Abstract
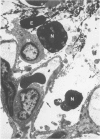
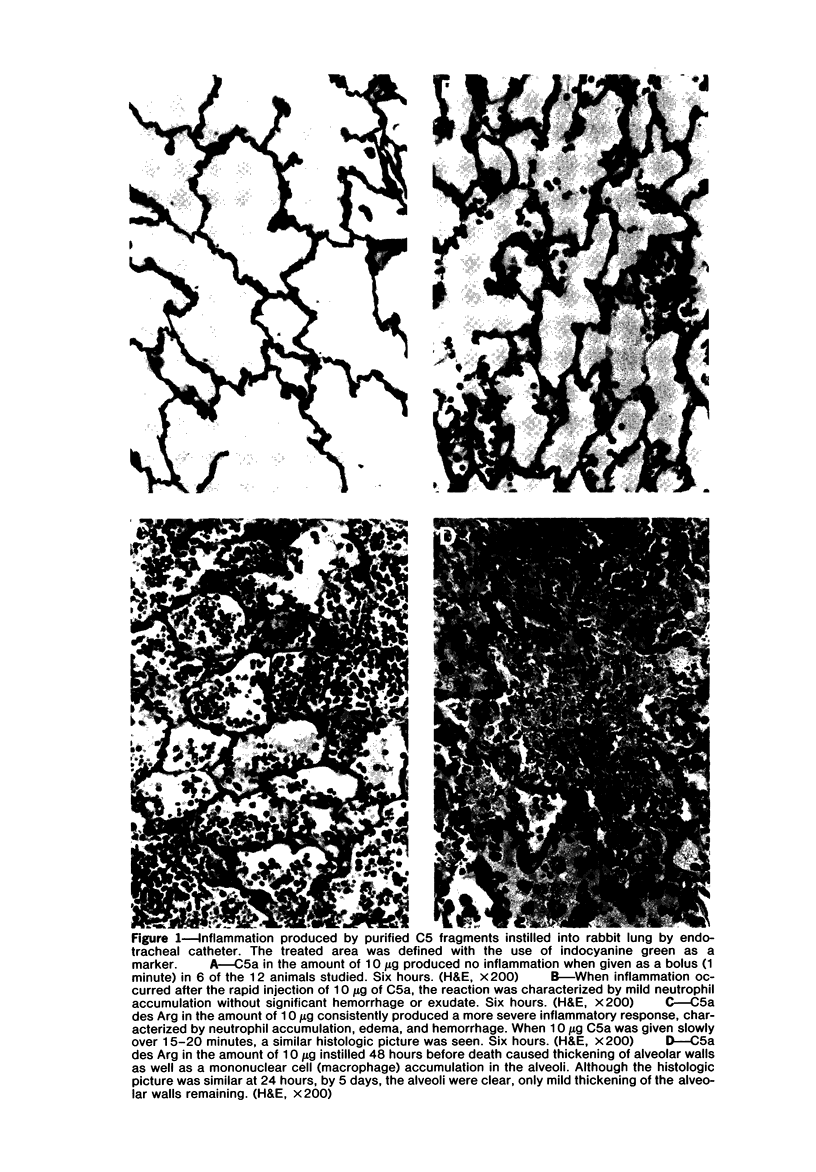
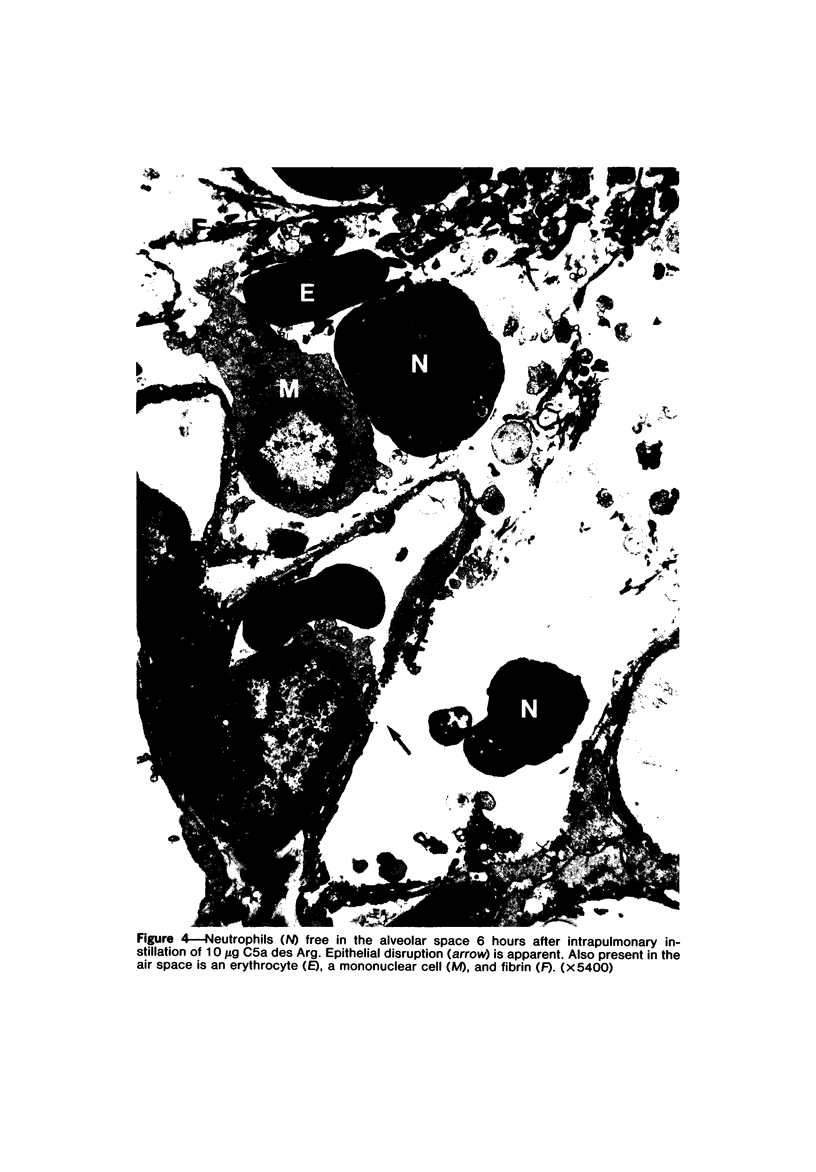
Earlier studies have shown that C5 fragments induce an inflammatory reaction when instilled into the rabbit lung. Because C5a is rapidly converted to C5a des Arg in vivo, experiments were performed to determine which fragment was most effective in producing pulmonary inflammation in this animal model. C5a des Arg consistently produced marked inflammation. This was characterized by neutrophil accumulation, edema, hemorrhage, fibrin formation, and damage to alveolar epithelium. The time course of the inflammatory reaction initiated by C5a des Arg showed pulmonary vascular sequestration of neutrophils with no intra-alveolar migration at 30 minutes after injection. By 2 hours, interstitial and alveolar neutrophils were numerous, with the accumulation of neutrophils in the alveoli increasing to a maximum at 6 hours. At 24 and 48 hours, the predominant cells were mononuclear (macrophages). By 120 hours, the lesions were resolving. In contrast, at all doses examined, a similar instillation of C5a induced either no inflammation or a milder, more focal response than C5a des Arg. This inability of C5a to initiate inflammation was not apparently due to the generation of inhibitors, since mixtures of C5a and C5a des Arg were phlogistic. A prolonged, intrapulmonary infusion of C5a (20 minutes), in contrast to a bolus instillation (1 minute), did initiate an inflammatory response, which may reflect the conversion of the C5a to C5a des Arg in the lung. This study points out the inflammatory potential of products of complement activation, particularly of the C5 fragment C5a des Arg, when applied to the airway side of the lungs. This inflammatory response raises the possibility that cleavage of intrapulmonary C5 may play an important role in the initiation of pulmonary inflammation.