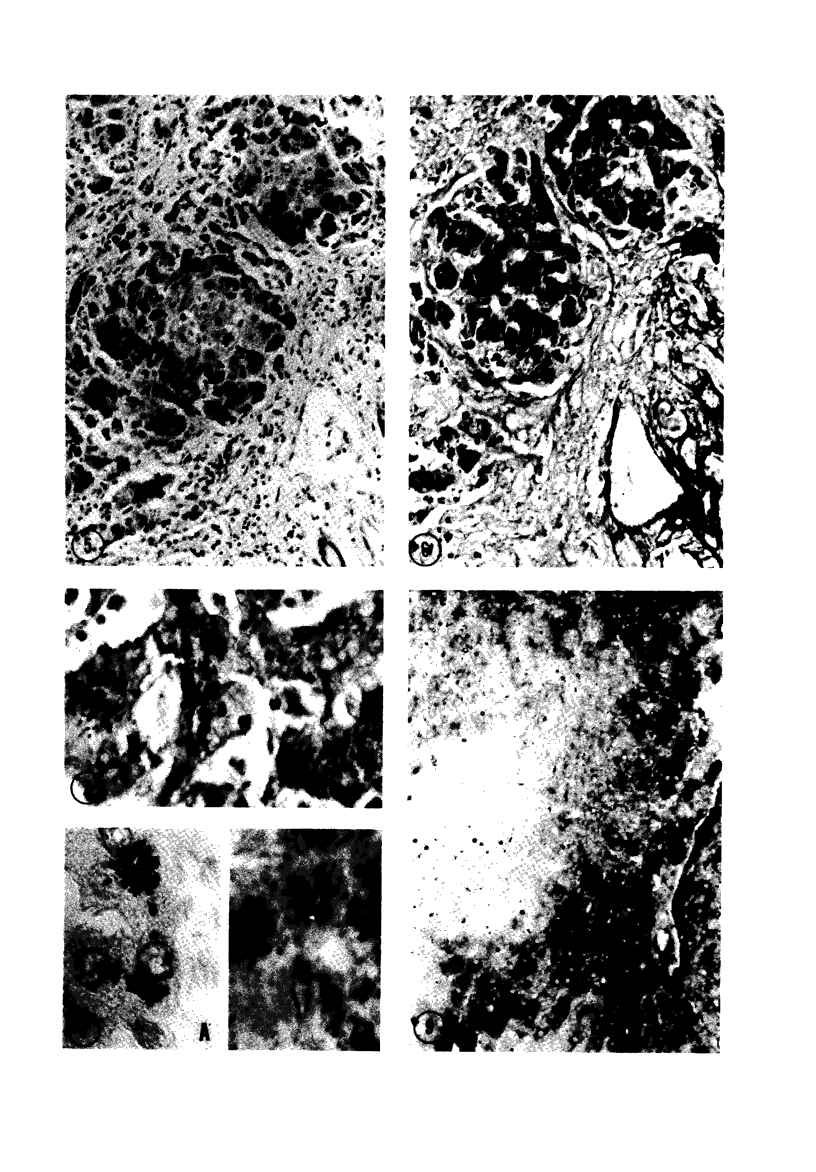
Biopsy and autopsy specimens of liver from patients with Wilson's disease in various stages, chronic cholestatic conditions (including primary biliary cirrhosis, extrahepatic biliary obstruction, sclerosing cholangitis, and biliary atresia), chronic active hepatitis, and Indian childhood cirrhosis, as well as normal neonates, were examined by means of histochemical techniques for copper and copper-associated protein. The intracellular localization of copper and the lobular distribution of the metal and its associated protein differed in these conditions. Periportal hepatocytes containing granules (lysosomes) that were reactive for copper and for copper-associated protein were characteristic of cholestasis and neonatal liver. However, in cholestasis extralysosomal copper was often present in the hepatocellular cytoplasm. In contrast, in Wilson's disease, despite very high concentrations of copper in the early stages, the metal was diffuse in the cytoplasm, and the histochemical reactions for granular copper and its associated protein were usually negative. Therefore, a failure to stain for copper does not exclude the diagnosis of Wilson's disease. In the late stages of Wilson's disease staining varied in different nodules. In Indian childhood cirrhosis copper was present throughout the parenchyma, with periportal predominance. Differences in the distribution of copper and the cellular changes associated with its deposition suggest that different pathogenetic mechanisms and possibly different intracellular targets are susceptible to the toxic effects of the metal. For diagnosis, staining for copper and for copper-associated protein may assist in the differentiation of primary biliary cirrhosis from chronic active hepatitis.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brückmann G., Zondek S. G. Iron, copper and manganese in human organs at various ages. Biochem J. 1939 Nov;33(11):1845–1857. doi: 10.1042/bj0331845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico R. J., Deutsch H. F. Isolation of human hepatocuprein and cerebrocuprein. Their identity with erythrocuprein. J Biol Chem. 1969 Nov 25;244(22):6087–6093. [PubMed] [Google Scholar]
- Fleming C. R., Dickson E. R., Baggenstoss A. H., McCall J. T. Copper and primary biliary cirrhosis. Gastroenterology. 1974 Dec;67(6):1182–1187. [PubMed] [Google Scholar]
- Frommer D. J. Defective biliary excretion of copper in Wilson's disease. Gut. 1974 Feb;15(2):125–129. doi: 10.1136/gut.15.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Bernstein J. Lipofuscin (aging) pigment granules of the newborn human liver. J Cell Biol. 1969 Jul;42(1):253–261. doi: 10.1083/jcb.42.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Moskal J. Electron probe microanalysis of liver in Wilson's disease. Simultaneous assay for copper and for lead deposited by acid phosphatase activity in lysosomes. Am J Pathol. 1966 Feb;48(2):305–315. [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Sternlieb I. Changes in the distribution of hepatic copper in relation to the progression of Wilson's disease (hepatolenticular degeneration). Am J Pathol. 1968 Dec;53(6):883–901. [PMC free article] [PubMed] [Google Scholar]
