Abstract
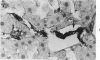
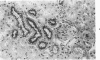
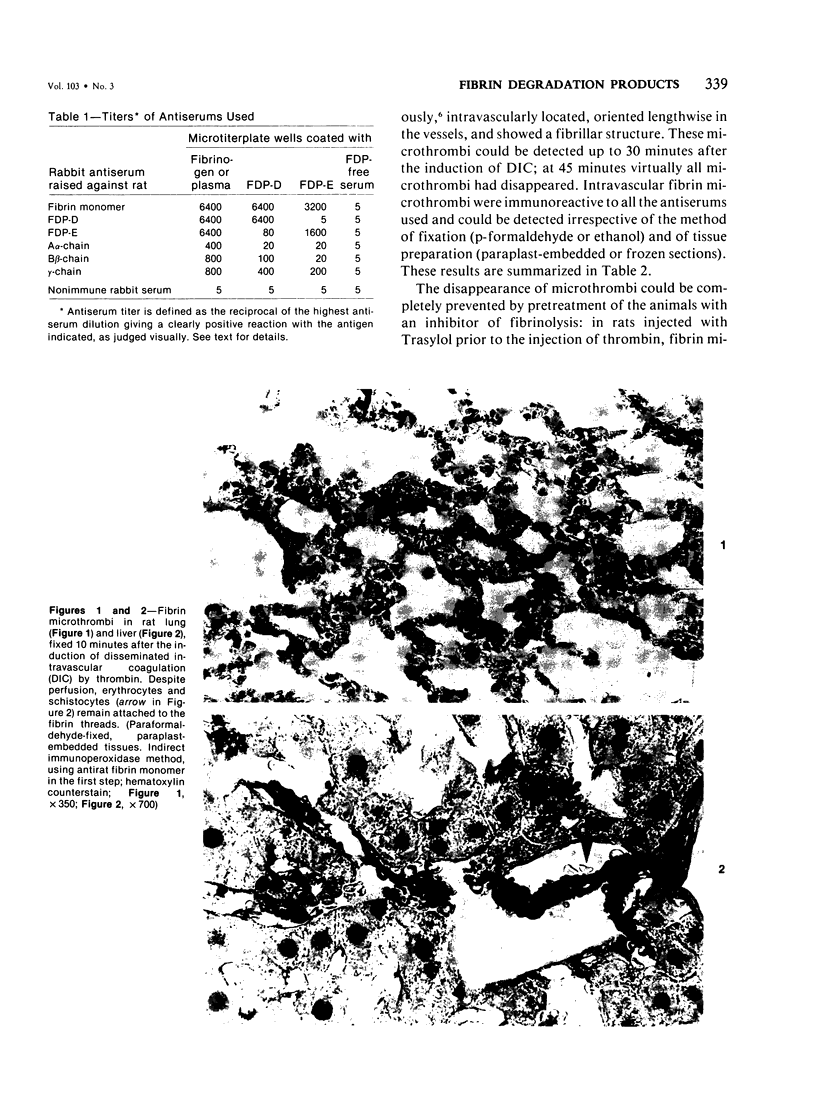
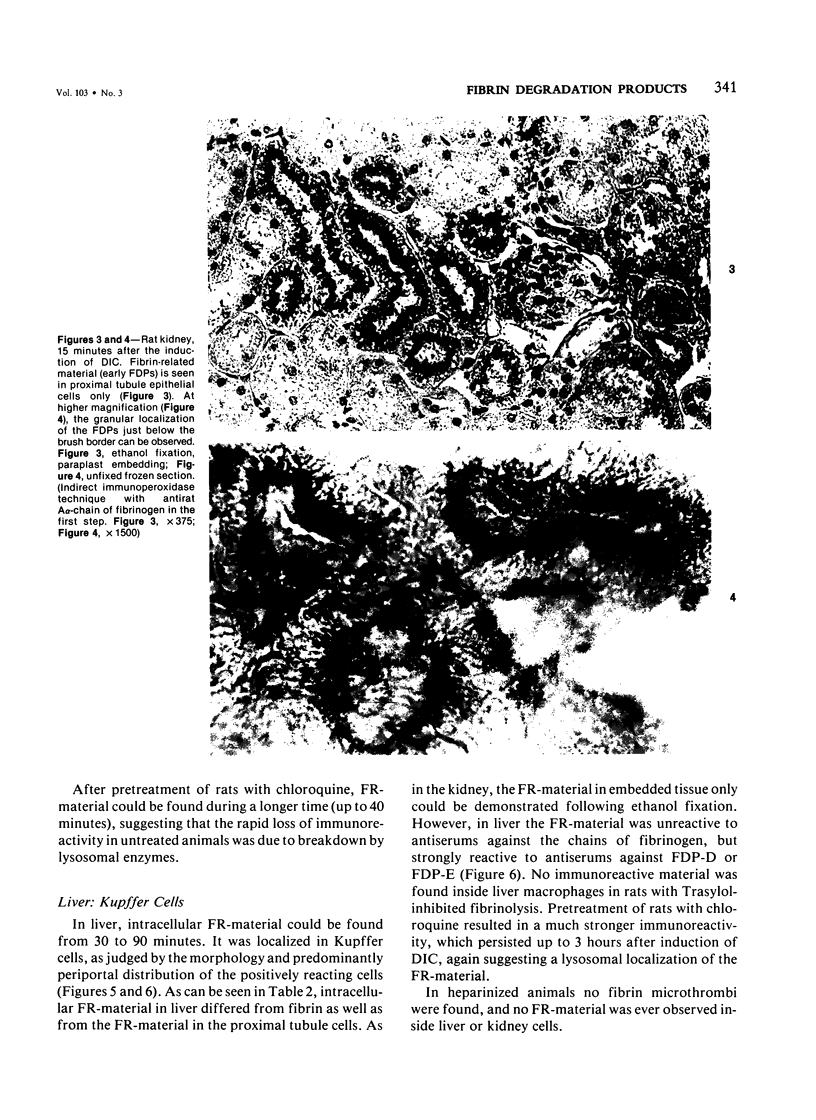
An immunoenzyme histochemical study was conducted to localize fibrin degradation products (FDPs) in rat tissues during disseminated intravascular coagulation (DIC). Serial measurements of FDP levels in serum after thrombin-induced DIC showed peak levels to be found at 30 minutes; the FDPs were rapidly cleared from the circulation (half-life about one hour). Rat tissues obtained from 10 minutes to 3 hours after the induction of DIC were studied by means of immunohistochemistry. A method was developed to differentiate FDPs from fibrin in tissue sections. This method is based on the observation that, in paraplast-embedded tissues, FDPs can be demonstrated following ethanol fixation only, and that fibrin is demonstrable after both paraformaldehyde fixation and ethanol fixation. Moreover, FDPs will react to some of the antiserums employed only, while fibrin will react to all antiserum used (antiserums against fibrin monomer, against the constituent chains of fibrinogen, and against FDP-D and -E). At 10-20 minutes after the induction of DIC, FDPs were found in kidney proximal tubule epithelial cells. These FDPs could be demonstrated using antiserum against the constituent chains of fibrinogen, but not by antiserums against FDP-D or -E. At 30-90 minutes, FDPs were found inside liver macrophages. The FDPs in liver did not react to anti-chain antiserums, though they did react to antiserums against FDP-D and -E. Since no FDPs were found in other tissues, rat FDPs are apparently cleared by kidney (earlier phase) and liver (later phase) only. In human cases of DIC, FDPs, could be demonstrated in kidney proximal tubules cells and in liver macrophages as well.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Busch C., Rammer L., Saldeen T. Quantitation of fibrin deposition and elimination in organs of rats injected with labelled fibrinogen and albumin. Thromb Diath Haemorrh. 1973 Feb 28;29(1):94–107. [PubMed] [Google Scholar]