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R., Glover A., Rabson A. R. The in vitro effects of histamine and metiamide on neutrophil motility and their relationship to intracellular cyclic nucleotide levels. J Immunol. 1977 May;118(5):1690–1696. [PubMed] [Google Scholar]
- Bachofen M., Weibel E. R. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977 Oct;116(4):589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Rowe J. G., Hugli T. E. A modified method for chemotaxis under agarose. J Immunol Methods. 1979;25(4):337–353. doi: 10.1016/0022-1759(79)90026-7. [DOI] [PubMed] [Google Scholar]
- Desai U., Kreutzer D. L., Showell H., Arroyave C. V., Ward P. A. Acute inflammatory pulmonary reactions induced by chemotactic factors. Am J Pathol. 1979 Jul;96(1):71–83. [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Fernandez H. N., Hugli T. E. Partial characterization of human C5a anaphylatoxin. I. Chemical description of the carbohydrate and polypeptide prtions of human C5a. J Immunol. 1976 Nov;117(5 Pt 1):1688–1694. [PubMed] [Google Scholar]
- Henson P. M., McCarthy K., Larsen G. L., Webster R. O., Giclas P. C., Dreisin R. B., King T. E., Shaw J. O. Complement fragments, alveolar macrophages, and alveolitis. Am J Pathol. 1979 Oct;97(1):93–110. [PMC free article] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Anderson T. P., Ward P. A. Suppression of immune complex-induced inflammation by the chemotactic factor inactivator. J Clin Invest. 1977 May;59(5):951–958. doi: 10.1172/JCI108717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Goldstein I. M., Chernoff D., Webster R. O., Henson P. M. Chemotactic activity of C5ades Arg: evidence of a requirement for an anionic peptide 'helper factor' and inhibition by a cationic protein in serum from patients with systemic lupus erythematosus. Mol Immunol. 1980 Feb;17(2):163–169. doi: 10.1016/0161-5890(80)90068-1. [DOI] [PubMed] [Google Scholar]
