Abstract
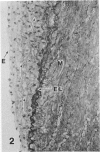
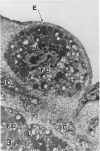
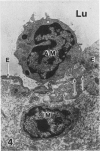
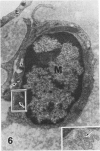
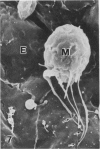
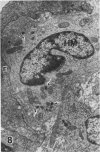
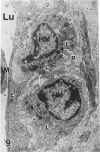
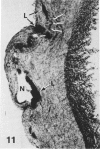
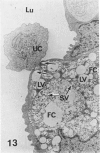
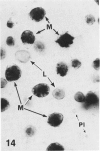
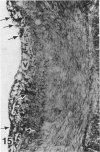
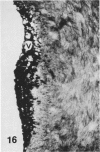
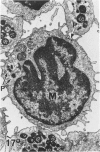
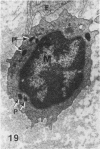
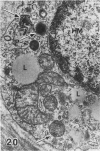
In a previous publication the author and his co-workers demonstrated that atherosclerotic lesion development in the aorta of hypercholesterolemic pigs was preceded by intimal penetration of blood-borne mononuclear cells, and that medial smooth muscle cells were not involved in the formation of early fatty lesions in this model. The current study shows that aortic arch lesions do not progress beyond the fatty cell lesion stage for up to 30 weeks of a moderate cholesterol/lard diet, although they become more extensive in area. Mononuclear cells were found adherent to the endothelium, in endothelial junctions, and in the intima during this period, and were ultrastructurally identified as monocytes by the presence of peroxidase-positive granules (peroxisomes) in their cytoplasm. In addition, lesion areas with