Abstract
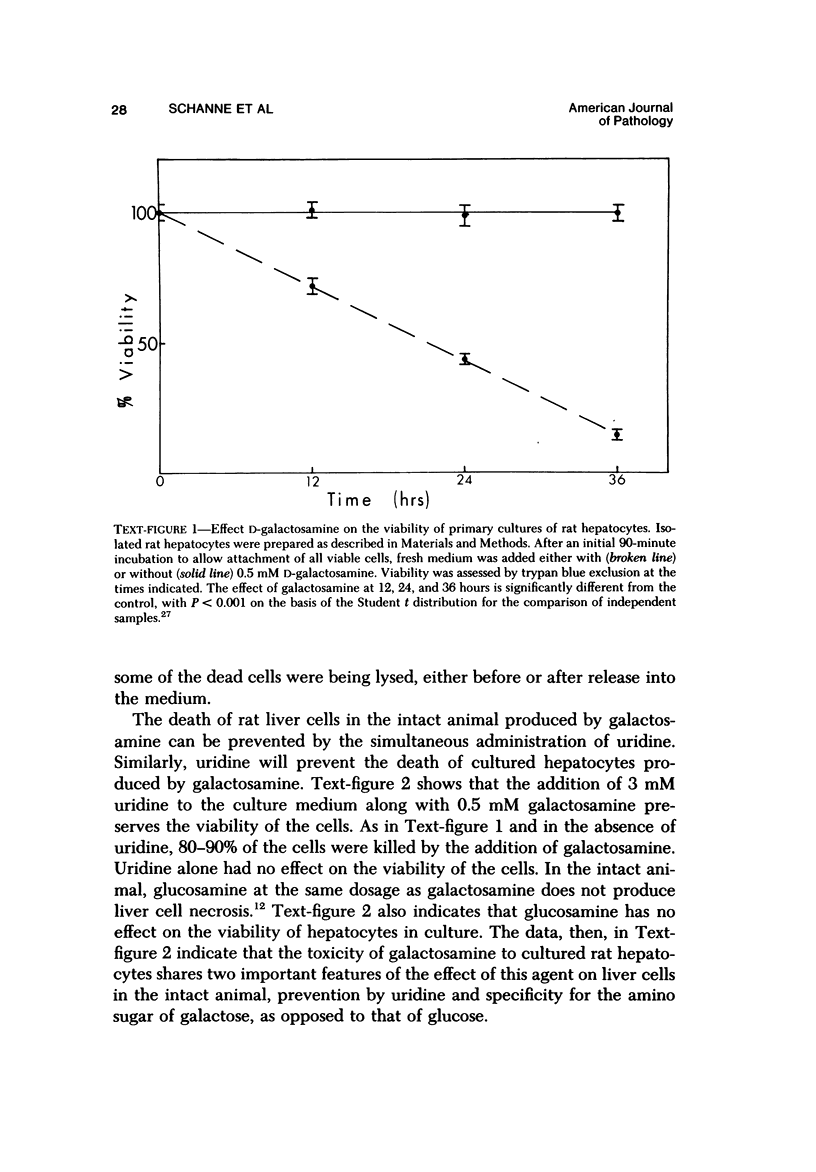
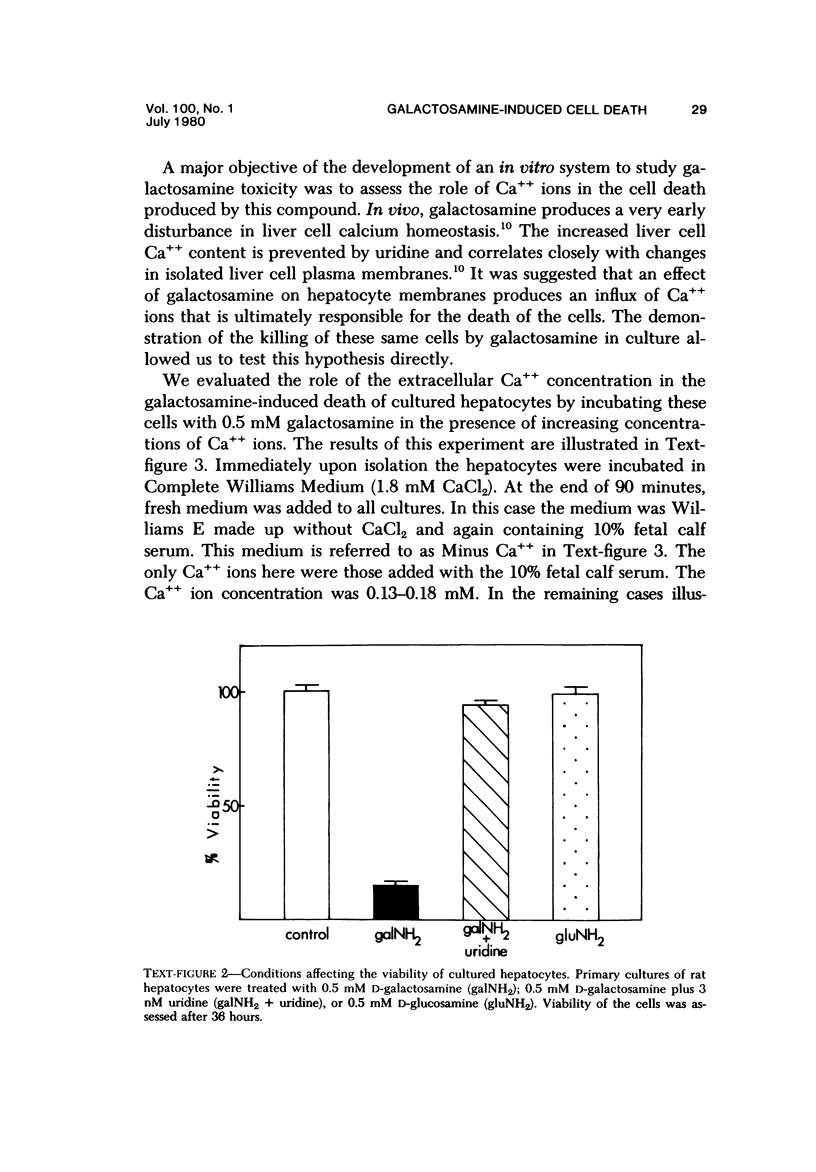
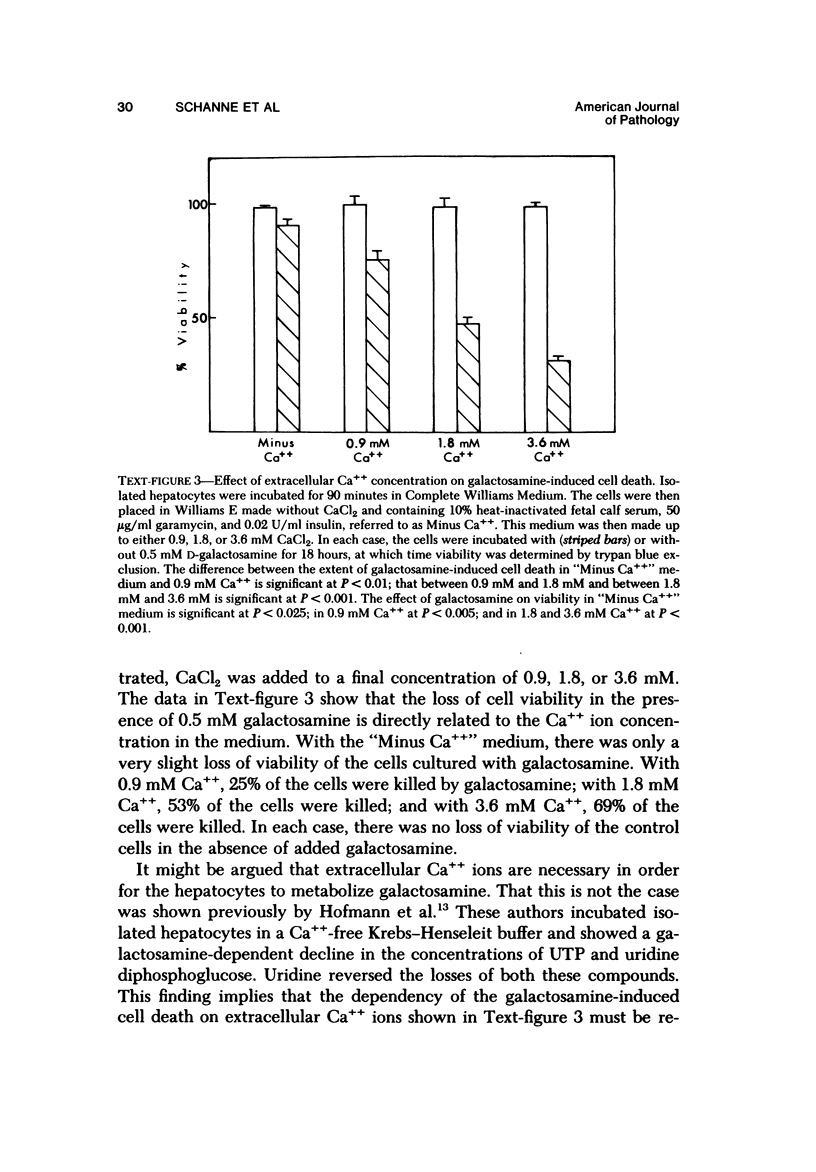
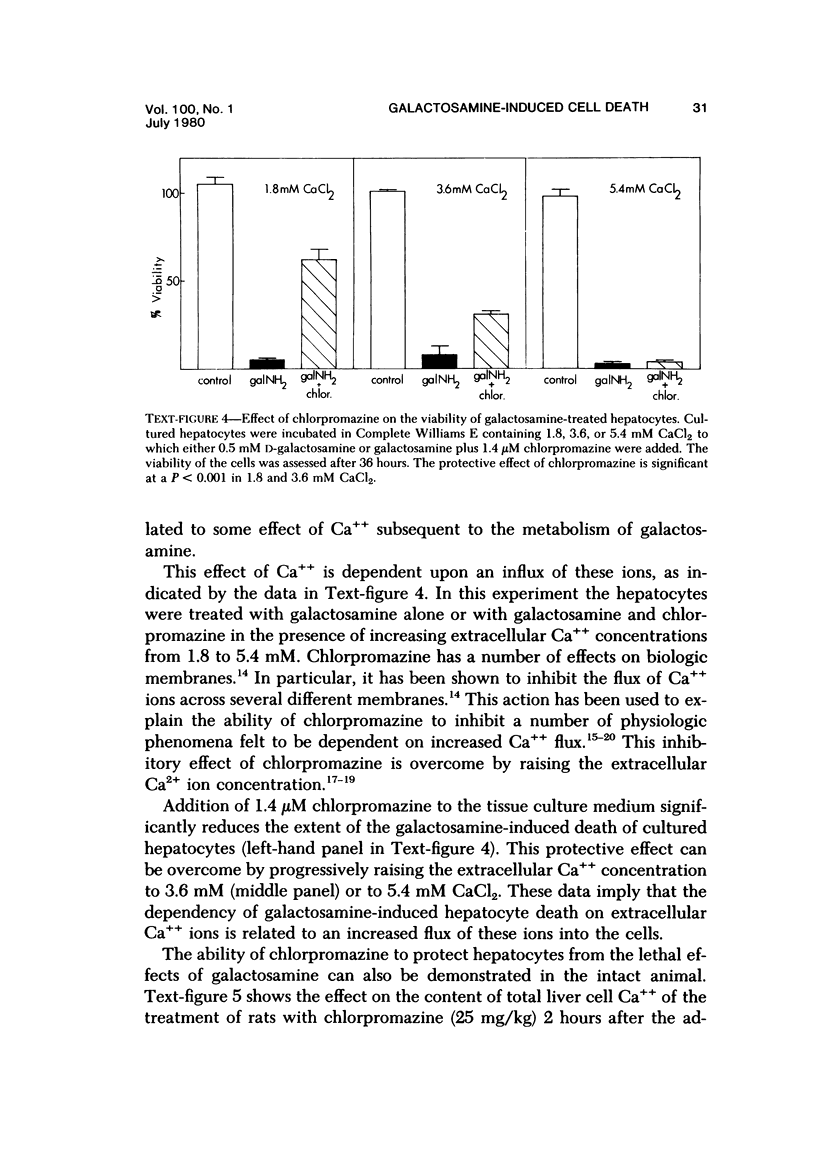
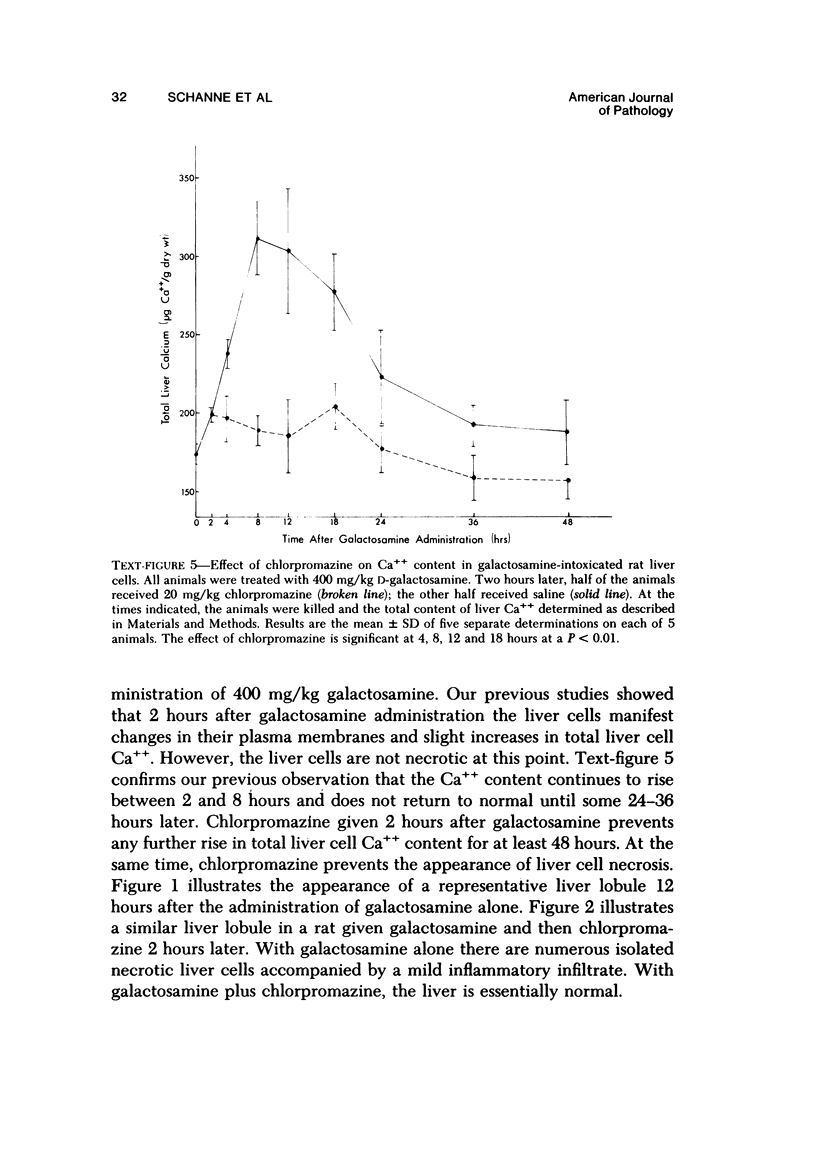
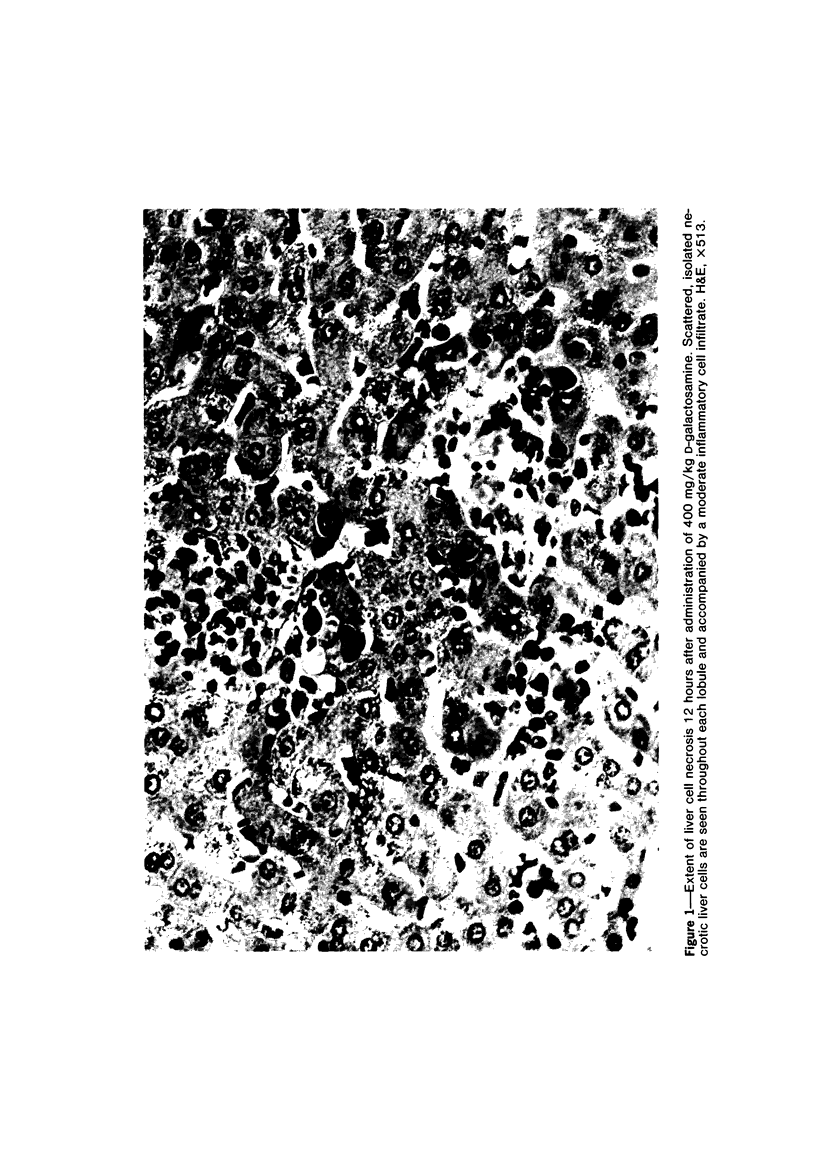
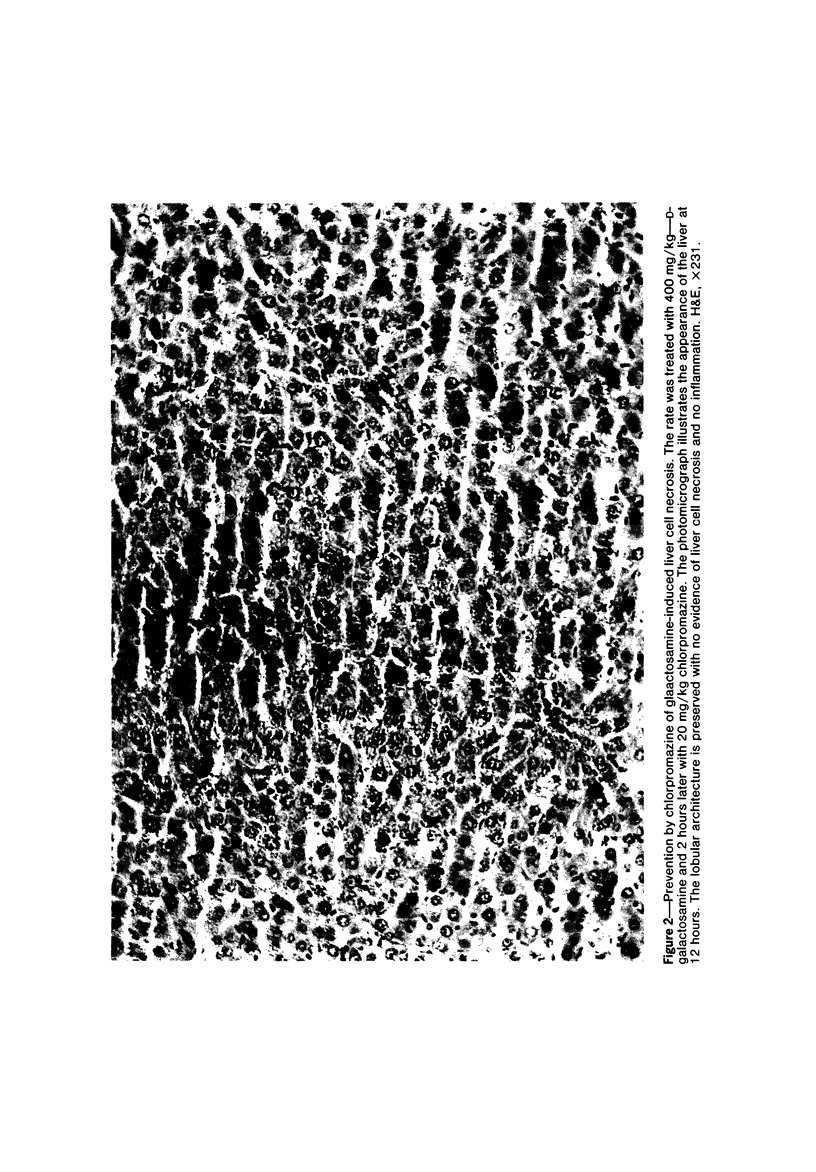
Primary cultures of rat hepatocytes were exposed to 0.5 mM D-galactosamine. After 36 hours, only 10-20% of the original cells were viable, as assessed by trypan blue exclusion. In the absence of galactosamine, there was no loss of viability over this same period. The addition of 3 mM uridine to the culture medium completely prevented the cell death produced by galactosamine. Glucosamine had no effect on the viability of the hepatocytes. The extent of galactosamine-induced cell death was dependent upon the concentration of Ca++ ions in the culture medium. With the only source of Ca++ that added with the fetal calf serum, galactosamine had only a very slight effect on viability. With higher