Abstract
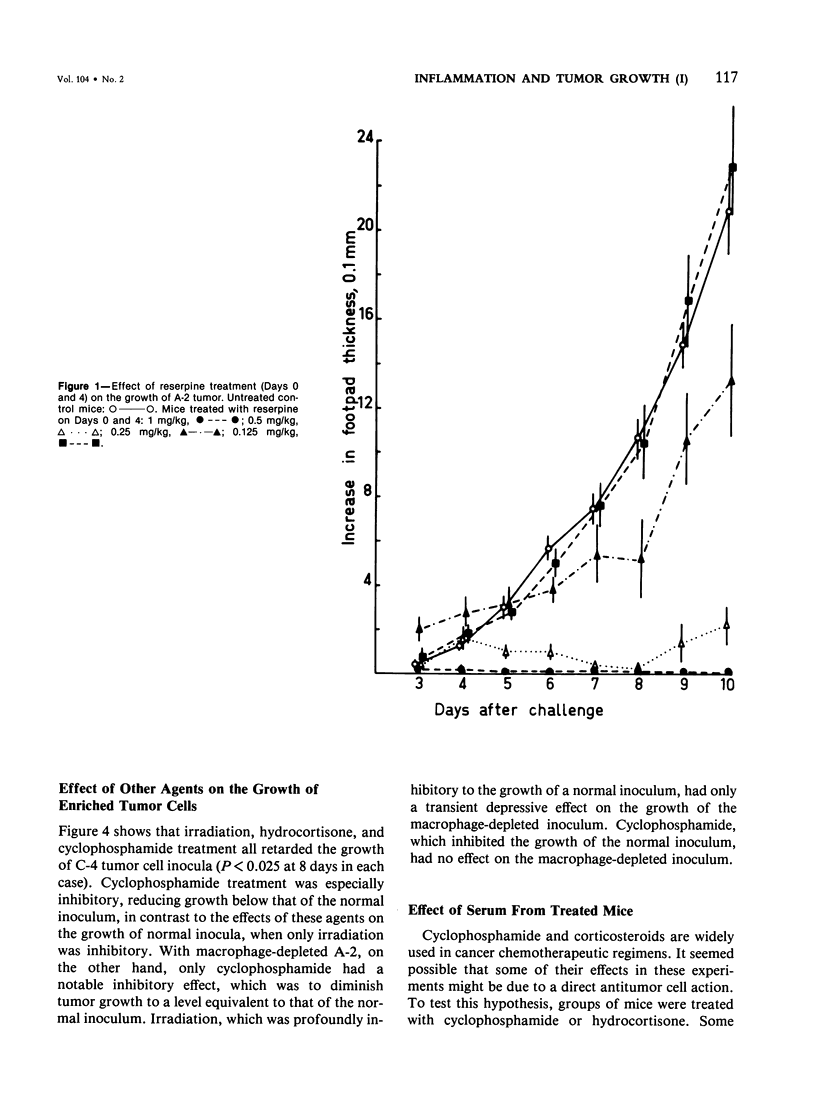
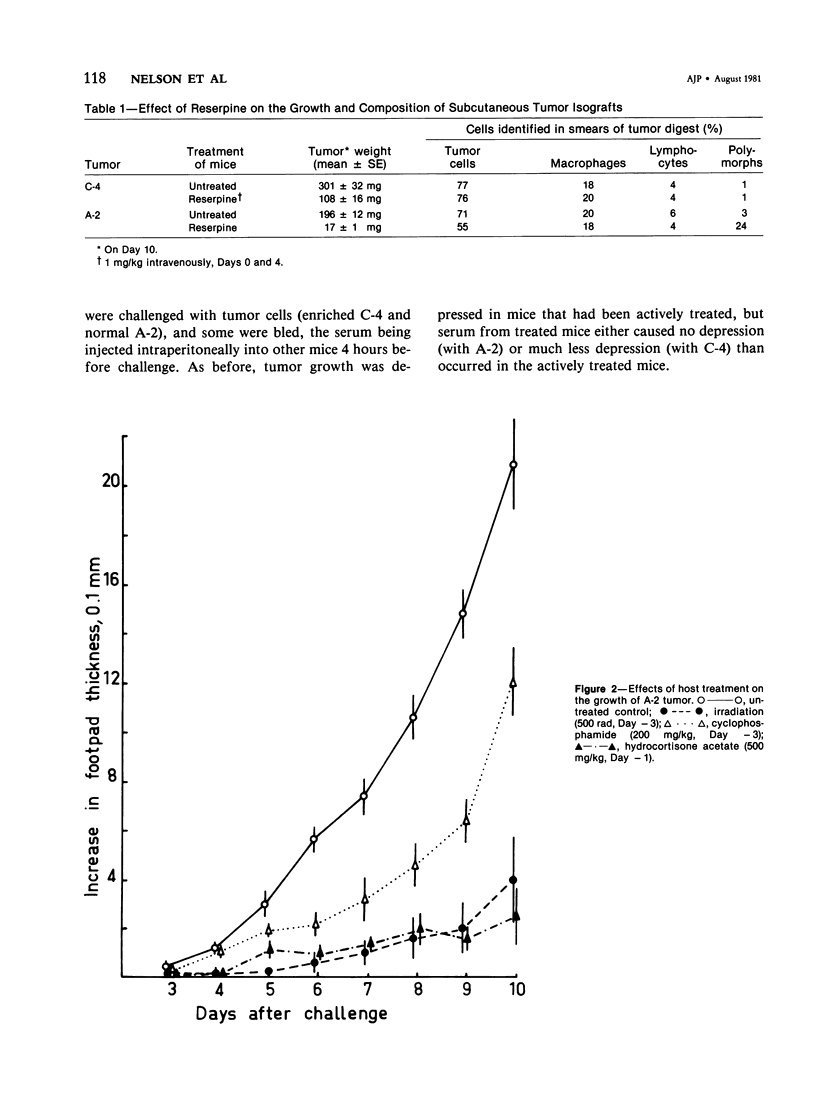
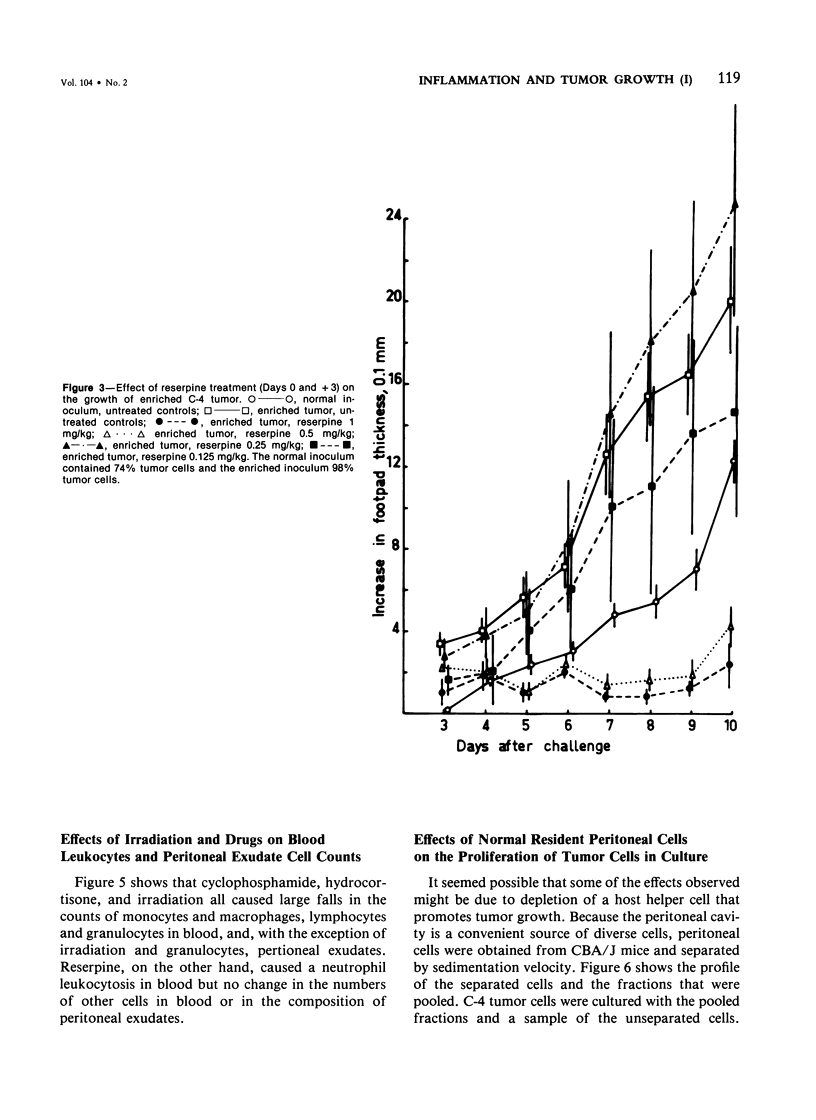
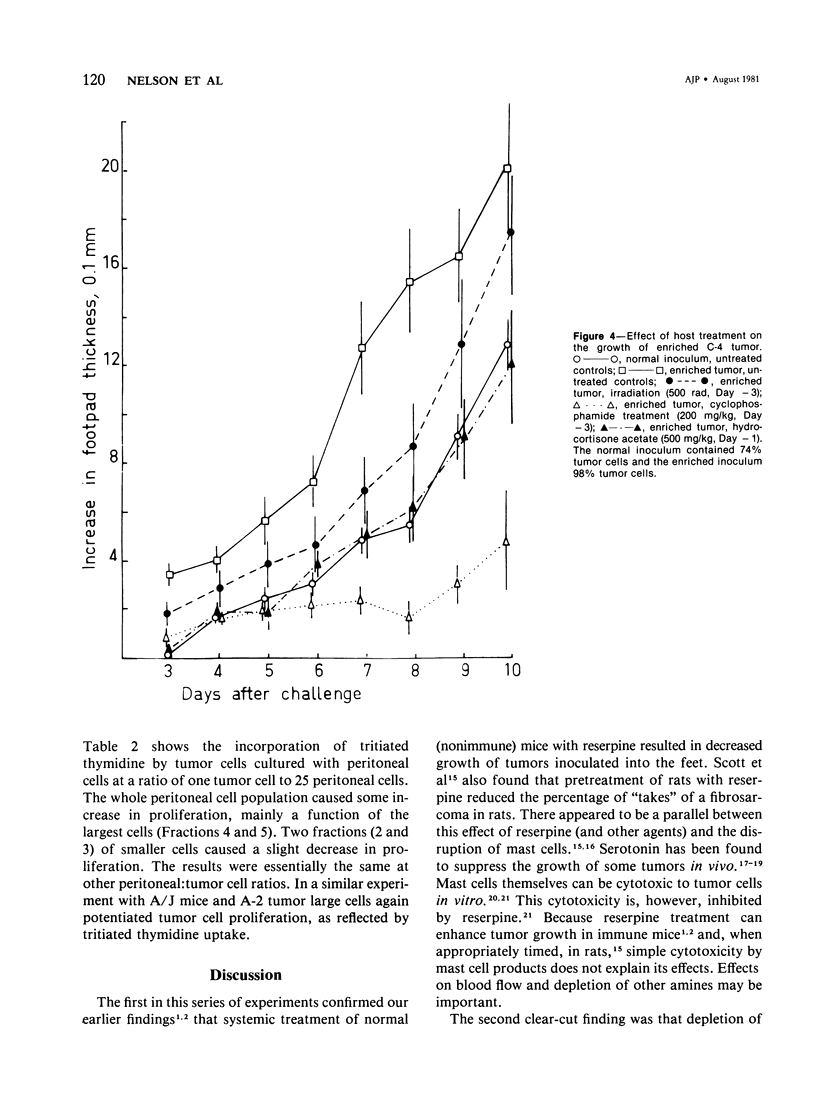
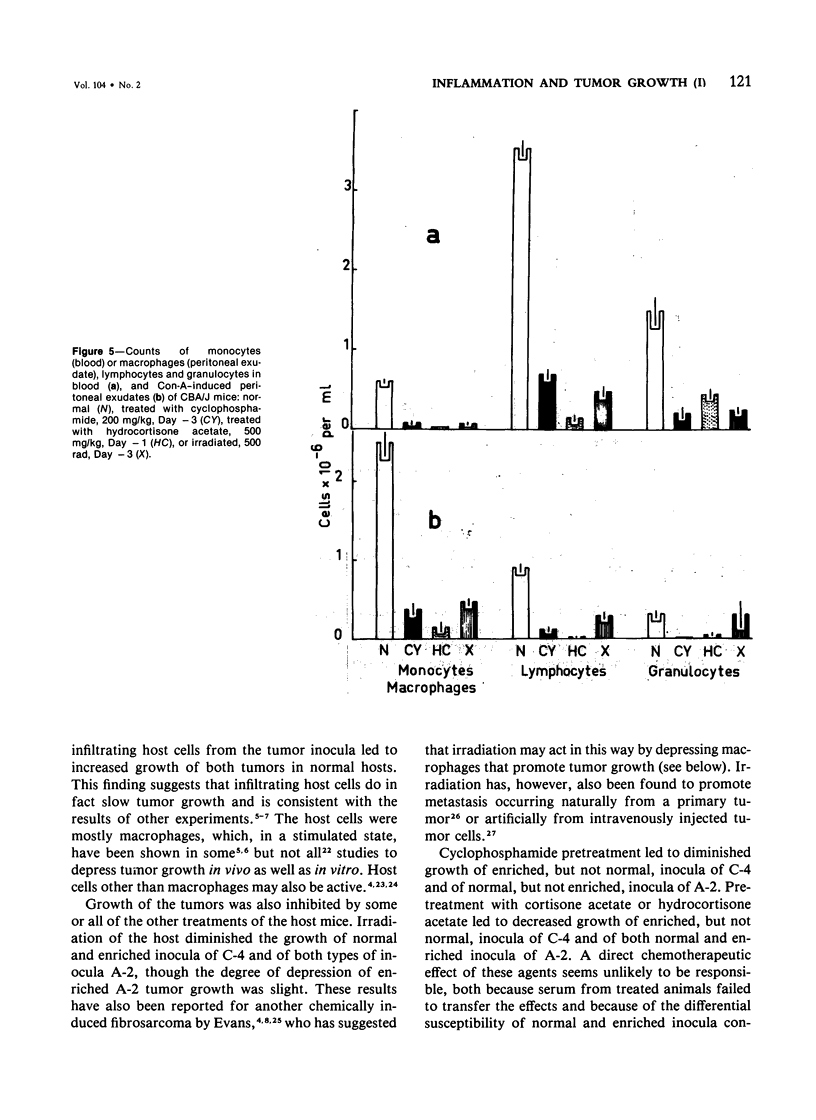
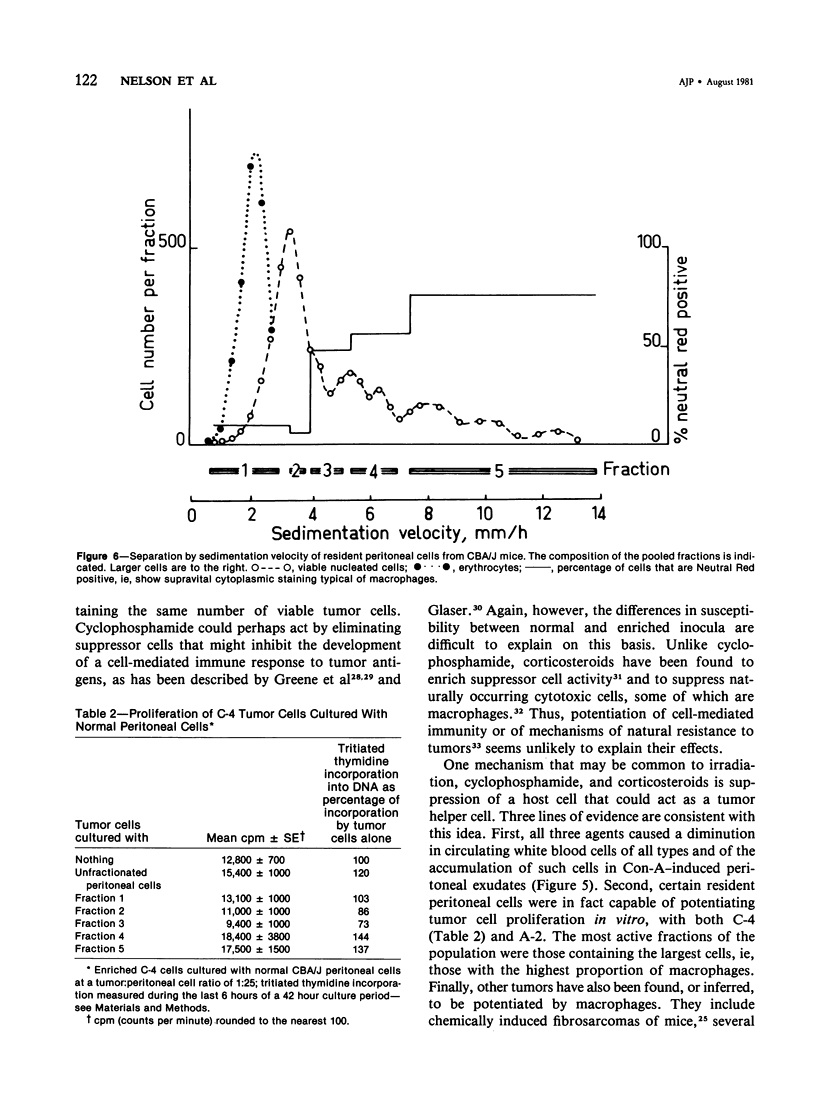
Studies were made of the effects of various treatments on the growth in mouse feet of isografts of two methylcholanthrene-induced fibrosarcomas: C-4, of CBA/J mice, and A-2, of A/J mice. The isografts were prepared by pronase digestion of subcutaneous tumors and were injected as unseparated cell suspensions or as tumor-cell-enriched suspensions after depletion of infiltrating host inflammatory cells. The recipient mice were untreated or treated with reserpine, sublethal whole body irradiation, cyclophosphamide, or corticosteroids. Depletion of host cells from the inoculum resulted in increased growth from the same number of tumor cells. Reserpine treatment decreased the growth of both tumors, whether unseparated or tumor-cell-enriched, and whether injected into the foot or the flank. Irradiation, cyclophosphamide pretreatment, and corticosteroid pretreatment decreased the growth of normal inocula or enriched inocula or both. The effects of cyclophosphamide and corticosteroids were apparently not due to cytotoxicity to tumor cells. Normal resident peritoneal cells increased tritiated thymidine uptake by tumor cells in vitro. Sedimentation velocity separation showed the largest cells to be the most potent. It is