Abstract
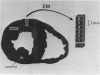
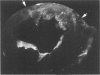
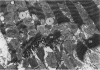
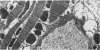
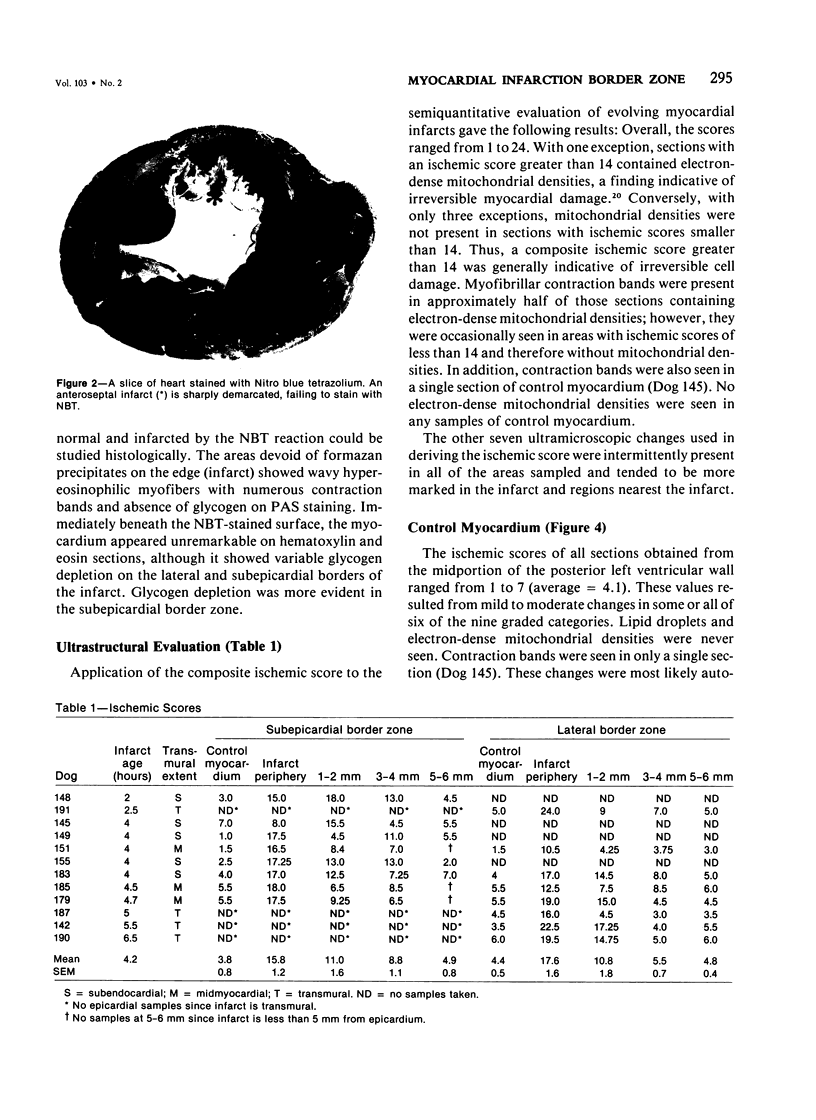
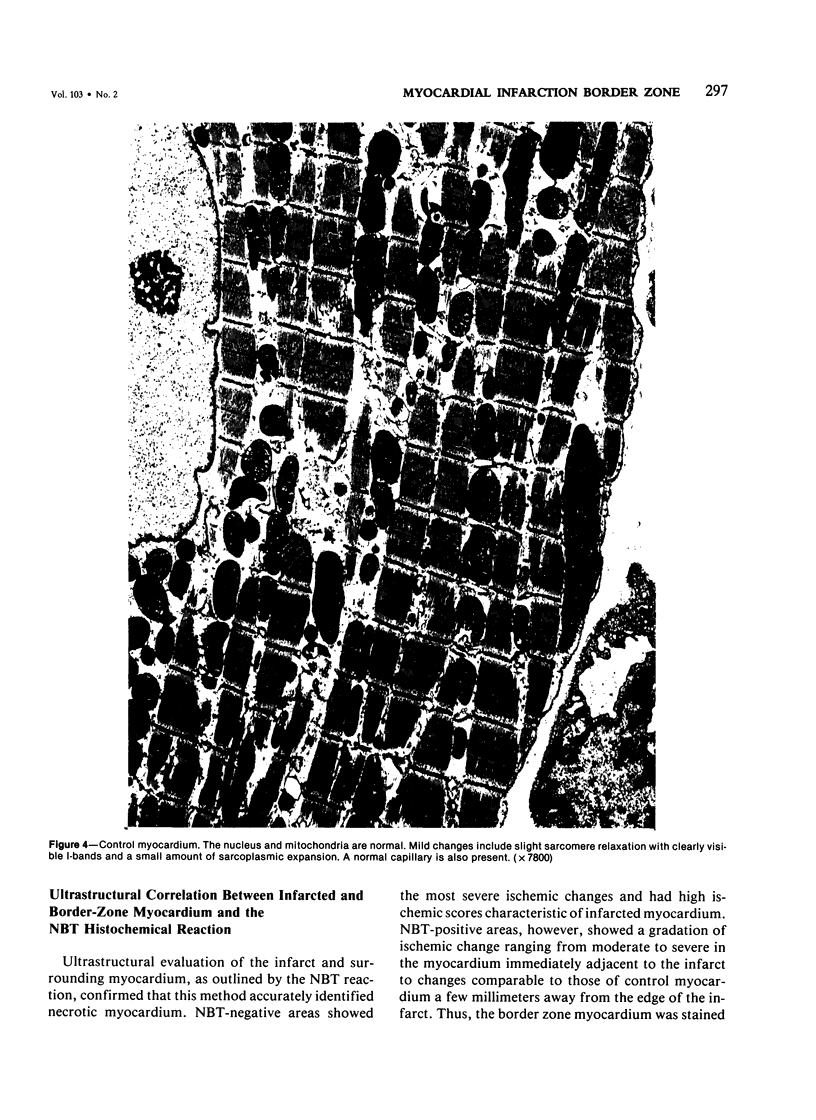
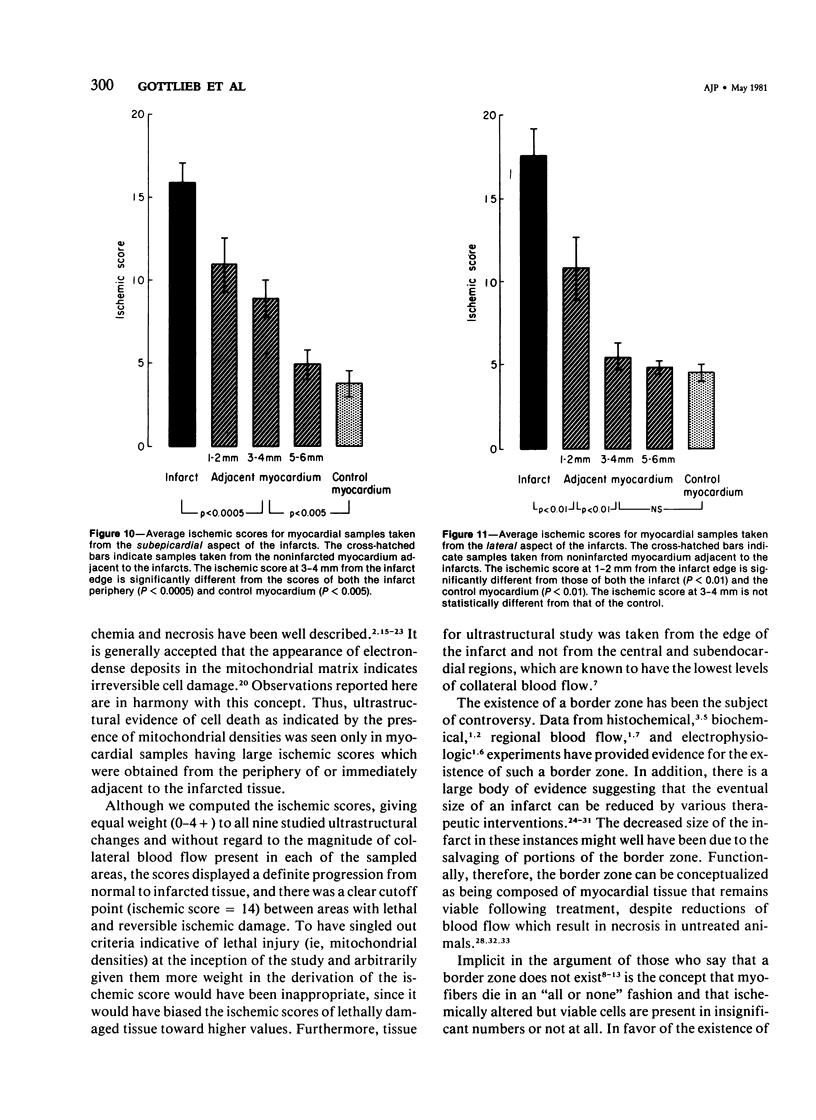
The existence of a border zone composed of reversibly injured myocardium surrounding an evolving infarct has been the subject of controversy. In experiments designed to search for such a border zone by electron microscopy, 12 mongrel dogs underwent permanent ligation of the left anterior descending coronary artery (LAD). Two to 6.5 (average = 4.2) hours later, the hearts were excised, the area at risk (myocardium perfused by the LAD) was outlined by injection of fluorescent microspheres, and the myocardial infarct was demonstrated by the nitro blue tetrazolium (NBT) gross histochemical method. Myocardial samples for electron-microscopic study were obtained from the periphery of the infarct (tissues unstained by NBT) and serially from the immediately adjacent myocardium, which was stained deep blue by NBT. Grossly, the infarcts always involved the subendocardial myocardium, extended for a variable distance in the epicardial direction, and closely approximated the lateral margins of the area at risk. When examined by electron microscopy, the infarct periphery showed evidence of irreversible damage, thus confirming the ability of NBT to detect early myocardial necrosis. Multiple samples of the NBT-stained myocardium immediately adjacent to the infarct showed varying degrees of reversible ischemia, thus demonstrating, at the ultrastructural level, the existence of a border zone of intermediate myocardial injury. This border zone was substantial (3--4 mm in width) along the subepicardial aspect of the infarct and very thin (1--2 mm) laterally. In conclusion, a significant border zone was demonstrable by electron microscopy in the subepicardial myocardium of 8 out or 12 canine hearts with recent coronary artery occlusion. In the remaining 4 hearts, the infarcts had already reached the epicardium at the time of study, and only a thin lateral border zone was present.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armiger L. C., Gavin J. B. Changes in the microvasculature of ischemic and infarcted myocardium. Lab Invest. 1975 Jul;33(1):51–56. [PubMed] [Google Scholar]