Ca++ than with the fetal calf serum, galactosamine had only a very slight effect on viability. With higher Ca++ concentrations, from 0.9 to 3.6 mM, the viability ranged from 75% to 31% 18 hours after treatment with galactosamine. The addition of 1.4 microM chlorpromazine to culture medium containing 1.8 mM Ca++ decreased the extent of the galactosamine-induced cell death. This protective effect was progressively reduced by raising the Ca++ concentration to 3.6 and 5.4 mM. Chlorpromazine given to intact rats 2 hours after treatment with 400 mg/kg galactosamine prevented the appearance of liver cell necrosis. At the same time, chlorpromazine prevented the increases in liver cell Ca++ content. These results indicate that many of the features of the effect of galactosamine on intact rat liver cells can be reproduced in primary cultures of these same cells. The data also support the hypothesis that a disturbance in intracellular Ca++ homeostasis leading to accumulations of these ions is causally related to the cell death produced by galactosamine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Decker K., Keppler D. Galactosamine induced liver injury. Prog Liver Dis. 1972;4:183–199. [PubMed] [Google Scholar]
- El-Mofty S. K., Scrutton M. C., Serroni A., Nicolini C., Farber J. L. Early, reversible plasma membrane injury in galactosamine-induced liver cell death. Am J Pathol. 1975 Jun;79(3):579–596. [PMC free article] [PubMed] [Google Scholar]
- Farber J. L., El-Mofty S. K. The biochemical pathology of liver cell necrosis. Am J Pathol. 1975 Oct;81(1):237–250. [PMC free article] [PubMed] [Google Scholar]
- Farber J. L., Gill G., Konishi Y. Prevention of galactosamine-induced liver cell necrosis by uridine. Am J Pathol. 1973 Jul;72(1):53–62. [PMC free article] [PubMed] [Google Scholar]
- GALLAGHER C. H., GUPTA D. N., JUDAH J. D., REES K. R. Biochemical changes in liver in acute thioacetamide intoxication. J Pathol Bacteriol. 1956 Jul;72(1):193–201. doi: 10.1002/path.1700720125. [DOI] [PubMed] [Google Scholar]