suggested that some hot inflammatory reaction is necessary for optimal tumor growth and that murine hosts produce not only cells with antitumor effects but also cells, possibly a subpopulation of macrophages, that potentiate tumor growth.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Snyderman R. Do macrophages destroy nascent tumors? J Natl Cancer Inst. 1979 Jun;62(6):1341–1345. [PubMed] [Google Scholar]
- Buick R. N., Fry S. E., Salmon S. E. Effect of host-cell interactions on clonogenic carcinoma cells in human malignant effusions. Br J Cancer. 1980 May;41(5):695–704. doi: 10.1038/bjc.1980.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRILE G., Jr The effects of heat and radiation on cancers implanted on the feet of mice. Cancer Res. 1963 Mar;23:372–380. [PubMed] [Google Scholar]
- Carswell E. A., Lerman S. P., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. II. Fate of labeled tumor cells in normal and irradiated syngeneic mice. Cell Immunol. 1976 Apr;23(1):39–52. doi: 10.1016/0008-8749(76)90170-2. [DOI] [PubMed] [Google Scholar]
- Clerici E., Schechter B., Feldman M. Enrichment of suppressor cell activity by hydrocortisone: suppression of in vitro activation of splenocytes from mice bearing Lewis lung carcinoma. Int J Cancer. 1980 Mar 15;25(3):349–354. doi: 10.1002/ijc.2910250308. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Galli S. J., Galli A. S., Hammond M. E., Churchill W. H., Jr, Dvorak H. F. Tumor-basophil interactions in vitro--a scanning and transmission electron microscopic study. J Immunol. 1979 Jun;122(6):2447–2457. [PubMed] [Google Scholar]