- Armiger L. C., Seelye R. N., Carnell V. M., Smith C. U., Gavin J. B., Herdson P. B. Morphologic and biochemical changes in autolysing dog heart muscle. Lab Invest. 1976 Apr;34(4):357–362. [PubMed] [Google Scholar]
- Barlow C. H., Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science. 1976 Sep 3;193(4256):909–910. doi: 10.1126/science.181843. [DOI] [PubMed] [Google Scholar]
- Bilheimer D. W., Buja L. M., Parkey R. W., Bonte F. J., Willerson J. T. Fatty acid accumulation and abnormal lipid deposition in peripheral and border zones of experimental myocardial infarcts. J Nucl Med. 1978 Mar;19(3):276–283. [PubMed] [Google Scholar]
- CAULFIELD J., KLIONSKY B. Myocardial ischemia and early infarction: an electron microscopic study. Am J Pathol. 1959 May-Jun;35(3):489–523. [PMC free article] [PubMed] [Google Scholar]
- Cox J. L., Daniel T. M., Boineau J. P. The electrophysiologic time-course of acute myocardial ischemia and the effects of early coronary artery reperfusion. Circulation. 1973 Nov;48(5):971–983. doi: 10.1161/01.cir.48.5.971. [DOI] [PubMed] [Google Scholar]
- Cox J. L., McLaughlin V. W., Flowers N. C., Horan L. G. The ischemic zone surrounding acute myocardial infarction. Its morphology as detected by dehydrogenase staining. Am Heart J. 1968 Nov;76(5):650–659. doi: 10.1016/0002-8703(68)90164-6. [DOI] [PubMed] [Google Scholar]
- Dusek J. Significance of morphological changes at the periphery of an experimental myocardial infarct. Recent Adv Stud Cardiac Struct Metab. 1972;1:430–438. [PubMed] [Google Scholar]
- Factor S. M., Sonnenblick E. H., Kirk E. S. The histologic border zone of acute myocardial infarction--islands or peninsulas? Am J Pathol. 1978 Jul;92(1):111–124. [PMC free article] [PubMed] [Google Scholar]
- Hearse J. D., Opie L. H., Katzeff I. E., Lubbe W. F., Van der Werff T. J., Peisach M., Boulle G. Characterization of the "border zone" in acute regional ischemia in the dog. Am J Cardiol. 1977 Nov;40(5):716–726. doi: 10.1016/0002-9149(77)90187-4. [DOI] [PubMed] [Google Scholar]