- Godfraind T., Kaba A. Blockade or reversal of the contraction induced by calcium and adrenaline in depolarized arterial smooth muscle. Br J Pharmacol. 1969 Jul;36(3):549–560. doi: 10.1111/j.1476-5381.1969.tb08010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grün M., Liehr H., Grün W., Rasenack U., Brunswig D. Influence of liver-RES on toxic liver damage due to galactosamine. Acta Hepatogastroenterol (Stuttg) 1974 Feb;21(1):5–15. [PubMed] [Google Scholar]
- Grün M., Liehr H., Rasenack U. Significance of endotoxaemia in experimental "galactosamine-hepatitis" in the rat. Acta Hepatogastroenterol (Stuttg) 1977 Apr;24(2):64–81. [PubMed] [Google Scholar]
- Gárdos G., Lassen U. V., Pape L. Effect of antihistamines and chlorpromazine on the calcium-induced hyperpolarization of the Amphiuma red cell membrane. Biochim Biophys Acta. 1976 Nov 2;448(4):599–606. doi: 10.1016/0005-2736(76)90113-9. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Wilkening J., Nowack J., Decker K. Response of isolated rat hepatocytes to D-galactosamine and uridine. Hoppe Seylers Z Physiol Chem. 1976 Mar;357(3):427–433. doi: 10.1515/bchm2.1976.357.1.427. [DOI] [PubMed] [Google Scholar]
- Kane A. B., Young E. E., Schanne F. A., Farber J. L. Calcium dependence of phalloidin-induced liver cell death. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1177–1180. doi: 10.1073/pnas.77.2.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppler D., Lesch R., Reutter W., Decker K. Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol. 1968 Oct;9(2):279–290. doi: 10.1016/0014-4800(68)90042-7. [DOI] [PubMed] [Google Scholar]
- Liehr H., Grün M., Seelig H. P., Seelig R., Reutter W., Heine W. D. On the pathogenesis of galactosamine hepatitis. Indications of extrahepatocellular mechanisms responsible for liver cell death. Virchows Arch B Cell Pathol. 