Abstract
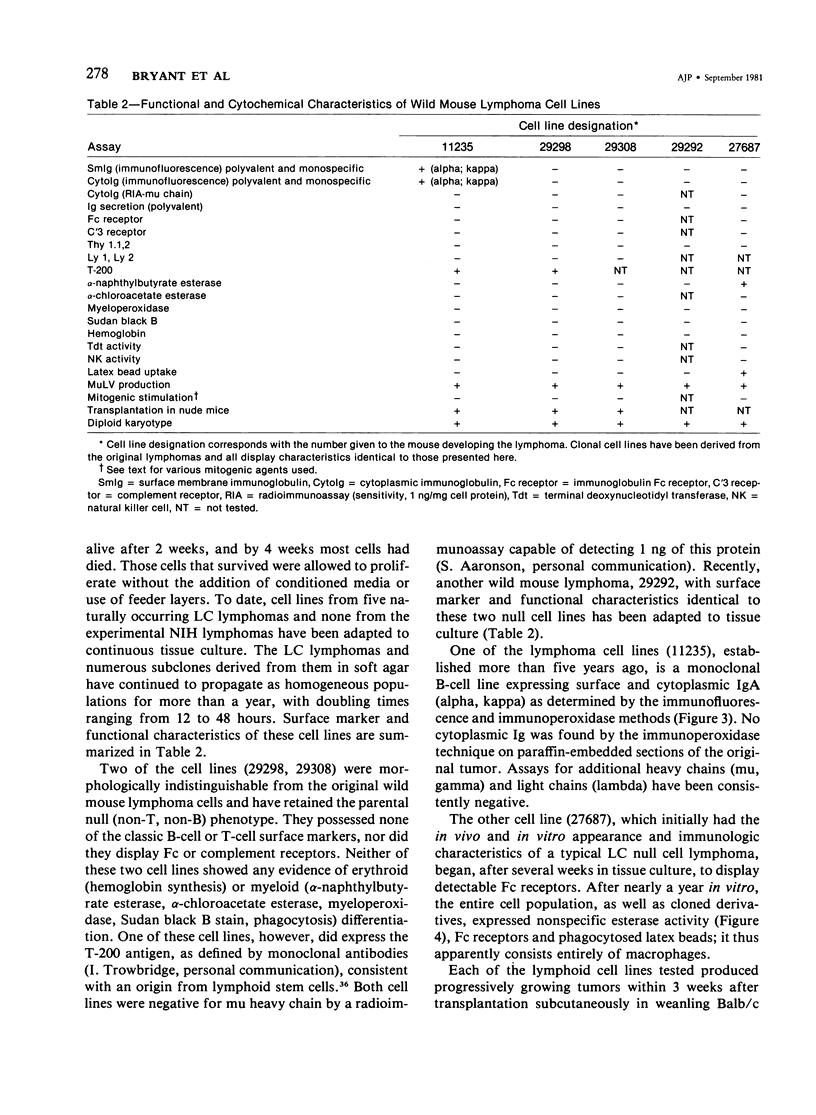
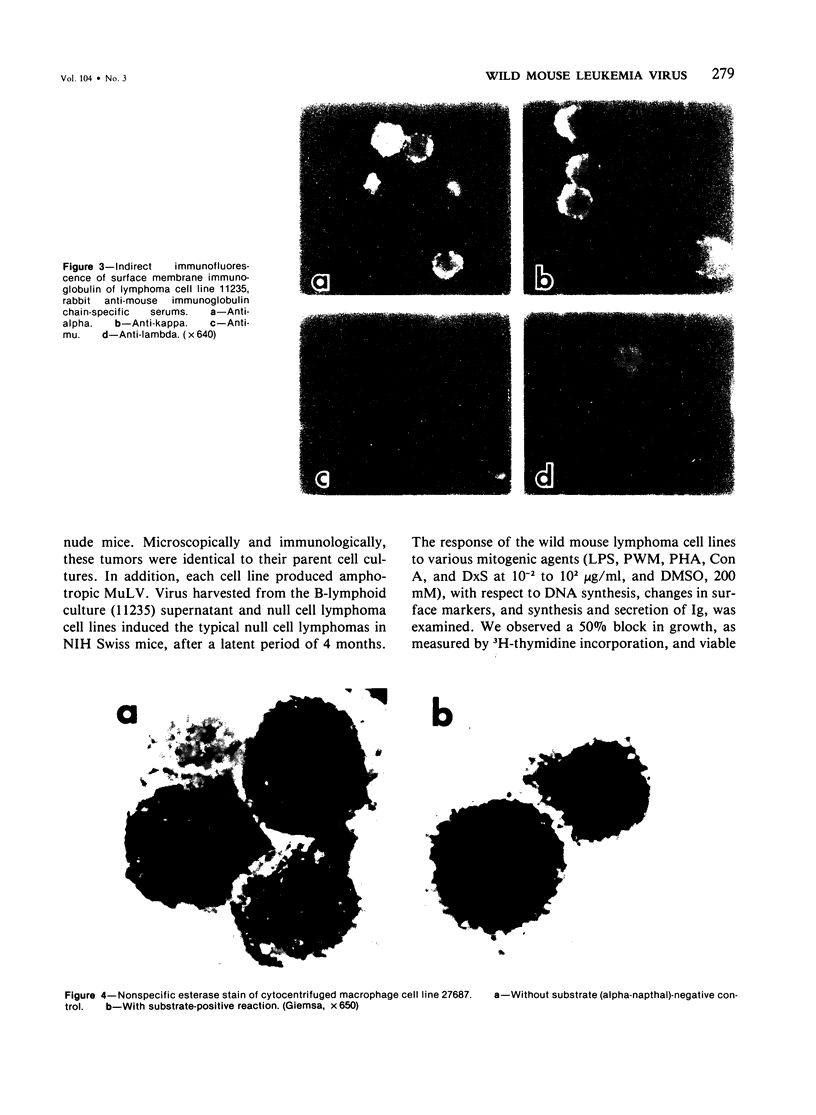
Naturally occurring lymphomas of Lake Casitas (LC) wild mice, and the lymphomas induced by LC murine leukemia virus (MuLV) in Swiss mice from the National Institutes of Health, displayed remarkably similar gross, microscopic, and functional characteristics. They spared the thymus, arose primarily in the splenic red pulp, became leukemic, and were comprised of stem cells lacking classic T- and B-cell markers. Cytoplasmic and surface immunoglobulin were undetectable in 34 of 35 spontaneous LC lymphomas and in any of ten LC MuLV-induced lymphomas in NIH Swiss mice. Assays for immunoglobulin secretion, complement (C'3) and Fc receptors, Thy 1.1,2 antigens, Ly 1,2 antigens, and erythroid and myeloid markers were negative on all of the spontaneous and experimental lymphomas. Cell lines were derived from five spontaneous lymphomas of LC mice. Three lines were characterized as null cells, one line as B cells, and one line as macrophages. All cell lines were diploid. The wild mouse spontaneous lymphomas, and lymphomas experimentally induced by LC MuLV in laboratory mice, provide a useful model for childhood acute lymphoblastic leukemia and for study of the early steps of B-lymphocyte differentiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Influence of prednisolone on Moloney leukemogenic virus in BALB-c mice. Cancer Res. 1970 Aug;30(8):2208–2212. [PubMed] [Google Scholar]
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Baltimore D., Rosenberg N., Witte O. N. Transformation of immature lymphoid cells by Abelson murine leukemia virus. Immunol Rev. 1979;48:3–22. doi: 10.1111/j.1600-065x.1979.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Blankenhorn E. P., Gardner M. B., Estes J. D. Immunogenetics of a thymus antigen in lymphoma-prone and lymphoma-resistant colonies of wild mice. J Natl Cancer Inst. 1975 Mar;54(3):665–672. [PubMed] [Google Scholar]
- Brouet J. C., Preud'homme J. L., Penit C., Valensi F., Rouget P., Seligmann M. Acute lymphoblastic leukemia with pre-B-cell characteristics. Blood. 1979 Jul;54(1):269–273. [PubMed] [Google Scholar]
- Bryant M. L., Klement V. Clonal heterogeneity of wild mouse leukemia viruses: host ranges and antigenicity. Virology. 1976 Sep;73(2):532–536. doi: 10.1016/0042-6822(76)90415-3. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chess L., Levine H., MacDermott R. P., Schlossman S. F. Immunologic functions of isolated human lymphocyte subpopulations. VI. Further characterization of the surface Ig negative, E rosette negative (null cell) subset. J Immunol. 1975 Dec;115(6):1483–1487. [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. A monoclonal antibody that recognizes B cells and B cell precursors in mice. J Exp Med. 1981 Feb 1;153(2):269–279. doi: 10.1084/jem.153.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foon K. A., Billing R. J., Terasaki P. I., Cline M. J. Immunologic classification of acute lymphoblastic leukemia. Implications for normal lymphoid differentiation. Blood. 1980 Dec;56(6):1120–1126. [PubMed] [Google Scholar]