- Hirzel H. O., Sonnenblick E. H., Kirk E. S. Absence of a lateral border zone of intermediate creatine phosphokinase depletion surrounding a central infarct 24 hours after acute coronary occlusion in the dog. Circ Res. 1977 Nov;41(5):673–683. doi: 10.1161/01.res.41.5.673. [DOI] [PubMed] [Google Scholar]
- JENNINGS R. B., BAUM J. H., HERDSON P. B. FINE STRUCTURAL CHANGES IN MYOCARDIAL ISCHEMIC INJURY. Arch Pathol. 1965 Feb;79:135–143. [PubMed] [Google Scholar]
- JENNINGS R. B., SOMMERS H. M., SMYTH G. A., FLACK H. A., LINN H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960 Jul;70:68–78. [PubMed] [Google Scholar]
- Janse M. J., Cinca J., Moréna H., Fiolet J. W., Kléber A. G., de Vries G. P., Becker A. E., Durrer D. The "border zone" in myocardial ischemia. An electrophysiological, metabolic, and histochemical correlation in the pig heart. Circ Res. 1979 Apr;44(4):576–588. doi: 10.1161/01.res.44.4.576. [DOI] [PubMed] [Google Scholar]
- Jennings R. B., Ganote C. E. Structural changes in myocardium during acute ischemia. Circ Res. 1974 Sep;35 (Suppl 3):156–172. [PubMed] [Google Scholar]
- Jennings R. B., Sommers H. M., Herdson P. B., Kaltenbach J. P. Ischemic injury of myocardium. Ann N Y Acad Sci. 1969 Jan 31;156(1):61–78. doi: 10.1111/j.1749-6632.1969.tb16718.x. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Braunwald E., Maroko P. R. Long-term preservation of ischemic myocardium in the dog by hyaluronidase. Circulation. 1978 Aug;58(2):220–226. doi: 10.1161/01.cir.58.2.220. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Korb G., Totović V. Electron microscopical studies on experimental ischemic lesions of the heart. Ann N Y Acad Sci. 1969 Jan 31;156(1):48–60. doi: 10.1111/j.1749-6632.1969.tb16717.x. [DOI] [PubMed] [Google Scholar]
- MAJNO G., LA GATTUTA M., THOMPSON T. E. Cellular death and necrosis: chemical, physical and morphologic changes in rat liver. Virchows Arch Pathol Anat Physiol Klin Med. 1960;333:421–465. doi: 10.1007/BF00955327. [DOI] [PubMed] [Google Scholar]