1978 Feb 14;26(4):331–344. doi: 10.1007/BF02889560. [DOI] [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- REES K. R., SINHA K. P., SPECTOR W. G. The pathogenesis of liver injury in carbon tetrachloride and thioacetamide poisoning. J Pathol Bacteriol. 1961 Jan;81:107–118. [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. I. INITIAL ALTERATIONS OF THE CELL FOLLOWING POISONING WITH CARBON TETRACHLORIDE. J Cell Biol. 1963 Oct;19:139–157. doi: 10.1083/jcb.19.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. LIVER PARENCHYMAL CELL INJURY. II. CYTOCHEMICAL EVENTS CONCERNED WITH MITOCHONDRIAL DYSFUNCTION FOLLOWING POISONING WITH CARBON TETRACHLORIDE. Lab Invest. 1964 Nov;13:1457–1470. [PubMed] [Google Scholar]
- Schanne F. A., Kane A. B., Young E. E., Farber J. L. Calcium dependence of toxic cell death: a final common pathway. Science. 1979 Nov 9;206(4419):700–702. doi: 10.1126/science.386513. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Unanue E. R. The disruption of immunoglobulin caps by local anesthetics. Clin Immunol Immunopathol. 1976 Sep;6(2):264–269. doi: 10.1016/0090-1229(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Seeman P., Chen S. S., Chau-Wong M., Staiman A. Calcium reversal of nerve blockade by alcohols, anesthetics, tranquilizers, and barbiturates. Can J Physiol Pharmacol. 1974 Jun;52(3):526–534. doi: 10.1139/y74-070. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Smuckler E. A. Studies on carbon tetrachloride intoxication. IV. Effect of carbon tetrachloride on liver slices and isolated organelles in vitro. Lab Invest. 1966 Jan;15(1 Pt 1):157–166. [PubMed] [Google Scholar]
- THIERS R. E., REYNOLDS E. S., VALLEE B. L. The effect of carbon tetrachloride poisoning on subcellular metal distribution in rat liver. J Biol Chem. 1960 Jul;235:2130–2133. [PubMed] [Google Scholar]
- Williams J. A., Poulsen J. H., Lee M. Effects of membrane stabilizers on pancreatic amylase release. J Membr Biol. 1977 May 6;33(1-2):185–195. doi: 10.1007/BF01869515. [DOI] [PubMed] [Google Scholar]