- Gardner M. B., Chiri A., Dougherty M. F., Casagrande J., Estes J. D. Congenital transmission of murine leukemia virus from wild mice prone to the development of lymphoma and paralysis. J Natl Cancer Inst. 1979 Jan;62(1):63–70. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Estes J. D., Menck H., Parker J. C., Huebner R. J. Unusually high incidence of spontaneous lymphomas in wild house mice. J Natl Cancer Inst. 1973 Jun;50(6):1571–1579. doi: 10.1093/jnci/50.6.1571. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Estes J. D., Rongey R. W., Casagrande J., Pike M., Huebner R. J. The epidemiology and virology of C-type virus-associated hematological cancers and related diseases in wild mice. Cancer Res. 1976 Feb;36(2 Pt 2):574–581. [PubMed] [Google Scholar]
- Gardner M. B., Henderson B. E., Officer J. E., Rongey R. W., Parker J. C., Oliver C., Estes J. D., Huebner R. J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J Natl Cancer Inst. 1973 Oct;51(4):1243–1254. doi: 10.1093/jnci/51.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B., Rasheed S., Pal B. K., Estes J. D., O'Brien S. J. Akvr-1, a dominant murine leukemia virus restriction gene, is polymorphic in leukemia-prone wild mice. Proc Natl Acad Sci U S A. 1980 Jan;77(1):531–535. doi: 10.1073/pnas.77.1.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner M. B. Type C viruses of wild mice: characterization and natural history of amphotropic, ecotropic, and xenotropic MuLv. Curr Top Microbiol Immunol. 1978;79:215–259. doi: 10.1007/978-3-642-66853-1_5. [DOI] [PubMed] [Google Scholar]
- Greenwood M. F., Coleman M. S., Hutton J. J., Lampkin B., Krill C., Bolium F. J., Holland P. Terminal deoxynucleotidyltransferase distribution in neoplastic and hematopoietic cells. J Clin Invest. 1977 May;59(5):889–899. doi: 10.1172/JCI108711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Haran-Chera N., Peled A. Thymus and bone marrow derived lymphatic leukaemia in mice. Nature. 1973 Feb 9;241(5389):396–398. doi: 10.1038/241396a0. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaak D. D., Price J. A., Reinisch C. L., Cerny J. Target cell heterogeneity in murine leukemia virus infection. I. Differences in susceptibility to infection with Friend leukemia virus between B lymphocytes from spleen, bone marrow and lymph nodes. J Immunol. 1979 Oct;123(4):1822–1828. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kaplan H. S. On the natural history of the murine leukemias: presidential address. Cancer Res. 1967 Aug;27(8):1325–1340. [PubMed] [Google Scholar]