- Marcus M. L., Kerber R. E., Ehrhardt J., Abboud F. M. Three dimensional geometry of acutely ischemic myocardium. Circulation. 1975 Aug;52(2):254–263. doi: 10.1161/01.cir.52.2.254. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Bernstein E. F., Libby P., DeLaria G. A., Covell J. W., Ross J., Jr, Braunwald E. Effects of intraaortic balloon counterpulsation on the severity of myocardial ischemic injury following acute coronary occlusion. Counterpulsation and myocardial injury. Circulation. 1972 Jun;45(6):1150–1159. doi: 10.1161/01.cir.45.6.1150. [DOI] [PubMed] [Google Scholar]
- Maroko P. R., Braunwald E. Modification of myocardial infarction size after coronary occlusion. Ann Intern Med. 1973 Nov;79(5):720–733. doi: 10.7326/0003-4819-79-5-720. [DOI] [PubMed] [Google Scholar]
- Mathur V. S., Guinn G. A., Burris W. H., 3rd Maximal revascularization (reperfusion) in intact conscious dogs after 2 to 5 hours of coronary occlusion. Am J Cardiol. 1975 Aug;36(2):252–261. doi: 10.1016/0002-9149(75)90534-2. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., SHNITKA T. K. Macroscopic identification of early myocardial infarcts by alterations in dehydrogenase activity. Am J Pathol. 1963 Apr;42:379–405. [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M. M., Reimer K. A., Kloner R. A., Jennings R. B. Infarct size reduction by propranolol before and after coronary ligation in dogs. Circulation. 1977 Nov;56(5):794–798. doi: 10.1161/01.cir.56.5.794. [DOI] [PubMed] [Google Scholar]
- Reimer K. A., Jennings R. B. The "wavefront phenomenon" of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979 Jun;40(6):633–644. [PubMed] [Google Scholar]
- Reimer K. A., Lowe J. E., Rasmussen M. M., Jennings R. B. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977 Nov;56(5):786–794. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- Smith H. J., Singh B. N., Nisbet H. D., Norris R. M. Effects of verapamil on infarct size following experimental coronary occlusion. Cardiovasc Res. 1975 Jul;9(4):569–578. doi: 10.1093/cvr/9.4.569. [DOI] [PubMed] [Google Scholar]
- Vokonas P. S., Malsky P. M., Paul S. J., Robbins S. L., Hood W. B., Jr Radioautographic studies in experimental myocardial infarction: profiles of ischemic blood flow and quantification of infarct size in relation to magnitude of ischemic zone. Am J Cardiol. 1978 Jul;42(1):67–75. doi: 10.1016/0002-9149(78)90987-6. [DOI] [PubMed] [Google Scholar]