- Goldfischer S., Villaverde H., Forschirm R. The demonstration of acid hydrolase, thermostable reduced diphosphopyridine nucleotide tetrazolium reductase and peroxidase activities in human lipofuscin pigment granules. J Histochem Cytochem. 1966 Sep;14(9):641–652. doi: 10.1177/14.9.641. [DOI] [PubMed] [Google Scholar]
- HUNT A. H., PARR R. M., TAYLOR D. M., TROTT N. G. RELATION BETWEEN CIRRHOSIS AND TRACE METAL CONTENT OF LIVER WITH SPECIAL REFERENCE TO PRIMARY BILIARY CIRRHOSIS AND COPPER. Br Med J. 1963 Dec 14;2(5371):1498–1501. doi: 10.1136/bmj.2.5371.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons R. D., Schenk E. A., Lee J. C. Cytochemical methods for copper. Semiquantitative screening procedure for identification of abnormal copper levels in liver. Arch Pathol Lab Med. 1977 Jun;101(6):298–301. [PubMed] [Google Scholar]
- Jain S., Scheuer P. J., Archer B., Newman S. P., Sherlock S. Histological demonstration of copper and copper-associated protein in chronic liver diseases. J Clin Pathol. 1978 Aug;31(8):784–790. doi: 10.1136/jcp.31.8.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist R. R. Studies on the pathogenesis of hepatolenticular degeneration. II. Cytochemical methods for the localization of copper. Arch Pathol. 1969 Apr;87(4):370–379. [PubMed] [Google Scholar]
- Ludwig J., McDonald G. S., Dickson E. R., Elveback L. R., McCall J. T. Copper stains and the syndrome of primary biliary cirrhosis. Evaluation of staining methods and their usefulness for diagnosis and trials of penicillamine treatment. Arch Pathol Lab Med. 1979 Aug;103(9):467–470. [PubMed] [Google Scholar]
- NOVIKOFF A. B., ESSNER E. The liver cell. Some new approaches to its study. Am J Med. 1960 Jul;29:102–131. doi: 10.1016/0002-9343(60)90011-5. [DOI] [PubMed] [Google Scholar]
- Owen C. A., Jr, Dickson E. R., Goldstein N. P., Baggenstoss A. H., McCall J. T. Hepatic subcellular distribution of copper in primary biliary cirrhosis. Comparison with other hyperhepatocupric states and review of the literature. Mayo Clin Proc. 1977 Feb;52(2):73–80. [PubMed] [Google Scholar]
- Popper H., Goldfischer S., Sternlieb I., Nayak N. C., Madhavan T. V. Cytoplasmic copper and its toxic effects. Studies in Indian childhood cirrhosis. Lancet. 1979 Jun 9;1(8128):1205–1208. doi: 10.1016/s0140-6736(79)91894-4. [DOI] [PubMed] [Google Scholar]
- Popper H. The problem of histologic evaluation of primary biliary cirrhosis. Virchows Arch A Pathol Anat Histol. 1978 Aug 22;379(2):99–102. doi: 10.1007/BF00432478. [DOI] [PubMed] [Google Scholar]
- Porter H. Neonatal hepatic mitochondrocuprein. 3. Solubilization of the copper and protein from mitochondria of newborn liver by reduction with mercaptoethanol. Biochim Biophys Acta. 1968 Jan 22;154(1):236–238. doi: 10.1016/0005-2795(68)90280-8. [DOI] [PubMed] [Google Scholar]