nonspecific esterase activity correlated well with Sudan IV staining. Intimal monocytes and altered intimal monocytes with an enlarged cytoplasm and containing a few lipid droplets were both shown to be phagocytic by their uptake of ferritin, which had penetrated the intima after intravenous injection. Circulating monocytes and those adherent to the endothelial surface did not contain ferritin in these animals. The results indicate that blood mononuclear cells associated with lesion formation in this model are, in fact, monocytes, which subsequently undergo transformation into macrophage foam cells in fatty streak lesions. The absence of medial cell involvement indicates that monocytes are the major foam cell precursor in these lesions.
Full text
PDF

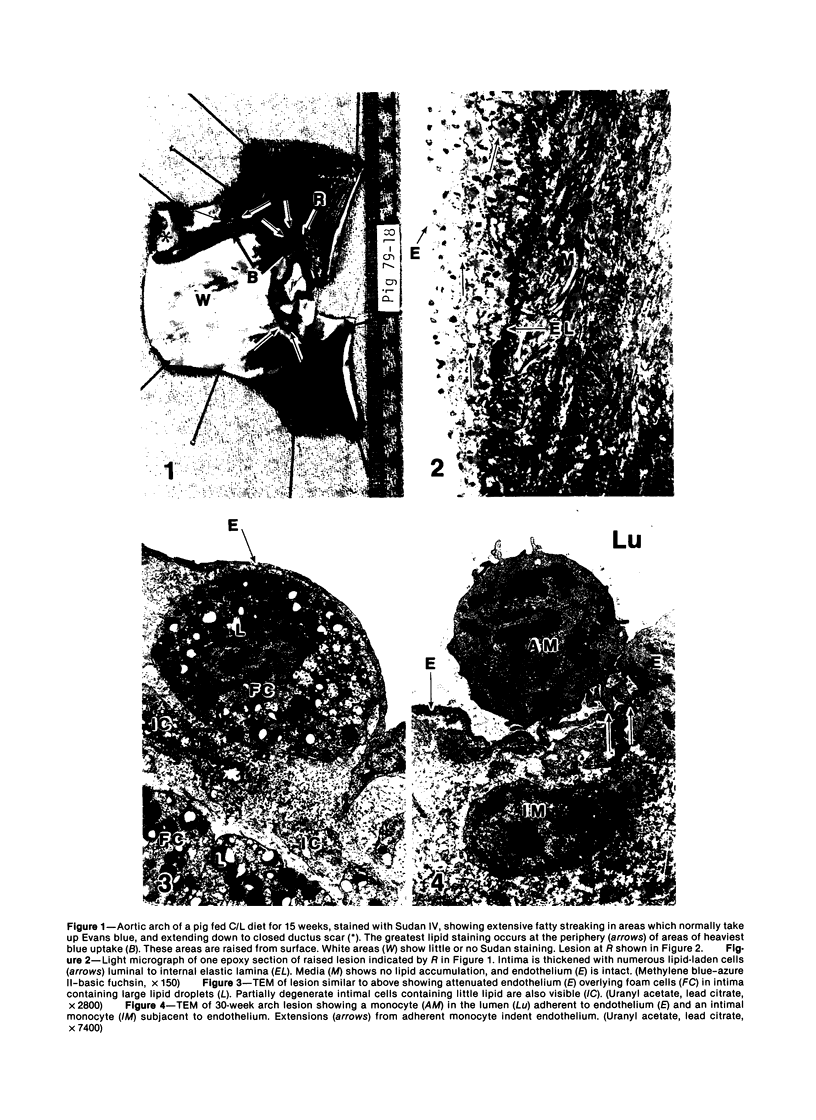
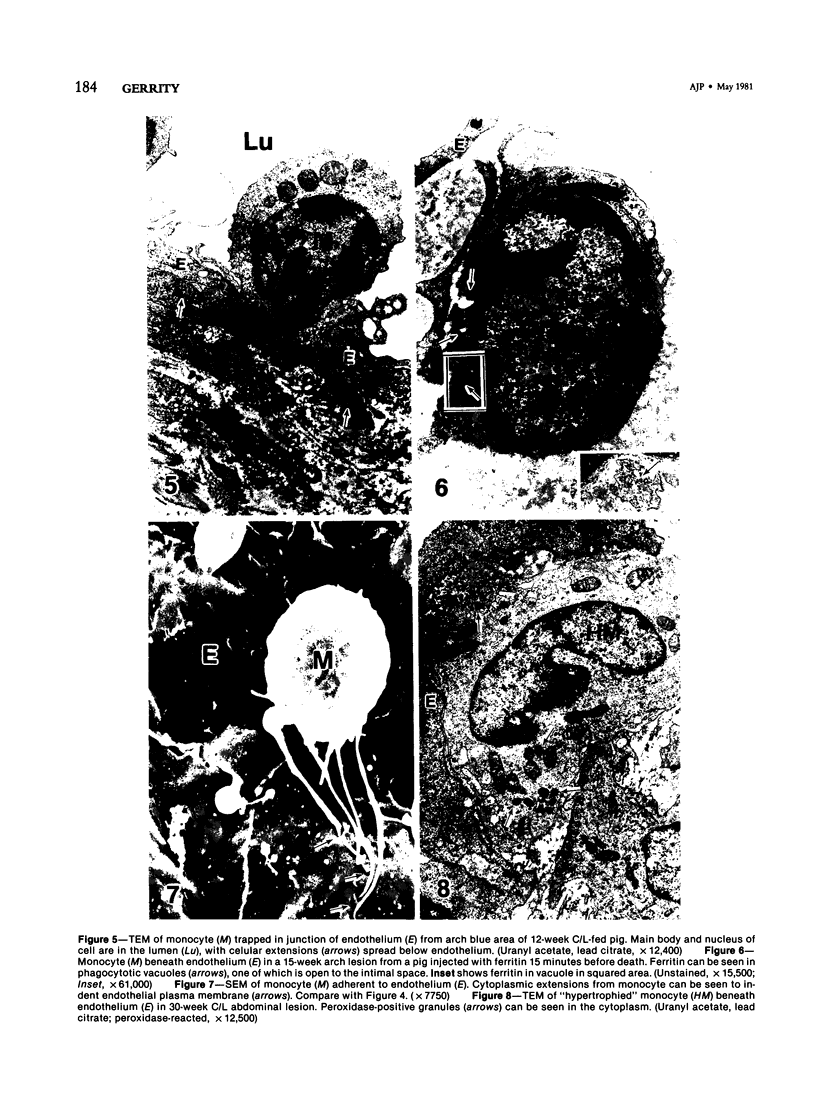
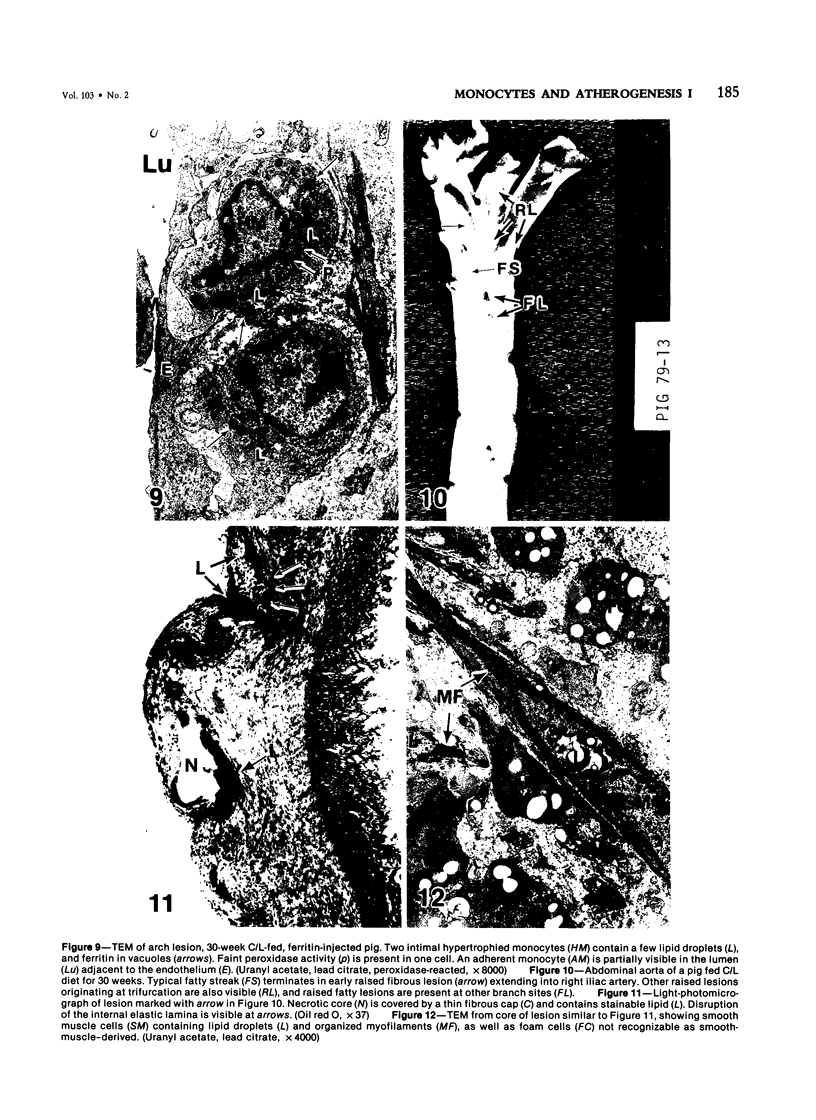



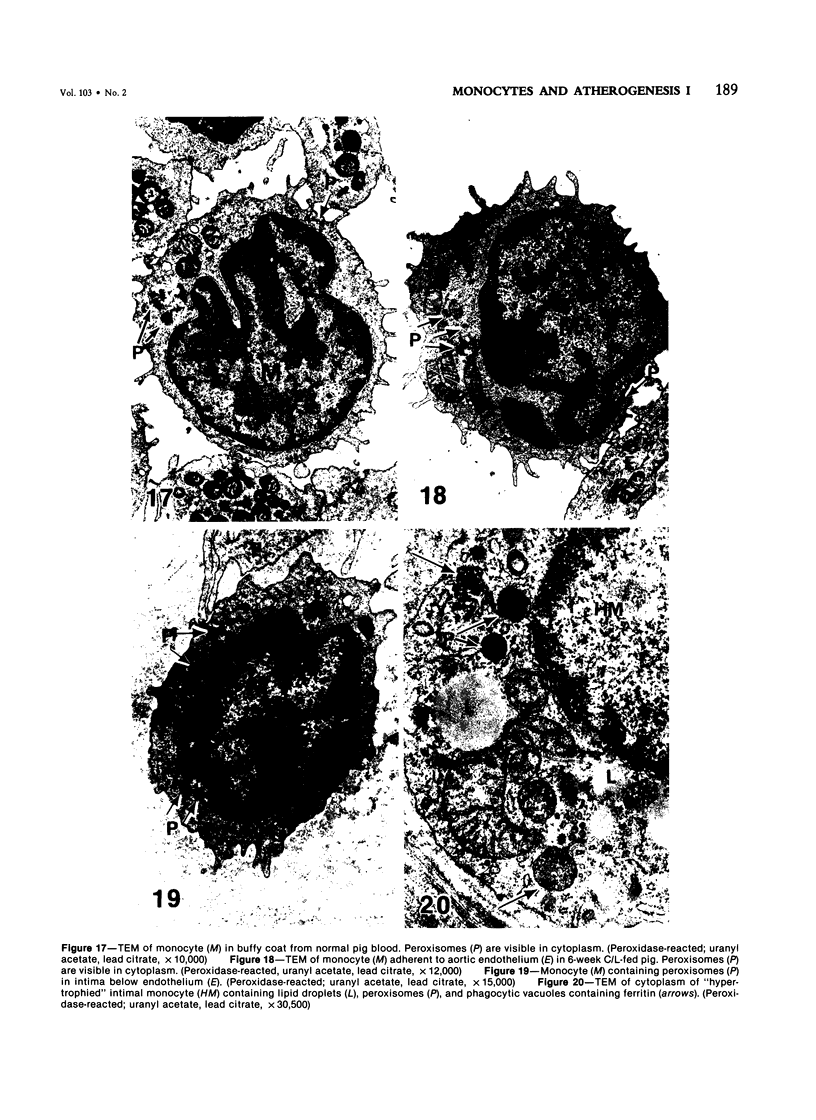

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Bayliss O. B. Detection of macrophages in atherosclerotic lesions with cytochrome oxidase. Br J Exp Pathol. 1976 Feb;57(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- Adams C. W., Bayliss O. B., Turner D. R. Phagocytes, lipid-removal and regression of atheroma. J Pathol. 1975 Aug;116(4):225–238. doi: 10.1002/path.1711160406. [DOI] [PubMed] [Google Scholar]