- Evans R., Booth C. G., Spencer F. Lack of correlation between in vivo rejection of syngeneic fibrosarcomas and in vitro non-specific macrophage cytotoxicity. Br J Cancer. 1978 Nov;38(5):583–590. doi: 10.1038/bjc.1978.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Effect of X-irradiation on host-cell infiltration and growth of a murine fibrosarcoma. Br J Cancer. 1977 May;35(5):557–566. doi: 10.1038/bjc.1977.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. Host cells in transplanted murine tumors and their possible relevance to tumor growth. J Reticuloendothel Soc. 1979 Oct;26(4):427–437. [PubMed] [Google Scholar]
- Evans R. Macrophage requirement for growth of a murine fibrosarcoma. Br J Cancer. 1978 Jun;37(6):1086–1089. doi: 10.1038/bjc.1978.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farram E., Nelson D. S. Mouse mast cells as anti-tumor effector cells. Cell Immunol. 1980 Oct;55(2):294–301. doi: 10.1016/0008-8749(80)90162-8. [DOI] [PubMed] [Google Scholar]
- Gershon R. K., Askenase P. W., Gershon M. D. Requirement for vasoactive amines for production of delayed-type hypersensitvity skin reactions. J Exp Med. 1975 Sep 1;142(3):732–747. doi: 10.1084/jem.142.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie G. Y., Russell S. W. Level of activation determines whether inflammatory peritoneal and intratumoral macrophages will promote or suppress in vitro development of cytolytic T lymphocyte activity. J Reticuloendothel Soc. 1980 May;27(5):535–545. [PubMed] [Google Scholar]
