Abstract
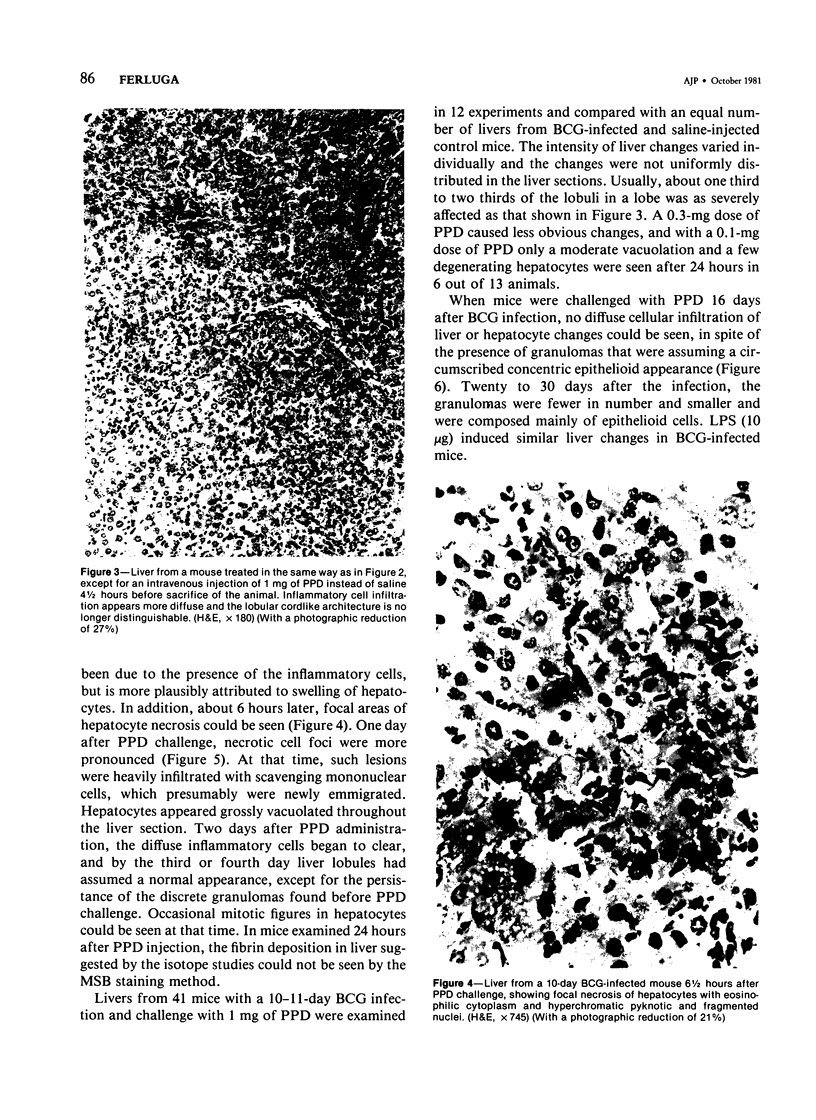
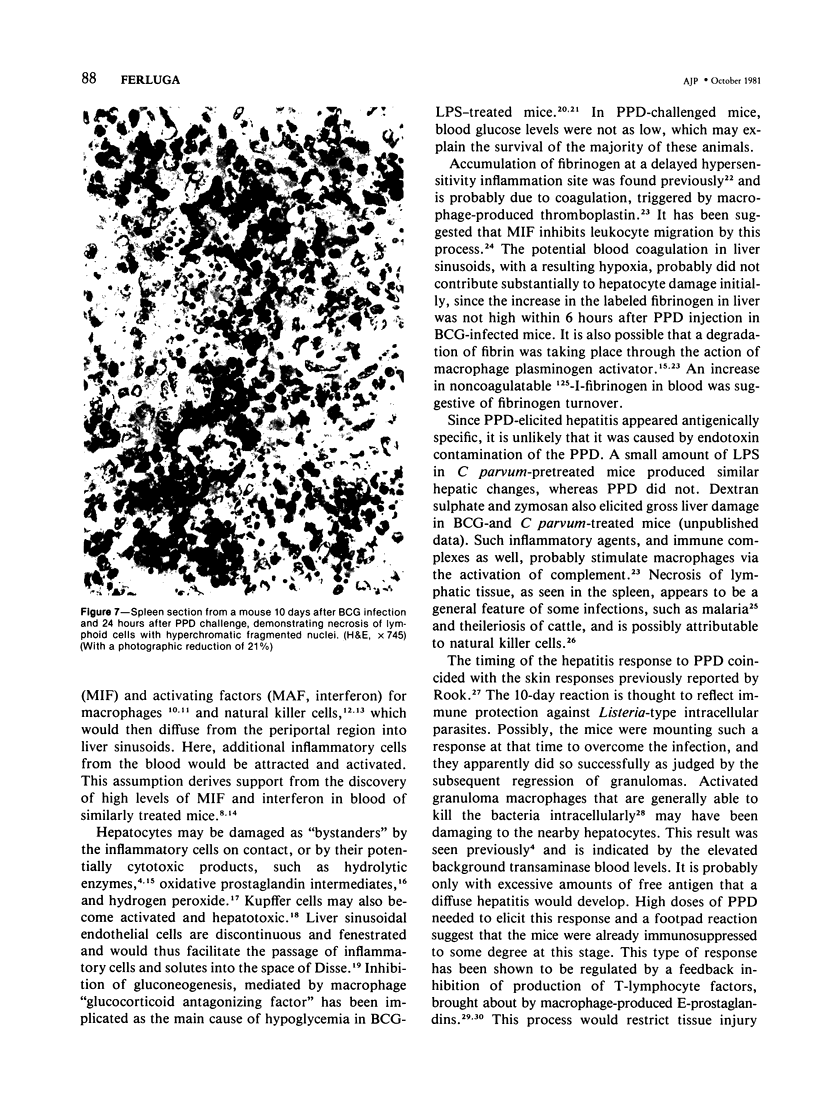
A systemic BCG infection in mice induced multiple small granulomas located mainly in the periportal areas of the liver. Following systemic challenge of such mice with purified protein derivative of tuberculin (PPD), a rapidly developing hepatitis with diffuse intralobular mononuclear cell infiltration was precipitated, accompanied by high levels of aspartate transaminase in peripheral blood, hypoglycemia, focal hepatocyte necrosis, and accumulation of fibrinogen in liver. Bacterial lipopolysaccharide (LPS) also provoked acute hepatic damage both in BCG-infected mice and in mice pretreated with Corynebacterium parvum. PPD was not active in the latter. There were also lymphoid cell destruction and fibrinogen accumulation in the spleen of BCG-PPD-treated mice. Possible involvement of inflammatory and hepatotoxic mediators is suggested, and a T-lymphocyte-macrophage regulatory role in the pathogenesis of hepatitis is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Macrophage activation and nonspecific immunity. Int Rev Exp Pathol. 1978;18:303–346. [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisari F. V., Castle K. L., Xavier C., Anderson D. S. Functional properties of lymphocyte subpopulations in hepatitis B virus infection. I. Suppressor cell control of T lymphocyte responsiveness. J Immunol. 1981 Jan;126(1):38–44. [PubMed] [Google Scholar]
- Clark I. A., Clouston W. M. Effects of endotoxin on the histology of intact and athymic mice infected with Plasmodium vinckei petteri. J Pathol. 1980 Jul;131(3):221–233. doi: 10.1002/path.1711310304. [DOI] [PubMed] [Google Scholar]