- Portmann B., Tanner M. S., Mowat A. P., Williams R. Orcein-positive liver deposits in Indian childhood cirrhosis. Lancet. 1978 Jun 24;1(8078):1338–1340. doi: 10.1016/s0140-6736(78)92407-8. [DOI] [PubMed] [Google Scholar]
- Reed G. B., Butt E. M., Landing B. H. Copper in childhood liver disease. A histologic, histochemical, and chemical survey. Arch Pathol. 1972 Mar;93(3):249–255. [PubMed] [Google Scholar]
- Rydén L., Deutsch H. F. Preparation and properties of the major copper-binding component in human fetal liver. Its identification as metallothionein. J Biol Chem. 1978 Jan 25;253(2):519–524. [PubMed] [Google Scholar]
- SCHEINBERG I. H., STERNLIEB I. Copper metabolism. Pharmacol Rev. 1960 Sep;12:355–381. [PubMed] [Google Scholar]
- Salaspuro M., Sipponen P. Demonstration of an intracellular copper-binding protein by orcein staining in long-standing cholestatic liver diseases. Gut. 1976 Oct;17(10):787–790. doi: 10.1136/gut.17.10.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikata T., Uzawa T., Yoshiwara N., Akatsuka T., Yamazaki S. Staining methods of Australia antigen in paraffin section--detection of cytoplasmic inclusion bodies. Jpn J Exp Med. 1974 Feb;44(1):25–36. [PubMed] [Google Scholar]
- Sipponen P. Orcein positive hepatocellular material in long-standing biliary diseases. I. Histochemical characteristics. Scand J Gastroenterol. 1976;11(6):545–552. [PubMed] [Google Scholar]
- Sipponen P., Salaspuro M. P., Makkonen H. Histological characteristics of chronic hepatides and primary biliary cirrhosis with special reference to orcein positive hepatocellular accumulations. Ann Clin Res. 1976 Jun;8(3):200–205. [PubMed] [Google Scholar]
- Sternlieb I. Evolution of the hepatic lesion in Wilson's disease (hepatolenticular degeneration). Prog Liver Dis. 1972;4:511–525. [PubMed] [Google Scholar]
- Sternlieb I., Goldfischer S. Heavy metals and lysosomes. Front Biol. 1976;45:185–200. [PubMed] [Google Scholar]
- Sternlieb I., Harris R. C., Scheinberg I. H. Le cuivre dans la cirrhose biliaire de l'enfant. Rev Int Hepatol. 1966;16(5):1105–1110. [PubMed] [Google Scholar]
- Sternlieb I., Scheinberg I. H. Prevention of Wilson's disease in asymptomatic patients. N Engl J Med. 1968 Feb 15;278(7):352–359. doi: 10.1056/NEJM196802152780702. [DOI] [PubMed] [Google Scholar]
- Sternlieb I., Van den Hamer C. J., Morell A. G., Alpert S., Gregoriadis G., Scheinberg I. H. Lysosomal defect of hepatic copper excretion in Wilson's disease (hepatolenticular degeneration). Gastroenterology. 1973 Jan;64(1):99–105. [PubMed] [Google Scholar]
- TIMM F. [Histochemical demonstration of copper in the brain]. Z Zellforch Microsk Anat Histochem. 1961;2:332–341. [PubMed] [Google Scholar]
- Tanner M. S., Portmann B., Mowat A. P., Williams R., Pandit A. N., Mills C. F., Bremner I. Increased hepatic copper concentration in Indian childhood cirrhosis. Lancet. 1979 Jun 9;1(8128):1203–1205. doi: 10.1016/s0140-6736(79)91893-2. [DOI] [PubMed] [Google Scholar]
- UZMAN L. L. Histochemical localization of copper with rubeanic acid. Lab Invest. 1956 May-Jun;5(3):299–305. [PubMed] [Google Scholar]