- BUCK R. C. The fine structure of the aortic endothelial lesions in experimental cholesterol atherosclerosis of rabbits. Am J Pathol. 1958 Sep-Oct;34(5):897–909. [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K., Herrlinger H., Luh S., Anzil A. P. Ultrastructural cytochemical demonstration of peroxidase-positive monocyte granules: an additional method for studying the origin of mononuclear cells in encephalitic lesions. Acta Neuropathol. 1978 Aug 7;43(1-2):55–61. doi: 10.1007/BF00684998. [DOI] [PubMed] [Google Scholar]
- Bodel P. T., Nichols B. A., Bainton D. F. Appearance of peroxidase reactivity within the rough endoplasmic reticulum of blood monocytes after surface adherence. J Exp Med. 1977 Feb 1;145(2):264–274. doi: 10.1084/jem.145.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day A. J. Lipid metabolism by macrophages and its relationship to atherosclerosis. Adv Lipid Res. 1967;5:185–207. doi: 10.1016/b978-1-4831-9941-2.50011-x. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Edwards P. A., Hokom M., Popják G. Cholesterol biosynthesis in human lymphocytes, monocytes, and granulocytes. Biochem Biophys Res Commun. 1977 May 9;76(1):167–173. doi: 10.1016/0006-291x(77)91682-5. [DOI] [PubMed] [Google Scholar]
- Gaton E., Wolman M. The role of smooth muscle cells and hematogenous macrophages in atheroma. J Pathol. 1977 Oct;123(2):123–128. doi: 10.1002/path.1711230208. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Richardson M., Somer J. B., Bell F. P., Schwartz C. J. Endothelial cell morphology in areas of in vivo Evans blue uptake in the aorta of young pigs. II. Ultrastructure of the intima in areas of differing permeability to proteins. Am J Pathol. 1977 Nov;89(2):313–334. [PMC free article] [PubMed] [Google Scholar]
- Gerrity R. G., Schwartz C. J. Structural correlates of arterial endothelial permeability in the Evans blue model. Prog Biochem Pharmacol. 1977;13:134–137. [PubMed] [Google Scholar]
- Ghidoni J. J., O'Neal R. M. Recent advances in molecular pathology: a review ultrastructure of human atheroma. Exp Mol Pathol. 1967 Dec;7(3):378–400. doi: 10.1016/0014-4800(67)90049-4. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Humphrey C. D., Pittman F. E. A simple methylene blue-azure II-basic fuchsin stain for epoxy-embedded tissue sections. Stain Technol. 1974 Jan;49(1):9–14. doi: 10.3109/10520297409116929. [DOI] [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Kim H. S., Suzuki M., O'Neal R. M. Leukocyte lipids of human blood. Am J Clin Pathol. 1967 Sep;48(3):314–319. doi: 10.1093/ajcp/48.3.314. [DOI] [PubMed] [Google Scholar]
- Marshall J. R., O'Neal R. M. The lipophage in hyperlipemic rats: an electron microscopic study. Exp Mol Pathol. 1966 Feb;5(1):1–11. doi: 10.1016/0014-4800(66)90002-5. [DOI] [PubMed] [Google Scholar]