- Colvin R. B., Johnson R. A., Mihm M. C., Jr, Dvorak H. F. Role of the clotting system in cell-mediated hypersensitivity. I. Fibrin deposition in delayed skin reactions in man. J Exp Med. 1973 Sep 1;138(3):686–698. doi: 10.1084/jem.138.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Ferluga J., Doenhoff M. J., Allison A. C. Increased hepatotoxicity of bacterial lipopolysaccharide in mice infected with Schistosoma mansoni. Parasite Immunol. 1979 Winter;1(4):289–294. doi: 10.1111/j.1365-3024.1979.tb00714.x. [DOI] [PubMed] [Google Scholar]
- Ferluga J., Kaplun A., Allison A. C. Protection of mice against endotoxin-induced liver damage by anti-inflammatory drugs. Agents Actions. 1979 Dec;9(5-6):566–574. doi: 10.1007/BF01968129. [DOI] [PubMed] [Google Scholar]
- Gordon D., Bray M. A., Morley J. Control of lymphokine secretion by prostaglandins. Nature. 1976 Jul 29;262(5567):401–402. doi: 10.1038/262401a0. [DOI] [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Kuehl F. A., Jr, Humes J. L., Egan R. W., Ham E. A., Beveridge G. C., Van Arman C. G. Role of prostaglandin endoperoxide PGG2 in inflammatory processes. Nature. 1977 Jan 13;265(5590):170–173. doi: 10.1038/265170a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Churchill W. H., Jr, David Macrophages activated in vitro with lymphocyte mediators kill neoplastic but not normal cells. J Immunol. 1975 Jan;114(1 Pt 2):293–299. [PubMed] [Google Scholar]
- Playfair J. H., De Souza J. B., Dockrell H. M., Agomo P. U., Taverne J. Cell-mediated immunity in the liver of mice vaccinated against malaria. Nature. 1979 Dec 13;282(5740):731–734. doi: 10.1038/282731a0. [DOI] [PubMed] [Google Scholar]
- Poste G. The tumoricidal properties of inflammatory tissue macrophages and multinucleate giant cells. Am J Pathol. 1979 Aug;96(2):595–610. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. Three forms of delayed skin-test response evoked by mycobacteria. Nature. 1978 Jan 5;271(5640):64–65. doi: 10.1038/271064a0. [DOI] [PubMed] [Google Scholar]