- Kaplan J., Ravindranath Y., Peterson W. D., Jr T and B lymphocyte antigen-positive null cell leukemias. Blood. 1977 Mar;49(3):371–378. [PubMed] [Google Scholar]
- Klein G. Lymphoma development in mice and humans: diversity of initiation is followed by convergent cytogenetic evolution. Proc Natl Acad Sci U S A. 1979 May;76(5):2442–2446. doi: 10.1073/pnas.76.5.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Ohno S., Rosenberg N., Wiener F., Spira J., Baltimore D. Cytogenetic studies on abelson-virus-induced mouse leukemias. Int J Cancer. 1980 Jun 15;25(6):805–811. doi: 10.1002/ijc.2910250617. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Kulenkampff J., Janossy G., Greaves M. F. Acid esterase in human lymphoid cells and leukaemic blasts: a marker for T lymphocytes. Br J Haematol. 1977 Jun;36(2):231–240. doi: 10.1111/j.1365-2141.1977.tb00644.x. [DOI] [PubMed] [Google Scholar]
- LAW L. W., MILLER J. H. Observations on the effect of thymectomy on spontaneous leukemias in mice of the highleukemic strains, RIL and C 58. J Natl Cancer Inst. 1950 Oct;11(2):253–262. [PubMed] [Google Scholar]
- Lanier L. L., Lynes M., Haughton G., Wettstein P. J. Novel type of murine B-cell lymphoma. Nature. 1978 Feb 9;271(5645):554–555. doi: 10.1038/271554a0. [DOI] [PubMed] [Google Scholar]
- METCALF D. THYMUS GRAFTS AND LEUKEMOGENESIS. Cancer Res. 1964 Dec;24:1952–1957. [PubMed] [Google Scholar]
- MOLONEY J. B. Biological studies on a lymphoid-leukemia virus extracted from sarcoma 37. I. Origin and introductory investigations. J Natl Cancer Inst. 1960 Apr;24:933–951. [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Lampert P. W., Lee S., Dixon F. J. Pathogenesis of the slow disease of the central nervous system associated with WM 1504 E virus. I. Relationship of strain susceptibility and replication to disease. Am J Pathol. 1977 Jul;88(1):193–212. [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Parker J. W., Taylor C. R., Pattengale P. K., Royston I., Tindle B. H., Cain M. J., Lukes R. J. Morphologic and cytochemical comparison of human lymphoblastoid T-cell and B-cell lines: light and electron microscopy. J Natl Cancer Inst. 1978 Jan;60(1):59–68. doi: 10.1093/jnci/60.1.59. [DOI] [PubMed] [Google Scholar]
- Premkumar E., Potter M., Singer P. A., Sklar M. D. Synthesis, surface deposition, and secretion of immunoglobulins by Abelson virus-transformed lymphosarcoma cell lines. Cell. 1975 Oct;6(2):149–159. doi: 10.1016/0092-8674(75)90005-7. [DOI] [PubMed] [Google Scholar]
- Ralph P. Functional subsets of murine and human B lymphocyte cell lines. Immunol Rev. 1979;48:107–121. doi: 10.1111/j.1600-065x.1979.tb00300.x. [DOI] [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Direct toxic effects of immunopotentiators on monocytic, myelomonocytic, and histiocytic or macrophage tumor cells in culture. Cancer Res. 1977 Feb;37(2):546–550. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Raschke W. C. Lymphosarcoma cell growth is selectively inhibited by B lymphocyte mitogens: LPS, dextran sulfate and PPD. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1268–1275. doi: 10.1016/s0006-291x(74)80421-3. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Chan E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J Virol. 1976 Jul;19(1):13–18. doi: 10.1128/jvi.19.1.13-18.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Gardner M. B., Rongey R. W., Nelson-Rees W. A., Arnstein P. Human bladder carcinoma: characterization of two new tumor cell lines and search for tumor viruses. J Natl Cancer Inst. 1977 Apr;58(4):881–890. doi: 10.1093/jnci/58.4.881. [DOI] [PubMed] [Google Scholar]
- Reddy E. P., Dunn C. Y., Aaronson S. A. Different lymphoid cell targets by transformation by replication-competent Moloney and Rauscher mouse leukemia viruses. Cell. 1980 Mar;19(3):663–669. doi: 10.1016/s0092-8674(80)80043-2. [DOI] [PubMed] [Google Scholar]
- Rice M. C., Gardner M. B., O'Brien S. J. Genetic diversity in leukemia-prone feral house mice infected with murine leukemia virus. Biochem Genet. 1980 Oct;18(9-10):915–928. doi: 10.1007/BF00500124. [DOI] [PubMed] [Google Scholar]
- Shevach E. M., Stobo J. D., Green I. Immunoglobulin and theta-bearing murine leukemias and lymphomas. J Immunol. 1972 May;108(5):1146–1151. [PubMed] [Google Scholar]
- Siegler R., Rich M. A. Pathogenesis of murine leukemia. Natl Cancer Inst Monogr. 1966 Sep;22:525–547. [PubMed] [Google Scholar]
- Slavin S., Strober S. Spontaneous murine B-cell leukaemia. Nature. 1978 Apr 13;272(5654):624–626. doi: 10.1038/272624a0. [DOI] [PubMed] [Google Scholar]
- Taylor C. R., Burns J. The demonstration of plasma cells and other immunoglobulin-containing cells in formalin-fixed, paraffin-embedded tissues using peroxidase-labelled antibody. J Clin Pathol. 1974 Jan;27(1):14–20. doi: 10.1136/jcp.27.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theilen G. H., Kawakami T. G., Rush J. D., Munn R. J. Replication of cat leukemia virus in cell suspension cultures. Nature. 1969 May 10;222(5193):589–590. doi: 10.1038/222589b0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S. Interspecies spleen-myeloma hybrid producing monoclonal antibodies against mouse lymphocyte surface glycoprotein, T200. J Exp Med. 1978 Jul 1;148(1):313–323. doi: 10.1084/jem.148.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. A slide centrifuge: an apparatus for concentrating cells in suspension onto a microscope slide. J Lab Clin Med. 1966 Sep;68(3):494–501. [PubMed] [Google Scholar]
- Wiener F., Babonits M., Spira J., Bregula U., Klein G., Merwin R. M., Asofsky R., Lynes M., Haughton G. Chromosome 15 trisomy in spontaneous and carcinogen-induced murine lymphomas of B-cell origin. Int J Cancer. 1981 Jan 15;27(1):51–58. doi: 10.1002/ijc.2910270109. [DOI] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]