- Glaser M. Augmentation of specific immune response against a syngeneic SV40-induced sarcoma in mice by depletion of suppressor T cells with cyclophosphamide. Cell Immunol. 1979 Dec;48(2):339–345. doi: 10.1016/0008-8749(79)90128-x. [DOI] [PubMed] [Google Scholar]
- Goldman R., Bar-Shavit Z. Dual effect of normal and stimulated macrophages and their conditioned media on target cell proliferation. J Natl Cancer Inst. 1979 Oct;63(4):1009–1016. [PubMed] [Google Scholar]
- Greene M. I., Dorf M. E., Pierres M., Benacerraf B. Reduction of syngeneic tumor growth by an anti-I-J-alloantiserum. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5118–5121. doi: 10.1073/pnas.74.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene M. I., Perry L. L., Benacerraf B. Regulation of the immune response to tumor antigen. Am J Pathol. 1979 Apr;95(1):159–169. [PMC free article] [PubMed] [Google Scholar]
- Haskill J. S., Häyry P., Radov L. A. Systemic and local immunity in allograft and cancer rejection. Contemp Top Immunobiol. 1978;8:107–170. doi: 10.1007/978-1-4684-0922-2_5. [DOI] [PubMed] [Google Scholar]
- Hersey P., MacLennan I. C. Macrophage dependent protection of tumour cells. Immunology. 1973 Feb;24(2):385–393. [PMC free article] [PubMed] [Google Scholar]