- POOLE J. C., FLOREY H. W. Changes in the endothelium of the aorta and the behaviour of macrophages in experimental atheroma of rabbits. J Pathol Bacteriol. 1958 Apr;75(2):245–251. doi: 10.1002/path.1700750202. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., O'NEAL R. M. ACCUMULATION OF LIPIDS IN THE LEUKOCYTES OF RATS FED ATHEROGENIC DIETS. J Lipid Res. 1964 Oct;5:624–627. [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Stary H. C. Coronary artery fine structure in rhesus monkeys: the early atherosclerotic lesion and its progression. Primates Med. 1976;9:359–395. [PubMed] [Google Scholar]
- Stary H. C., Strong J. P. Coronary artery fine structure in rhesus monkeys: nonatherosclerotic intimal thickening. Primates Med. 1976;9:321–358. [PubMed] [Google Scholar]
- Stary H. C., Strong J. P. The fine structure of nonatherosclerotic intimal thickening, of developing, and of regressing atherosclerotic lesions at the bifurcation of the left coronary artery. Adv Exp Med Biol. 1976;67(00):89–108. doi: 10.1007/978-1-4614-4618-7_5. [DOI] [PubMed] [Google Scholar]
- Suzuki M., O'Neal R. M. Circulating lipophages, serum lipids, and atherosclerosis in rats. Arch Pathol. 1967 Feb;83(2):169–174. [PubMed] [Google Scholar]
- Taylor K., Schaffner T., Wissler R. W., Glagov S. Immuno-morphologic identification and characterization of cells derived from experimental atherosclerotic lesions. Scan Electron Microsc. 1979;(3):815–822. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D., Grusky G., Marcus A. Transformation of monocytes into "fat" cells. Lab Invest. 1978 May;38(5):620–628. [PubMed] [Google Scholar]