- Coombey D., Tyler H. M. Quantitative monitoring of intravascular coagulation and fibrinolysis in the lungs of rats. Thromb Diath Haemorrh. 1972 Apr 30;27(2):241–245. [PubMed] [Google Scholar]
- Craane H., Emeis J. J., Lindeman J., Nieuwenhuizen W. Immunoenzymehistochemical detection of fibrin microthrombi during disseminated intravascular coagulation rats. Histochemistry. 1978 Aug 25;57(2):97–105. doi: 10.1007/BF00496674. [DOI] [PubMed] [Google Scholar]
- Diffang C., Saldeen T. Effect of Trasylol on fibrin deposition and elimination in the lungs of rats with intravascular coagulation induced by thrombin or thromboplastin. Thromb Res. 1974 Sep;5(3):263–276. doi: 10.1016/0049-3848(74)90166-2. [DOI] [PubMed] [Google Scholar]
- Emeis J. J., Lindeman J. Rat liver macrophages will not phagocytose fibrin during disseminated intravascular coagulation. Haemostasis. 1976;5(4):193–210. doi: 10.1159/000214135. [DOI] [PubMed] [Google Scholar]
- Gaffney P. J. Structure of fibrinogen and degradation products of fibrinogen and fibrin. Br Med Bull. 1977 Sep;33(3):245–251. doi: 10.1093/oxfordjournals.bmb.a071443. [DOI] [PubMed] [Google Scholar]
- Hayne O. A., Sherman L. A. In vivo behavior of fibrinogen fragment D in experimental renal, hepatic and reticuloendothelial dysfunction. Am J Pathol. 1973 May;71(2):219–238. [PMC free article] [PubMed] [Google Scholar]
- Iio A., Rutherford W. E., Wochner R. D., Spilberg I., Sherman L. A. The roles of renal catabolism and uremia in modifying the clearance of fibrinogen and its degradative fragments D and E. J Lab Clin Med. 1976 Jun;87(6):934–946. [PubMed] [Google Scholar]
- Lindsey G. G., Brown G., Merrifield E. H., Purves L. R. The carbohydrate prosthetic groups of rat fibrinogen plasmic fragment E. Biochim Biophys Acta. 1979 Mar 27;577(1):110–116. doi: 10.1016/0005-2795(79)90012-6. [DOI] [PubMed] [Google Scholar]
- Markwardt F., Drawert J., Perlewitz J. Pharmakologische Beeinflussung der sekundären generalisierten Fibrinolyse. Acta Biol Med Ger. 1979;38(8):1201–1209. [PubMed] [Google Scholar]