- Hewlett G., Opitz H. G., Flad H. D., Schlumberger H. D. Macrophages/monocytes require cell-to-cell contact in order to regulate the growth of a murine lymphoma cell line. J Immunol. 1979 Nov;123(5):2265–2269. [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Chapman H. A., Jr, Weinberg J. B. The macrophage as an antineoplastic surveillance cell: biological perspectives. J Reticuloendothel Soc. 1978 Nov;24(5):549–570. [PubMed] [Google Scholar]
- Hochman P. S., Cudkowicz G. Suppression of natural cytotoxicity by spleen cells of hydrocortisone-treated mice. J Immunol. 1979 Sep;123(3):968–976. [PubMed] [Google Scholar]
- Hopper K. E., Harrison J., Nelson D. S. Partial characterization of anti-tumor effector macrophages in the peritoneal cavities of concomitantly immune mice and mice injected with macrophage-stimulating agents. J Reticuloendothel Soc. 1979 Sep;26(3):259–271. [PubMed] [Google Scholar]
- Hopper K. E., Wood P. R., Nelson D. S. Macrophage heterogeneity. Vox Sang. 1979;36(5):257–274. doi: 10.1111/j.1423-0410.1979.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Kearney R., Nelson D. S. Concomitant immunity to syngeneic methylcholanthrene-induced tumours in mice. Occurrence and specificity of concomitant immunity. Aust J Exp Biol Med Sci. 1973 Dec;51(6):723–735. doi: 10.1038/icb.1973.70. [DOI] [PubMed] [Google Scholar]
- Keller R. Promotion of tumor growth in vivo by antimacrophage agents. J Natl Cancer Inst. 1976 Dec;57(6):1355–1361. doi: 10.1093/jnci/57.6.1355. [DOI] [PubMed] [Google Scholar]