- Pratt P. C. Pathology of pulmonary oxygen toxicity. Am Rev Respir Dis. 1974 Dec;110(6 Pt 2):51–57. doi: 10.1164/arrd.1974.110.6P2.51. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R. C., Moore V. L. Immunopathogenesis of hypersensitivity pneumonitis. Am Rev Respir Dis. 1977 Dec;116(6):1075–1090. doi: 10.1164/arrd.1977.116.6.1075. [DOI] [PubMed] [Google Scholar]
- Robertson J., Caldwell J. R., Castle J. R., Waldman R. H. Evidence for the presence of components of the alternative (properdin) pathway of complement activation in respiratory secretions. J Immunol. 1976 Sep;117(3):900–903. [PubMed] [Google Scholar]
- Roska A. K., Garancis J. C., Moore V. L., Abramoff P. Immune-complex disease in guinea pig lungs. I. Elicitation by aerosol challenge, suppression with cobra venom factor, and passive transfer with serum. Clin Immunol Immunopathol. 1977 Sep;8(2):213–224. doi: 10.1016/0090-1229(77)90111-8. [DOI] [PubMed] [Google Scholar]
- Ryan G. B., Majno G. Acute inflammation. A review. Am J Pathol. 1977 Jan;86(1):183–276. [PMC free article] [PubMed] [Google Scholar]
- Schatz M., Patterson R., Fink J. Immunopatholgenesis of hypersensitivity pneumonitis. J Allergy Clin Immunol. 1977 Jul;60(1):27–37. doi: 10.1016/0091-6749(77)90079-3. [DOI] [PubMed] [Google Scholar]
- Scherzer H., Ward P. A. Lung and dermal vascular injury produced by preformed immune complexes. Am Rev Respir Dis. 1978 Mar;117(3):551–557. doi: 10.1164/arrd.1978.117.3.551. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Shin H., Dannenberg A. M., Jr Macrophage proteinase and inflammation: the production of chemotactic activity from the fifth complement by macrophage proteinase. J Immunol. 1972 Oct;109(4):896–898. [PubMed] [Google Scholar]