- Salvin S. B., Youngner J. S., Lederer W. H. Migration inhibitory factor and interferon in the circulation of mice with delayed hypersensitivity. Infect Immun. 1973 Jan;7(1):68–75. doi: 10.1128/iai.7.1.68-75.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Chirigos M. A. Similarities among factors that render macrophages tumoricidal in lymphokine and interferon preparations. Cancer Res. 1978 Apr;38(4):1003–1007. [PubMed] [Google Scholar]
- Senik A., Stefanos S., Kolb J. P., Lucero M., Falcoff E. Enhancement of mouse natural killer cell activity by type II interferon. Ann Immunol (Paris) 1980 May-Jun;131C(3):349–361. [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W., Jr Endotoxin induced metabolic alterations in BCG infected (hyperreactive) mice. Proc Soc Exp Biol Med. 1978 Oct;159(1):69–74. doi: 10.3181/00379727-159-40286. [DOI] [PubMed] [Google Scholar]
- Shands J. W., Jr, Senterfitt V. C. Endotoxin-induced hepatic damage in BCG-infected mice. Am J Pathol. 1972 Apr;67(1):23–40. [PMC free article] [PubMed] [Google Scholar]
- Tavassoli M. Selected items from the history of pathology -- Ignaz Philipp Semmelweis (1818-1865). Am J Pathol. 1980 Oct;101(1):114–114. [PMC free article] [PubMed] [Google Scholar]
- Trinder P. Determination of blood glucose using 4-amino phenazone as oxygen acceptor. J Clin Pathol. 1969 Mar;22(2):246–246. doi: 10.1136/jcp.22.2.246-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe S. A., Tracey D. E., Henney C. S. BCG-induced murine effector cells. II. Characterization of natural killer cells in peritoneal exudates. J Immunol. 1977 Sep;119(3):1152–1158. [PubMed] [Google Scholar]
- de Moura M. C., Vernace S. J., Paronetto F. Cell-mediated immune reactivity to hepatitis B surface antigen in liver diseases. Gastroenterology. 1975 Aug;69(2):310–317. [PubMed] [Google Scholar]