- Keller R. Suppression of natural antitumour defence mechanisms by phorbol esters. Nature. 1979 Dec 13;282(5740):729–731. doi: 10.1038/282729a0. [DOI] [PubMed] [Google Scholar]
- Lerman S. P., Carswell E. A., Chapman J., Thorbecke G. J. Properties of reticulum cell sarcomas in SJL/J mice. III. Promotion of tumor growth in irradiated mice by normal lymphoid cells. Cell Immunol. 1976 Apr;23(1):53–67. doi: 10.1016/0008-8749(76)90171-4. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Terry W. D. Differential stimulation of murine lymphoma growth in vitro by normal and BCG-activated macrophages. J Exp Med. 1975 Oct 1;142(4):887–902. doi: 10.1084/jem.142.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. II. Tumor growth at sites of inflammation induced by mitogens in mice. Am J Pathol. 1981 Aug;104(2):125–131. [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumors. IV. Influence of age on susceptibility of mice to anti-inflammatory and antimacrophage effects of tumor cell products. J Natl Cancer Inst. 1980 Oct;65(4):781–789. doi: 10.1093/jnci/65.4.781. [DOI] [PubMed] [Google Scholar]
- Nelson M., Nelson D. S. Macrophages and resistance to tumours: influence of agents affecting macrophages and delayed-type hypersensitivity on resistance to tumours inducing concomitant immunity. Aust J Exp Biol Med Sci. 1978 Apr;56(2):211–223. [PubMed] [Google Scholar]