- Markwardt F., Nowak G., Meerbach W., Rüdiger K. D. Studies in experimental animals on disseminated intravascular coagulation (DIC). Thromb Diath Haemorrh. 1975 Nov 15;34(2):513–521. [PubMed] [Google Scholar]
- Merskey C., Lalezari P., Johnson A. J. A rapid, simple, sensitive method for measuring fibrinolytic split products in human serum. Proc Soc Exp Biol Med. 1969 Jul;131(3):871–875. doi: 10.3181/00379727-131-33998. [DOI] [PubMed] [Google Scholar]
- Saldeen T. Quantiative determination of intravascular coagulation in the lungs of experimental animals. Scand J Haematol. 1969;6(3):205–215. doi: 10.1111/j.1600-0609.1969.tb01827.x. [DOI] [PubMed] [Google Scholar]
- Saldeen T. Trends in microvascular research. The microembolism syndrome. Microvasc Res. 1976 Mar;11(2):227–259. doi: 10.1016/0026-2862(76)90054-6. [DOI] [PubMed] [Google Scholar]
- Sherman L. A. Catabolism of fibrinogen and its derivatives. Thromb Haemost. 1977 Dec 15;38(4):809–822. [PubMed] [Google Scholar]
- Stein Y., Ebin V., Bar-On H., Stein O. Chloroquine-induced interference with degradation of serum lipoproteins in rat liver, studied in vivo and in vitro. Biochim Biophys Acta. 1977 Feb 23;486(2):286–297. doi: 10.1016/0005-2760(77)90024-8. [DOI] [PubMed] [Google Scholar]
- Van Ruijven-Vermeer I. A., Nieuwenhuizen W. Purification of rat fibrinogen and its constituent chains. Biochem J. 1978 Mar 1;169(3):653–658. doi: 10.1042/bj1690653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh R. T., Barnhart M. I. Clearance of coagulation and fibrinolysis products by the reticuloendothelial system. Thromb Diath Haemorrh Suppl. 1969;36:83–97. [PubMed] [Google Scholar]
- van Ruijven-Vermeer I. A., Nieuwenhuizen W., Haverkate F., Timan T. A novel method for the rapid purification of human and rat fibrin(ogen) degradation products in high yields. Hoppe Seylers Z Physiol Chem. 1979 May;360(5):633–637. doi: 10.1515/bchm2.1979.360.1.633. [DOI] [PubMed] [Google Scholar]