- Palladino M. A., Galton J. E., Hochwald G. M., Thorbecke G. J. Immunity to carcinogen-induced transplantable fibrosarcomas in B2/B2 chickens. IV. Effect of whole-body gamma radiation on localization and growth of intravenously injected tumor cells. J Natl Cancer Inst. 1979 Sep;63(3):737–743. doi: 10.1093/jnci/63.3.737. [DOI] [PubMed] [Google Scholar]
- Prehn R. T. Immunomodulation of tumor growth. Am J Pathol. 1974 Oct;77(1):119–122. [PMC free article] [PubMed] [Google Scholar]
- Prehn R. T. Immunostimulation of the lymphodependent phase of neoplastic growth. J Natl Cancer Inst. 1977 Oct;59(4):1043–1049. doi: 10.1093/jnci/59.4.1043. [DOI] [PubMed] [Google Scholar]
- Robins R. A., Flannery G. R., Baldwin R. W. Tumour-derived lymphoid cells prevent tumour growth in Winn assays. Br J Cancer. 1979 Dec;40(6):946–949. doi: 10.1038/bjc.1979.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell S. W., Doe W. F., Hoskins R. G., Cochrane C. G. Inflammatory cells in solid murine neoplasms. I. Tumor disaggregation and identification of constituent inflammatory cells. Int J Cancer. 1976 Sep 15;18(3):322–330. doi: 10.1002/ijc.2910180309. [DOI] [PubMed] [Google Scholar]
- SCOTT K. G., SCHELINE R. R., STONE R. S. Mast cells and sarcoma growth in the rat. Cancer Res. 1958 Sep;18(8 Pt 1):927–931. [PubMed] [Google Scholar]
- SCOTT K. G., STONE R. S. The anti-tumor action of lysergic acid derivatives and their serotonin-blocking effect as reflected by the I 131 distribution in rats. Cancer Res. 1959 Aug;19:783–787. [PubMed] [Google Scholar]
- SOKOLOFF B., FUNAOKA K., FUJISAWA M., SAELHOF C. C., TANIGUCHI E., BIRD L., MILLER C. An oncostatic factor present in the bark of Hippophae rhamnoides. Growth. 1961 Dec;25:401–409. [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W. Immunoproliferation and cancer: a common macrophage-derived promoter substance. Lancet. 1978 Jun 17;1(8077):1289–1290. doi: 10.1016/s0140-6736(78)91270-9. [DOI] [PubMed] [Google Scholar]
- Seshadri M., Poduval T. B., Sundaram K. Studies on metastases. I. Role of sensitization and immunosuppression. J Natl Cancer Inst. 1979 Nov;63(5):1205–1210. [PubMed] [Google Scholar]
- Small M., Trainin N. Separation of populations of sensitized lymphoid cells into fractions inhibiting and fractions enhancing syngeneic tumor growth in vivo. J Immunol. 1976 Jul;117(1):292–297. [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Trejdosiewicz A. J., Ling N. R., Dykes P. W. Growth enhancing property of human monocytes from normal donors and cancer patients. Immunology. 1979 May;37(1):247–252. [PMC free article] [PubMed] [Google Scholar]
- Umetsu D. T., Lerman S. P., Thorbecke G. J. Accessory cell requirements for lymphoma growth in vitro and in irradiated mice. Cell Immunol. 1979 Jan;42(1):139–154. doi: 10.1016/0008-8749(79)90228-4. [DOI] [PubMed] [Google Scholar]
- Wood G. W., Gillespie G. Y. Studies on the role of macrophages in regulation of growth and metastasis of murine chemically induced fibrosarcomas. Int J Cancer. 1975 Dec 15;16(6):1022–1029. doi: 10.1002/ijc.2910160616. [DOI] [PubMed] [Google Scholar]


